Trans. Nonferrous Met. Soc. China 24(2014) 455-461
Influence of heat-treatment on oxidation-resistance of phosphate-coating for C/C composite
Yi-cheng GE, Ling-yun YANG, Shuai WU, Chan LI, Jian LUO, Mao-zhong YI
State Key Laboratory for Powder Metallurgy, Central South University, Changsha 410083, China
Received 5 January 2013; accepted 22 September 2013
Abstract:
Phosphate-coating was prepared for C/C composite using liquid-impregnation and different heat-treatment. The results show that the mass-loss rate of sample A with 1-2 °C/min slow-cooling rate technology is 47% after oxidation at 700 °C for 20 h, while that of sample B with air-fast-cooling one is only 0.98%. SEM images reveal that the coating of sample A is full of micro-holes, micro-cracks and many piece-like crystal particles, while that of sample B is integrated and compacted in glassy state with a few of micro-cracks. The coating of sample A is almost exhausted only in 8 h oxidized-test at 700 °C, while that of sample B remains integrated after 8 h test at 700 °C and becomes loose due to much small pores generated after 20 h test at 700 °C.
Key words:
C/C composite; phosphate-coating; oxidation-resistance; heat-treatment;
1 Introduction
Carbon fiber reinforced carbon matrix composite (C/C) is a special carbon-graphite material with low density, low thermal expansion coefficient, high specific strength and modulus, high specific heat capacity, excellent tribological behavior and properties designed ability [1-3], has been widely used as brake material for modern aircraft. But one large defect of C/C composite, easily being oxidized in air above 370 °C [4], reduces significantly the performance and threatens the safe applications. Generally, the normal application temperature for C/C brake material is 550-750 °C [5,6]. Therefore, it is necessary to take proper oxidation- resistance in this situation.
Recently, many kinds of oxidation-resistance coatings have been applied for C/C composites. For example, phosphate is a commonly used material because it is easily to be prepared with good property. LU and CHUNG [7] found that the coating, made from the phosphate precursor with mole ratio 12:1 of phosphate to aluminum-phosphate (AlPO4) and some aromatic hydrocarbons additives, had better oxidation-resistance than the similar one without additives. TRICOT et al [8] investigated the influence of snow removal agent with potassium, sodium and calcium additives, and made a conclusion that the performance of phosphate coating relies on the heat treatment. WU and RADOVIC [9] concluded that C—O—PO3 and C—PO3 ionic-groups at active point in carbon atomic-layer are effective physical oxygen barrier. ZHAO et al [10] prepared the coating with calcium phosphate as main ingredient and discussed the mechanism of calcium.
Many studies on coating of C/C revealed that micro-holes and micro-cracks [11-13], usually being found due to unsuitable heat-treatment, are fatal defects to threaten the application. Since phosphate can be generated into different products with a variety of forms and performance, we prepared phosphate coating on C/C with different heat-treatment and investigated the influence mechanism.
2 Experimental
C/C composite used in this work was reinforced by T300 polyacrylonitrile(PAN) derived carbon fiber preform made from a non-woven duplex cloth composed of a continuous PANCF layer attached to a short-cut PAN carbon fiber felt layer by needling. It was densified by chemical vapor infiltration in C3H6+N2 atmosphere to the density of 1.5 g/cm3 at first, followed by impregnation and carbonization of Furan resin to (1.85±0.03) g/cm3. The final heat-treatment temperature was 2100 °C. The volume fraction of carbon fibers in C/C composite was about 33%. And then the composite was machined to samples with dimensions of 15 mm × 15 mm × 15 mm, polished with 3000# SiC sand paper, cleaned in alcohol using KQ2200E ultrasonic cleaner, dried in DHG-9070A drying oven and stored in a drying can.
Liquid phosphate precursor was prepared using chemically-pure liquid H3PO4 as solvent, solid AlPO4, Mn(H2PO4)2·4H2O, Zn3(PO4)2·4H2O, Ca3(PO4)2, and some other reagents as solid additives, by heating the H3PO4 in water bath at first, and then adding the additives gradually, keeping stirring until it was dissolved completely.
C/C samples were impregnated in the precursor for 30 min, and then removed from it and put on high purity graphite. At first, those impregnated-samples were dried in DHG-9070A oven at 150 °C for 2 h. Afterwards, those samples were divided into two parts, A and B. Sample A was heated at 650 °C in Ar for 1 h with heating rate of 5-10 °C/min and cooling rate of 1-2 °C /min. Sample B was also heated at 650 °C in Ar for 1 h with the same heating rate, but the sample was directly removed from the furnace into air to ensure its fast cooling rate.
The oxidation test was taken at 700 °C with air flow rate of 2 L/min. The masses of samples are measured using FA2104N electronic balance every one hour. The test was repeated three times and then the average mass loss rate was calculated.
The microstructure and composition of the coating were detected using a FEI-Nova Nano 230 SEM, and the crystal structure of the oxidized sample was detected using a D/max 2550 XRD.

Fig. 1 SEM images of phosphate coating (a, b, c) and EDAX result (d) of flake component in marked-box in Fig. 1(c) of sample A with low cooling rate
3 Results and discussion
3.1 Results of heat treatment
Figure 1 presents SEM images and EDAX result of sample A with slow cooling rate. As shown in Fig. 1(a), many small pores are found, which indicates that the vaporization of residual water, low-melting component in the impregnated-coating, is the main reason for such defects with different shape and size, which will result in a loose coating. As shown in Fig. 1(b), many particles with different sizes accumulate and pack loosely, which leads to an uneven and non-compacted coating. As shown in Fig. 1(c), some flaky crystalline components exist in a big pore surrounded by loosely linked and stacked coating layer. As shown in Fig. 1(d), the EDAX result reveals that the flaky component is composed of carbon, phosphorus, oxygen and calcium. Therefore, it can be concluded that the component is some sort of calcium phosphate with good crystal structure [10].
Figure 2 presents SEM images of sample B with fast cooling rate. As shown in Fig. 2(a), the coating is integrated and compacted except for some little cracks, which is distinct from sample A. As shown in Fig. 2(b), some irregular, gray, scaly and interleaved stacked compounds layer spreads on top of the surface. Under this layer, it is a black coating without any clear crystalline particles.

Fig. 2 SEM images of phosphate coating of sample B with air-cooling
In many researches, thermal stress, mainly caused by different coefficient of thermal expansion (CTE) between coating and substrate, can be partially released by slow cooling technology. But for the impregnated- phosphate-coating, it would experience a series of reactions, such as polycondensation, polymerization, cyclization, re-nucleation and growth [15]. Therefore, the coating of sample A, treated with slow cooling rate, easily nucleates, and the crystalline grain grows to generate phosphate particles with different stoichiometric, shape and characteristics. For example, calcium hydrogen phosphate, dissolved in H3PO4 from calcium phosphate, is easily decomposed at 203 °C to form calcium phosphate again with flake-shaped grain in slow cooling, as shown in Fig. 1(c). However, the phosphate particles, with good crystallite and significantly anisotropic characteristics, cannot be stacked and combined tightly to form a compacted coating. In addition, a large amount of low melting component will volatile during the slow cooling process. This is another reason to bring about so many pores and reduce the self-healing ability of coating.
For sample B with air-fast-cooling, the component in coating is difficult to spread, segregate, nucleate and grow. Therefore, it ensures the uniformity of component and structure, depresses the local difference and suppresses the volatilization of low-melting component in the coating. As a result, it reduces the defects such as holes and cracks and then improves the oxidation-resistance of the coating. However, the thermal stress, caused by different CTE and fast cooling, can only be released by the generation and growth of micro-cracks [12]. It should be noted that in the gray layer on the top of the coating the component can generate some phosphate with good crystallite due to easier moving and diffusing than those inside the coating. As a whole, it is easy to form a tight glassy phosphate coating by fast cooling technology.
3.2 Results of oxidation-test
Figure 3 presents the curves of mass loss rate of samples A and B oxidized at 700 °C. As shown in Fig. 3, the mass loss rate of sample B is much less than that of sample A. The curve of sample B increases as a linear growth trend with prolonging the time and the highest value is only 0.98%. It proves that the coating with a fast cooling rate has a good thermal stability, excellent oxidation-resistance. The curve of sample A increases as a typical exponential growth with prolonging the time. The mass loss rate of sample A is more than 2% only after 5-6 h test and then increases quickly to 47% at the 20th hour, which indicates that the coating has lost its function only after 5-6 h test.
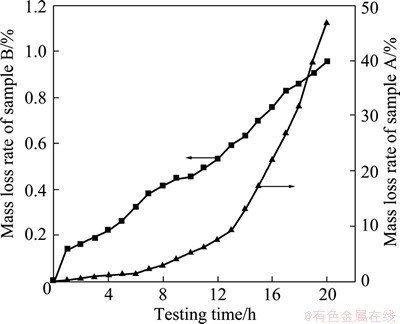
Fig. 3 Mass loss rate of samples A and B oxidized at 700 °C with time
Figure 4 presents SEM image of sample A after the oxidation at 700 °C for 8 h. As shown in Fig. 4(a), the residual coating is seemed as a broken fishing net with some glassy state, which is not the same as the initial one. The image also indicates that the residual coating still hold good surface wettability with C/C. As shown in Fig. 4(b), the coating on fiber bundle is almost exhausted, the residual phosphate spread on the bundle like small beads. As a whole, C/C has been exposed to air and the coating has lost the function.
Figure 5 presents SEM images of sample B after the oxidation at 700 °C for 8 h and 20 h, respectively. As shown in Fig. 5(a), the coating is still smooth and integrated. But a few small pores indicate the loss of some low-melting component. In addition, some micro-cracks reveal the effect of thermal stress in the initial coating. As shown in Fig. 5(b), a big hole, coming from C/C itself, is still covered by the phosphate though it is thinner than the surface coating. As shown in Fig. 5(c), the coating is no longer integrated as the initial one due to the significant increasing of small pores after 20 h test. As shown in Fig. 5(d), almost half coating, located on fiber bundles with the axis parallel to surface, has lost. The residual coating shrinks, accumulates but still adheres to the fibers. The nearby coating, located on carbon fiber felt, is integrated with some glassy state and part shrinkage. It reveals that the coating still maintain some oxidation-resistance. The oxidation-model is shown in Fig. 6.
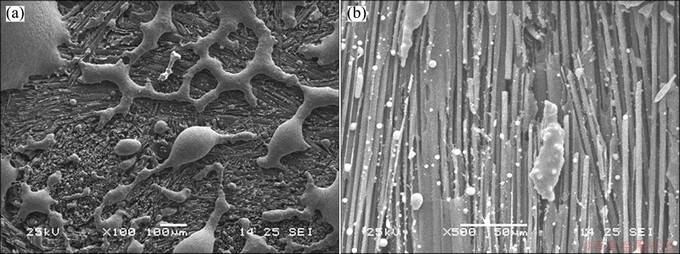
Fig. 4 SEM images of sample A after oxidation at 700 °C for 8 h
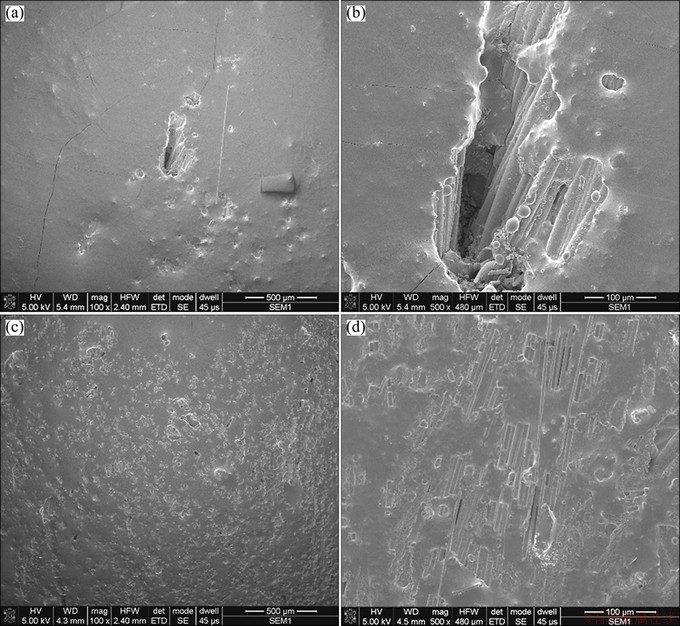
Fig. 5 SEM images of sample B after oxidation at 700 °C for 8 h (a, b) and 20 h (c, d)

Fig. 6 Model of carbon layer (a), phosphate coated sample (b) and oxidized sample (c)
Figure 7 presents XRD pattern of sample B after oxidization for 8 h. As shown in Fig. 7, many sharp peaks reveal that some crystallized components, such as Zn3(PO4)2, Al(PO3)3 and Mn2P2O7, are generated. Furthermore, MnO2, not existing in the raw material, is found, which indicates that some components in the coating were oxidized during the test.
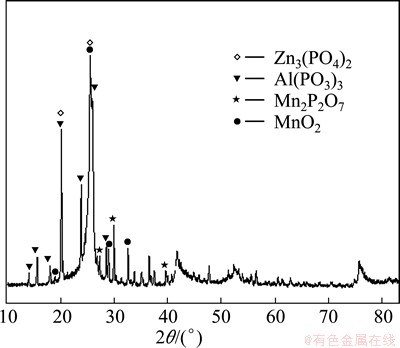
Fig. 7 XRD pattern of sample B after being oxidized at 700 °C for 8 h
3.3 Mechanism of heat treatment and oxidation- resistance of phosphate coating
In phosphate coating, C—O—PO3 and C—PO3 groups act as key role in combining coating with C/C composite [9,10,15]. Such groups can restrain oxidation by sealing the active-point in carbon atomic layer in C/C composite. When being heated at different temperatures, such as 213 °C, 300 °C and 480 °C, phosphoric acid would dehydrate gradually and react as follows:
H3PO4→H4P2O7+H2O
H3PO4→HPO3+H2O
H3PO4→ P2O5+H2O
Since the sublimation and melting temperatures of P2O5, 300 °C and 585 °C, are lower than the heat-treatment and oxidation ones in this work, it is difficult for pure P2O5 to maintain the oxidation- resistance for a long time. Therefore, it is a key to prevent the loss of P2O5 by using some suitable solid particles to improve the oxidation-resistance.
AlPO4, Mn3(PO4)2 and Zn3(PO4)2 are widely used oxidation-resistant materials for C/C composite [8-10]. Since these phosphates should be converted into liquid precursors to prepare coating, the heat-treatment would induce the decomposition, dehydration, polymerization and crystallization. For example, Al(H2PO4)3, made from AlPO4, would react as follows [8,14]:
2Al(H2PO4)3→Al2(H2P2O7)3+3H2O→Al2(H2P2O7)3→Al2(H2PO10)+Al(PO3)2+nH2O→Al(PO3)n
Similarly, zinc and manganese would also bring about such reaction to produce poly-phosphate at last.
CHEN and TAN [14] thought that soluble phosphate, in certain heat-treat temperature range, could be converted into glassy state, and then decomposed to bring about solid phosphate with different stoichiometric. Excessive H3PO4 was used as solvent to prepare the precursor, it could be bonded with Al2O3, MnO and ZnO to form a complicated and multi-dimensional phosphate system to suppress the rapid evaporation of P2O5. Therefore, the coating is composed of such phosphate in glassy state with complicated stoichiometry. As a result, the coating can be partly melted at the oxidation temperature, which makes it hold some fluidity and self-heal ability to fill those cracks and pores to ensure the oxidation-resistance. It should be noted that the MnO, with melting point of 1650 °C, can be oxidized to MnO2 with melting point of 535 °C. It is positive additive to act as low-melting component and enhance self-heal property in the coating.
During the oxidation, the samples were removed from muffle furnace to air directly like fast cooling by treatment to measure the mass, which induces the phosphates to form glassy state coating. This is the reason for sample A to have the glassy one after the test for 8 h.
After a long time of oxidation, the coating lost the low-melting components at first, followed by thinning gradually, shrinking with metal-oxide as core due to surface tension [10,14,15]. Therefore, some pores generated on the surface of fiber bundle or in some large holes of C/C itself at first, which reduces the integration and results in an island-shape residual coating at last. The residual phosphate, with high-melting temperature, has lost the vitrifiable, self-heal ability to repair the coating and release the thermal shock during the test.
Since the density of non-woven carbon fiber cloth reinforced area in C/C composite is higher than that of fiber felt [2,5], it is difficult for the precursor to impregnate in it. Thus, it becomes a shortage to decrease the combination between the coating and the area in C/C composite. In addition, CTE of PAN carbon fiber, -0.5-1×10-6 °C-1 along the axis, is lower than that of phosphate [14,16]. A big thermal stress, between the coating and fiber bundle, would be generated in the repeated weighing with quickly temperature changes. Therefore, the coating on such place is more easily to be peeled off than it on other one.
For sample A, the small pores and cracks in the initial coating, are permeating-way of oxygen, which results in higher mass loss rate at first than sample B. During the test, oxidized-products, such as MnO2 from MnO, might be important components to make up the self-heal ability of coating. So, sample A can remain good oxidation-resistance in the first 5-6 h test, followed by rapidly decreased one until failure. For sample B, fast cooling technology is a key to remain low-melting component in coating to ensure its long-time oxidation-resistance.
4 Conclusions
1) C/C sample with phosphate coating cooled in air has good oxidation-resistance. Its mass loss rate is only 0.98% after being tested at 700 °C for 20 h, while that with slow cooling rate of 1-2 °C/min is 47%.
2) The initial coating with fast cooling rate is integrated and compacted with glassy state, which remains integrated with some small pits after 8 h oxidation and then part of it is consumed after 20 h test. But the one with slow cooling rate is full of many different-shape particles and pores, which is almost exhausted only in 8 h test.
References
[1] LEI Bao-ling, HE Lian-long, YI Mao-zhong, RAN Li-ping, XU Hui-juan, GE Yi-cheng, PENG Ke. New insights into the microstructure of the friction surface layer of C/C composites [J]. Carbon, 2011, 49(11): 4554-4562.
[2] POLICANDRIOTES T, FILIP P. Effects of selected nanoadditives on the friction and wear performance of carbon–carbon aircraft brake composites [J]. Wear, 2011, 271( 9-10): 2280-2289.
[3] LIU Ye-qun, HE Lian-long, LU Xue-feng, XIAO Peng. Transmission electron microscopy study of the microstructure of carbon/carbon composites reinforced with in situ grown carbon nanofibers [J]. Carbon, 2012, 50(7): 2424-2430.
[4] SARKAR S, BADISCH E, MITRA R, ROY M. Impact abrasive wear response of carbon/carbon composites at elevated temperatures [J]. Tribological Letter, 2010, 37(2): 445-451.
[5] GUO Rui, XU Hui-juan, YI Mao-zhong, LEI Bao-ling. Effect of thermal expansion on stress field of C/C composites during braking [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 1031-1037. (in Chinese)
[6] XU Hui-juan, YI Mao-zhong, XIONG Xiang, HUANG Bai-yun, LIANG Yue-ming, GUO Rui. Effect of thermal stress on worn surface morphology of C/C composites during braking [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(1): 131-137. (in Chinese)
[7] LU Wei-ming, CHUNG D D L. Oxidation protection of carbon materials by acid phosphate impregnation [J]. Carbon, 2002, 40(5): 1249-1254.
[8] GREGORY T, NATHALIE N, PASCAL D, LIONEL M. Inhibition of the catalytic oxidation of carbon/carbon composite materials by an aluminophosphate coating [J]. Carbon, 2012, 50(12): 3440-3445.
[9] WU X X, RADOVIC L R. Inhibition of catalytic oxidation of carbon/carbon composites by phosphorus [J]. Carbon, 2006, 44(1): 141-151.
[10] ZHAO Xue-ni, LI He-jun, CHEN Meng-di, LI Ke-zhi, WANG Bin, XU Zhan-wei, CAO Sheng, ZHANG Lei-lei, DENG Hai-liang, LU Jin-hua. Strong-bonding calcium phosphate coatings on carbon/carbon composites by ultrasound-assisted anodic oxidation treatment and electrochemical deposition [J]. Applied Surface Science, 2012, 258(12): 5117-5125.
[11] LIN Y C, RUIZ E M, RATEICK R G Jr, McGINN P J, MUKASYAN A S. One-step synthesis of a multi-functional anti-oxidation protective layer on the surface of carbon/carbon composites [J]. Carbon, 2012, 50(3): 557-565.
[12] SU Zhe-an, YANG Xin, HUANG Qi-zhong, HUANG Bai-yun, LI Jian-li, ZHANG Ming-yu, XIE Zhi-yong. Effect of SiC coating on ablation resistance of C/C composites under oxyacetylene torch flame [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2838-2845. (in Chinese)
[13] FU Qian-gang, XUE Hui, LI He-jun, LI Ke-zhi, SHI Xiao-hong, ZHAO Hua. Anti-oxidation property of a multi-layer coating for carbon/carbon composites in a wind tunnel at 1500 °C[J]. New Carbon Materials, 2010, 25(4): 279-284. (in Chinese)
[14] CHEN Jia-fu, TAN Guang-xun. Production and application of phosphate [M]. Chengdu: Press of Chengdu University of Science and Technology, 1989. (in Chinese)
[15] ROSAS J M, RUIZ-ROSAS R, RODRIGUEZ-MIRASOL J, CORDERO T. Kinetic study of the oxidation resistance of phosphorus-containing activated carbons [J]. Carbon, 2012, 50(5): 1523-1537.
[16] HUANG Qi-zhong. Fabrication, structure and application of high-performance carbon/carbon composites [M]. Changsha: Central South University Press, 2010. (in Chinese).
热处理技术对C/C复合材料磷酸盐涂层抗氧化性能的影响
葛毅成,杨凌云,武 帅,李 禅,罗 健,易茂中
中南大学 粉末冶金国家重点实验室,长沙 410083
摘 要:采用液态磷酸盐浸渍及不同的热处理技术在C/C复合材料表面制备抗氧化涂层。实验结果表明:采用1~ 2 °C/min慢速冷却制备的材料A在700 °C氧化20 h后的氧化质量损失达到47%,而采用快速气冷技术制备的材料B的氧化质量损失仅仅为0.98%。SEM形貌观察表明:材料A的磷酸盐涂层表面疏松,充满大量孔洞、裂纹,以及片状结晶、团聚的磷酸盐,而材料B的涂层致密、完整,为玻璃态。氧化实验后,材料A的涂层在8 h氧化阶段就已消耗殆尽,抗氧化能力基本消失;而材料B的涂层在8 h 实验后表面涂层完整、致密,无明显损伤,在20 h实验后出现了较多的孔洞,抗氧化能力逐渐降低。
关键词:C/C复合材料;磷酸盐涂层;抗氧化性能;热处理
(Edited by Hua YANG)
Foundation item: Projects (09JJ4027) supported by the Natural Science Foundation of Hunan Province, China; Project (201206375003) supported by China Scholarship Council
Corresponding author: Yi-cheng GE; Tel: +86-731-88877700; E-mail: hncsgyc@163.com
DOI: 10.1016/S1003-6326(14)63082-X
Abstract: Phosphate-coating was prepared for C/C composite using liquid-impregnation and different heat-treatment. The results show that the mass-loss rate of sample A with 1-2 °C/min slow-cooling rate technology is 47% after oxidation at 700 °C for 20 h, while that of sample B with air-fast-cooling one is only 0.98%. SEM images reveal that the coating of sample A is full of micro-holes, micro-cracks and many piece-like crystal particles, while that of sample B is integrated and compacted in glassy state with a few of micro-cracks. The coating of sample A is almost exhausted only in 8 h oxidized-test at 700 °C, while that of sample B remains integrated after 8 h test at 700 °C and becomes loose due to much small pores generated after 20 h test at 700 °C.


