
Co-intensification of cyanide leaching gold by mercury ions and oxidant
LI Qian(李 骞), JIANG Tao(姜 涛), YANG Yong-bin(杨永斌),
LI Guang-hui(李光辉), GUO Yu-feng(郭宇峰), QIU Guan-zhou(邱冠周)
School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 4 January 2010
Abstract:The effects of mercury ions on gold cyanidation were studied. The results show that under low cyanide concentration, gold cyanide process is controlled by CN- transfer, while at higher cyanide concentration, there forms passivation on gold surface. Therefore, chemical oxidation of gold in cyanide solution of higher concentration is controlled by surface reaction. Small quantity of additions of mercury ions bring about great increases in anodic gold dissolution rate, decreases the passivation and reduces the equilibrium activated energy. In addition, they also markedly change the effect pattern of cyanide concentration. Mercury ions show positive effects on cathodic reduction of oxygen and raise the rate of electrochemical step of the cathodic reduction of oxygen. Addition of a certain amount of hydrogen peroxide is confirmed to be an effective way for intensification of cathodic process on gold electrode. Active potential range and current peak on anodic dissolution are enlarged when being co-intensified with Hg2+ and hydrogen peroxide. Co-intensifying effect may be obtained and gold leaching rate is considerably increased on cyanide leaching of gold from gold concentrates.
Key words:
co-intensification; cyanide leaching; mercury ions; hydrogen peroxide;
1 Introduction
It is well known that the dissolution of gold in aqueous cyanide solutions is an electrochemical process[1-2] in which the anodic reaction is gold oxidation and formation of Au(CN-)2 while the cathodic reaction is oxygen reduction. The equations are as follows:
Anodic area:![]() (1)
(1)
Cathodic area: O2+2H2O+2e→H2O2+2OH- (2)
The essential of intensification of gold cyanidation is to strengthen the electrode processes. The application of alternative oxidants such as permanganates, peroxides, ozone, dichromates, bromides and hydrogen peroxide as oxidizing agents belongs to strengthening the cathodic process[3]. The anodic process must be strengthened to obtain maximum dissolution rate of gold.
It is known that gold dissolution is unexpectedly passified in cyanide solutions[4]. The mechanism for this passivation of gold is the formation of polymeric AuCN or the adsorption of AuOH on the surface of gold[5]. It has been reported[6-8] that a small amount of heavy metallic ions such as thallium(Ⅰ), lead(Ⅱ), mercury(Ⅰ) and bismuth(Ⅲ) in alkaline cyanide solutions considerably accelerate the rate of gold dissolution. JIN et al[9] found that the presence of heavy mental salts in sodium cyanide solutions decreases the time for gold dissolution, for instance, a lead concentration of the order of 5 mg/L is able to multiply the reaction rate by four. CATHRO and KOCH[10] studied the thallium effect on the anodic peak at 0.35 V and found that gold passivation at this peak is prevented. YANG et al[11] found that heavy mental ions greatly affected the character of the peak at -0.3 V. It is almost universally agreed that these metals modify the gold surface by cementation, thus destroying any passive film, such as AuCN, which may exist on the surface[12].
The effect of heavy metals on anodic oxidation of gold and oxidant on cathodic reduction of oxygen has been studied, but the electrochemical kinetics of gold anodic dissolution intensified by these metals and oxidant have not been investigated, and there is less report about its industrial practice. In this work, the mechanism and application of co-intensification of cyanide leaching gold by mercury ions and oxidant were studied.
2 Experimental
Sodium cyanide, mercury nitrate, peroxide, oxygen and sodium hydroxide are of analytical grade and doubly distilled water, filtered through a Millipore Milli-Q system, was used in all experiments.
The electrochemical experiments were carried out on an EG & G PAR 273A potentiostat and a rotating disc apparatus to obtain the polarization data, which is controlled by the CorrWare for windows 1.2 Corrosion. Gold of electrolytic purity (999.5/1 000) was used in all experiments. A Ag/AgCl/KCl(3 mol/L) reference electrode was connected via a Luggin capillary tube. The Pt flake was used as the counter-electrode. All potentials were reported vs the SHE. The pH of the electrolyte was adjusted to 11 with NaOH solution by using a model pHS-3C pH meter and the dissolved oxygen concentration was measured by a model JPB-607 DO meter. Oxygen was removed from the solutions by sparging with pure nitrogen before each anodic experiment, which was conducted under the protection of nitrogen atmosphere. The 500 mL cylindrical reactor was put into a constant-temperature bath to maintain the temperature.
Leaching experiments were carried out on agitator of the model XJT. The ores, 50 g each time, were ground under 0.074 mm by d150 mm×50 mm ball grinder. Then, the slurry was put into 400 mL glass container and adjusted to the given pulp density. Leaching pH was adjusted by quicklime (CaO). After leaching and filtering, the gold content in tailing and solution was analyzed by atomic absorption spectrometry.
The sample is some flotation sulphide gold concentrate. The multielement analysis result and mineral composition are shown in Table 1 and Table 2, respectively. Gold content is 57.8 g/t as listed in Table 1. The contents of Cu, Fe and S are very high, which would consume cyanide and lead adverse effect on cyanidation.
Table 1 Compositions of gold concentrate

Table2 Mineral compositions of gold concentrate

As indicated in Table 2, the main minerals of the concentrate are pyrite, quartz and feldspar. In addition, there are a large amount of pyrrhotite and marcasite, which are oxygen consuming minerals, and they would be oxidized before gold cyanidation and consume oxygen in the leaching solution.
3 Results and discussion
3.1 Anodic processes of gold
The anodic characteristics of gold were studied, and the results are shown in Fig.1. There are three current peaks in the rangs from -0.8 to 0.8V. It suggests that there occur three active dissolution and passivation process in this potential range. The three peaks are at -0.3, 0 and 0.5 V, respectively. The results are in good agreement with those reported by LI and WANG[13].
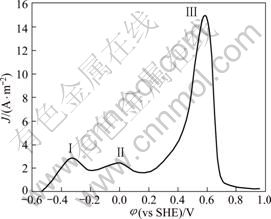
Fig.1 Anodic polarization plot of gold in cyanide solution with pH 11.0, temperature 25 °C, disc rotation speed 400 r/min, scan rate 1mV/s and c(NaCN) 0.02 mol/L
Since leaching reaction is taken place at the first active zone, the following investigation of kinetic characteristics, mechanism and strengthening measures of gold dissolution will focus on this zone.
The accepted reaction mechanism at first peak is as follows[14-15]:
![]() (3)
(3)
![]() (4)
(4)
Equation (3) shows that there first forms absorbed species AuCNads and at the same time it releases electrons. The reaction is determined by the anodic current density. Equation (4) shows that the AuCNads is dissolved as Au(CN)2. When AuCNads covers on the gold surface and could not be dissolved, the equation (4) is the rate-determining step. When AuCNads accumulates to some extent, the reaction is blocked, the current density decreases and the passivation occurs.
The effect of cyanide concentration on the anodic process was studied under the condition of pH 11, agitator speed 400 r/min and temperature 25 °C in the cyanidation concentration range of 0.002-0.1 mol/L. The results are shown in Fig.2.
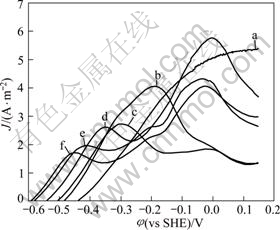
Fig.2 Anodic polarization plots of gold in solutions of various cyanide concentrations with pH 11.0, temperature 25 °C, disc rotation speed 400 r/min and scan rate 1 mV/s: (a) 0.002 mol/L; (b) 0.005 mol/L; (c) 0.01 mol/L; (d) 0.02 mol/L; (e) 0.05 mol/L; (f) 0.1 mol/L
The cyanide concentration has a great effect on gold dissolution, as indicated in Fig.2. The initial anodic reaction equilibrium potential is negative transference and the anodic current density is improved before it reaches peak potential with the cyanide concentrate increasing. The results suggest that gold would be dissolved at low potential and the dissolution rate increases before the passivation potential if raising the concentrates of cyanide. Moreover, the peak potential and the peak current density drop with the cyanide concentrates increasing, which indicates that the passivation would occur at the low potential and current density if raising the cyanide concentrates, that is to say, higher cyanide concentrates accelerate the passivation. This shows that CN- concentration affect is greater to the discharge reactor rate than to AuCNads dissolution rate.
The results also indicate that under low cyanide concentration, gold cyanide process is controlled by CN- transfer, while at higher cyanide concentration, there forms passivation on gold surface. Therefore, chemical oxidation of gold in cyanide solution of higher concentration is controlled by surface reaction.
The effect of mercury ions and cyanide concentration on anodic processes was studied under the conditions of 400 r/min, 25 °C and 10-5 mol/L mercury ions concentrations. The results are shown in Fig.3. Small quantity of additions of mercury ions greatly enhances the current density of anode and gold dissolution process is intensified, which is in agreement with the Refs.[5, 13].
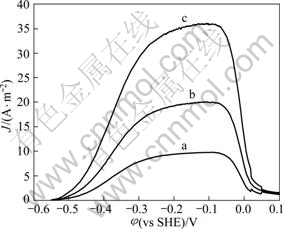
Fig.3 Anodic polarization plots in presence of Hg2+ for various cyanide concentrations with pH 11.0, temperature 25 °C, disc rotation speed 400 r/min, scan rate 1 mV/s and Hg2+content 10-5 mol/L: (a) 0.005 mol/L; (b) 0.01 mol/L; (c) 0.02 mol/L
The effect of cyanide concentration on the anodic gold dissolution with mercury ions is very different from that without mercury ions. With cyanide concentration increasing, the peak current density is dramatically improved while the peak potential is not changed. Besides, the dissolving activity potential zone is widened. So, the mercury ions not only eliminate the passivation potential negative transference led by higher cyanide concentration, but also improve the anodic gold dissolution rate at higher cyanide concentration.
3.2 Cathode processes of oxygen
With respect to the mechanism of gold cyanidation, it was considered that the final product of the oxygen reduction is hydrogen peroxide[15]:
![]() (5)
(5)
While an oxygen reduction was proposed to hydroxide via four electrons:
![]() (6)
(6)
Three mechanistic models for oxygen adsorption and subsequent reduction on different electroactive surfaces in alkaline solutions were also reported.
The effect of oxygen concentration on cathode processes was studied at standard atmosphere, 400 r/min, pH 11, 25 °C and scan rate 1 mV/s, and the corresponding results are plotted in Fig.4.
The cathode current density is greatly improved with the oxygen concentration of the solution increasing (in Fig. 4). So, oxygenation process during gold cyanidation is an effective strengthen measure.
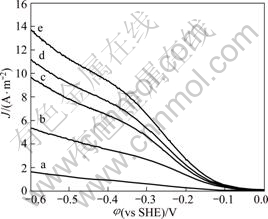
Fig.4 Cathodic polarization plots of oxygen reduction on gold disc at various dissolved oxygen concentrations with pH 11.0, temperature 25 °C, disc rotation speed 400 r/min and scan rate 1 mV/s: (a) 1.0 mg/L; (b) 7.5 mg/L; (c) 13.0 mg/L; (d) 15.0 mg/L; (e) 18.0 mg/L
The cathode current density is obviously affected and the characteristics of polarization curves are changed when adding small quantity of mercury ions (see Fig.5). The cathode current density increases sharply before -0.3 V, then the current density keeps constant with the potential negative removal. Because gold anodic dissolution occurs at -0.3 V or so, improving the cathode current density by mercury ions has great effect on the gold dissolution in this potential zone.
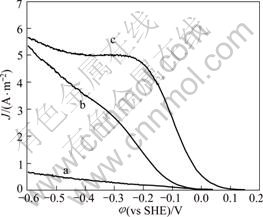
Fig.5 Effect of Hg2+ on cathodic reduction of oxygen on gold disc electrode with pH 11.0, temperature 25 °C, disc rotation speed 400 r/min and scan rate 1 mV/s: (a) Hg2+10-5 mol/L, no oxygen; (b) Oxygen7.5 mg/L, no mercury ion; (c) Hg2+ 10-5 mol/L, oxygen 7.5 mg/L
3.3 Mixed potential model of co-intensification
The intersection points of anodic and cathodic polarization curves for gold at different mercury ion concentrations and different dissolved oxygen levels were determined from Fig.6. Curve 1and curve 2 show the anodic dissolution characteristic without and with mercury ions, respectively. Curves a and b represent the cathode reaction characteristic with 3.5 mg/L and 7.5 mg/L dissolved oxygen, curve c expresses adding 0.02 mol/L H2O2 in the solution. The intersection points of E1, E2, E3 and E4 stand for the mixed potential of the corresponding system, where there are the potential and current density of gold dissolution.
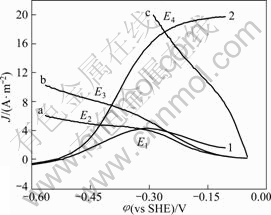
Fig.6 Mixed potential of gold dissolution at different conditions, with pH 11.0, temperature 25 °C, disc rotation speed 400 r/min and scan rate 1 mV/s: 1—NaCN 0.01 mol/L; 2—NaCN 0.01 mol/L, Hg 2+ 10-5 mol/L; (a) 3.5 mg/L dissolved oxygen; (b) 7.5 mg/L dissolved oxygen; (c) 0.02 mol/L H2O2
Although anodic process is strengthened by mercury ions, the current densities at E1 and E2 are almost at the same level. At this time, dissolved oxygen concentration is very low and it is only 3.5 mg/L, so, the current density is low. When the dissolved oxygen concentrations rise to 7.5 mg/L, compared with the current density of E2 and E3, it enhances evidently. Adding 0.02 mol/L H2O2, the cathodic processes are greatly strengthened and the current density is enhanced markedly, as seen from E4.
Analyses above show that although the mercury ions can significantly strengthen the anodic processes, in order to play an important role in gold leaching, the cathodic processes would be strengthened at the same time by oxidant such as pure oxygen or hydrogen peroxide.
3.4 Cyanide leaching gold concentrates
The investigation of cyanide leaching gold concentrates was done under the condition with or without mercury ions and hydrogen peroxide, and the results are shown in Fig.7. The leaching rate is very slow without mercury ions and hydrogen peroxide. The rate is 31.88% after 8 h leaching. When the leaching time increases to 12 h, the rate reaches 43.29%. Even if the time prolongs to 24 h, the leaching rate is only 45.68%. The results show that the gold concentrates are very refractory. The reason is that the concentrates contain magnetic pyrite and marcasite, which consume a plenty of oxygen and reduce the oxygen concentration in the solution. At the same time, the gold particle is very fine, which also leads to difficulty in increasing the leaching rate.
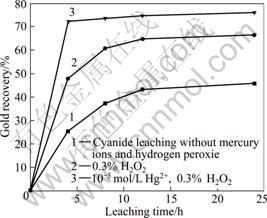
Fig.7 Effect of leaching time on gold leaching under co-intensification, with grinding solid-to-liquid ratio 1:5, leaching solid-to-liquid ratio 5:1, 0.2% NaCN,pH 11 and stirring rate 1 000 r/min
The leaching rate is increased substantially when adding hydrogen peroxide in the leaching system. The rate reaches 66.41% with 24 h intensified by hydrogen peroxide. On the basis of hydrogen peroxide, the leaching rate reaches 72.33% with 4 h co-intensification by mercury ions.
After two-stage agitation leaching with co- intensification, the rate is over 90% as indicated in Fig.8.
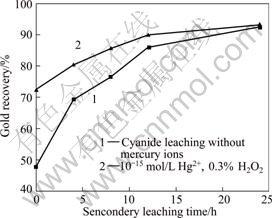
Fig.8 Effect of leaching time on leaching rate of second stage leaching (First stage leaching: 4 h, 0.2% NaCN, 0.3% H2O2, pH 11; Second stage leaching: 0.2% NaCN, 0.3% H2O2, pH 11)
4 Conclusions
1) Under the low cyanide concentration, gold cyanide process is controlled by CN- transfer, while at higher cyanide concentration, there forms passivation on the gold surface. Therefore, chemical oxidation of gold in cyanide solution of higher concentration is controlled by surface reaction.
2) Mercury ions increase the anodic gold dissolution rate and decrease the passivation. In addition, it also markedly changes the effect pattern of cyanide concentration.
3) Mercury ions show positive effects on cathodic reduction of oxygen and raise the rate of electrochemical step of the anodic reduction of oxygen.
4) Adding a certain amount of hydrogen peroxide is an effective way to intensify cathodic process on gold electrode. Active potential range and current peak on anodic dissolution are enlarged while being co- intensified with Hg2+ and hydrogen peroxide.
5) Co-intensifying increases the cyanide leaching of gold from gold concentrates significantly.
References
[1] SENANAYAKE G. Kinetics and reaction mechanism of gold cyanidation: Surface reaction model via Au(I)-OH-CN complexes [J]. Hydrometallurgy, 2005, 80(1):1-12.
[2] LU Pan. Solvent extraction of gold(I) from alkaline cyanide solution by dibutylcarbitol (DBC) with noctanol [J]. Journal of Chemical Technology and Biotechnology, 2008, 83(10): 1428-1432.
[3] YANG Yong-bin, LI Qian, JIANG Tao, JIN Yong-shi. Cyanide leaching of gold ores by heavy metal ions [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(8): 1283-1288.(in Chinese)
[4] KHMELNITSKAYA O D, BESKROVNAYA V P. A technology of precious metals recovery from gold-bismuth ores [C]//Australasian Institute of Mining and Metallurgy Publication Series, World Gold 2007 By the Co-Products and the Environment– Proceedings, Cairns, QLD, Australia, 2007: 245-250.
[5] MATTHEW I, JEFFREY, RITCHIE I M. Leaching of gold in cyanide solutions in the presence of impurities. II—The effect of silver [J]. Journal of the Electrochemical Society, 2000, 147(9): 3272-3276.
[6] SANDENBERGH R F, MILLER J D. Catalysis of the leaching of gold in cyanide solutions by lead, bismuth and thallium [J]. Minerals Engineering, 2001, 14(11): 1379-1386.
[7] BEK R Y, ZELINSKIJ A G, OVCHINNIKOVA S N, VAJS A A. Comparative characteristics of catalytic activity of thallium, lead, and bismuth adatoms in reaction of gold dissolution in cyanide solutions [J]. Elektrokhimiya, 2004, 40(2): 148-154.
[8] BEK R YU, SHURAEVA L I. Electrocatalysis by adatoms at the gold and silver dissolution in cyanide solutions [J]. Russian Journal of Electrochemistry, 2008, 44(1): 113-122.
[9] JIN O, MAY E, GHALI G, DESCHENE S. Investigation on the mechanism of the catalytical effect of lead salts on gold dissolution in cyanide solution [C]//Proceedings of the Third International Conference on Hydrometallurgy, 1998: 666-679.
[10] CATHRO K J, KOCH D F A. Electrocatalytic properties of surfaces modified by foreign metal adatoms [J]. J Electrochem Soc, 1964, 111: 1416-1420.
[11] YANG Yong-bin, LI Qian, LI Guang-hui, GUO Yu-feng, HUANG Zhu-cheng, JIANG Tao. An electrochemical investigation on intensification of gold cyanidation by heavy metal ions [C]//EPD Congress 2005—Proceedings of Sessions and Symposia Sponsored by the Extraction and Processing Division of The Minerals, Metals and Materials Society, San Francisco, CA, United states, 2005: 977-984.
[12] CHIMENOS J M, SEGARRA M, GUZMAN L, KARAGUEORGUIEVA A. ESPIELL F. Kinetics of the reaction of gold cyanidation in the presence of a thallium(I) salt [J]. Hydrometallurgy, 1997, 44: 269-286.
[13] LI Ding-xin, WANG Yong-lu. The extraction and refinery of precious metals [M]. Changsha: Central South University Press, 2003. (in Chinese)
[14] WANG Yu, CHEN Jing, WEI Qun-yan, XIE Qi-ying. Comparison of cyanidation rates of gold, silver and copper and influences of sulfion on them [J]. The Chinese Journal of Nonferrous Metals, 2007,17(1): 172-177.(in Chinese)
[15] QIU Ting-sheng, NIE Guang-hua, ZHANG Qiang, CHEN Jing-he, ZOU Lai-chang. Mechanism of oxidation and leaching for copper-bearing gold ores [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(12): 2028-2033. (in Chinese)
Foundation item: Project(50725416) supported by the National Science Fund for Distinguished Young Scholars, China
Corresponding author: LI Qian; Tel: +86-13574199228; E-mail: csuliqian@126.com
DOI: 10.1016/S1003-6326(09)60332-0


