文章编号:1004-0609(2009)05-0955-05
CaO-Cu2O-Fe2O3三元渣系组元活度计算模型
汪金良1, 2,张传福1,张文海1, 3
(1. 中南大学 冶金科学与工程学院,长沙 410083;
2. 江西理工大学 材料与化学工程学院,赣州 341000;
3. 中国瑞林工程技术有限公司,南昌 330002)
摘 要:
基于炉渣结构共存理论,建立1 200~1 300 ℃下CaO-Cu2O-Fe2O3三元渣系组元活度的计算模型,计算并绘制渣中CaO、Cu2O和Fe2O3的等活度曲线,考察碱度B和温度t对组元活度α(CaO)、α(Cu2O)和α(Fe2O3)的影响。结果表明:模型计算值α(Cu2O)与文献实测值α′(Cu2O)吻合程度高,说明模型能较好地反映该渣系的结构本质;α(Cu2O)呈拉乌尔正偏差,在B=1.54时出现最大值,当B>1.54时随着B和t的增大而降低,当B<1.54时趋势正好相反,且受碱度影响更为显著;α(CaO)和α(Fe2O3)分别随CaO和Fe2O3在炉渣中含量的升高而增大,但受温度的影响都不明显。研究结果可用于采用铁酸钙渣系的炼铜新工艺热力学研究。
关键词:
中图分类号:TF 801 文献标识码:A
Activity calculation model for slag system of CaO-Cu2O-Fe2O3
WANG Jin-liang1, 2, ZHANG Chuan-fu1, ZHANG Wen-hai1, 3
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Faculty of Material and Chemistry Engineering, Jiangxi University of Science and Technology,
Ganzhou 341000, China;
3. China Nerin Engineering Co., Ltd., Nanchang 330002, China)
Abstract: According to the coexistence theory of slag structure, the activity calculation model for slag system of CaO-Cu2O-Fe2O3 was built from 1 200 ℃ to 1 300 ℃. Then, the activities of CaO, Cu2O and Fe2O3 in the slag were calculated, and their equal activity curves were drawn. The influence of basicity B and temperature t on the activities α(Cu2O), α(CaO) and α(Fe2O3) was also analyzed. The results show that the calculated α(Cu2O) is in good agreement with the reported measured α′(Cu2O), showing that the model can wholly embody the slag structural characteristics. α(Cu2O) departures positively from Raoult values and reaches the maximum value when B is 1.54, then decreases with the increase of B and t when B>1.54, but increases when B<1.54. α(CaO) and α(Fe2O3) increase with the increasing contents of CaO and Fe2O3 in slag, but change little with change of temperature. The results can be applied to the thermodynamic research of new copper smelting technologies adopting calcium ferrite slag.
Key words: calcium ferrite slag; coexistence theory; activity; calculation model
闪速吹炼、三菱连续吹炼、Glogow直接闪速炼铜等现代火法炼铜工艺因具有节能、环保、高效等优点,近年来在世界各地得到推广应用[1]。随着氧势的提高,吹炼或造粗铜炉渣中Fe3O4和Cu2O的含量不断增加,为了避免形成固体磁铁矿,同时最大限度减少泡沫渣的形成,这些工艺往往采用铁酸钙渣型[2]。含铁酸盐而不含SiO2的CaO-Cu2O-Fe2O3 渣系就是其中重要渣型之一[3]。
由于火法炼铜过程属高温、多相、多组分复杂体系,在一般实验室条件下其研究工作很难开展,往往借助数学和计算机技术来模拟其过程[4-6],多相平衡计算即为重要手段之一[7]。由于多相平衡计算过程需要反复迭代各相各组分的活度等热力学数据,研究炉渣组分活度与各组分间的关系对开展炼铜新工艺的热力学研究具有重大意义。
近年来,ILYUSHECHKIN等[8]、SAKAI等[9]、FLORIAN和AKIRA[10]、SANG等[11]、PAVOL等[12]、FAHEY等[13]对铁酸钙渣系的相平衡、炉渣的密度、表面张力、黏度等进行了研究,但由于活度的测定工作较为复杂,目前,除YAZAWA等[14]测定了1 250 ℃时CaO- Cu2O-Fe2O3渣系中Cu2O的部分活度外,有关该渣系组元活度及其计算模型的文献未见报道。
ZHANG[15]提出了炉渣结构共存理论,其实质是基于溶液中同时存在离子、简单分子和复合分子的假设,建立离子、简单分子和复合分子的化学平衡关系,根据已有的化学平衡热力学数据计算组元的摩尔分数,并将其定义为作用浓度(活度)。共存理论在以冶金炉渣、金属熔体和熔盐为代表的溶液体系已得到良好应用,取得了与实际相符的结果[16]。
为此,本文作者基于炉渣结构共存理论,建立CaO-Cu2O-Fe2O3三元渣系组元活度的计算模型,绘制各组元的等活度曲线,考察碱度和温度对组元活度的影响,为采用铁酸钙渣系的炼铜新工艺热力学研究和过程模拟提供理论依据和热力学数据。
1 组元活度计算模型
查阅CaO-Fe2O3[17]、Cu2O-CaO[18]、Cu2O- Fe2O3[8]及CaO-Cu2O-Fe2O3[19]相图,根据炉渣结构的共存理论,确定在1 200~1 300 ℃之间CaO-Cu2O-Fe2O3渣系的组元为:Cu2+、Ca2+、O2-、Fe2O3、Cu2O?Fe2O3、CaO?Fe2O3和2CaO?Fe2O3。
渣中各组元之间的反应及其达到平衡时的![]() 如下[20-21]:
如下[20-21]:

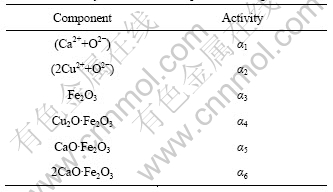
假定CaO-Cu2O-Fe2O3三元系成渣前的CaO、Cu2O、Fe2O3的摩尔数为n1、n2、n3,成渣后总摩尔数为∑n,各组元的活度为αi,如表1所列。
表1 渣系各组元活度定义
Table 1 Activity definition of components in slag
根据质量作用定律,以下关系式成立:

由式(1)~(7)组成的高次方程组即为求解CaO-Cu2O-Fe2O3渣系组元活度的计算模型,其中K为上述各组元之间的反应平衡常数。
2 计算及结果分析
从上述所建炉渣组元活度模型可以看出,求解过程是典型的非线性方程组求解过程,首先需要将高次方程分别对α1、α2和α3求偏导,从而构成线性方程组,再用高斯消去法迭代求解。计算流程如图1所示,其中ε是精度控制常量,其值取10-4。
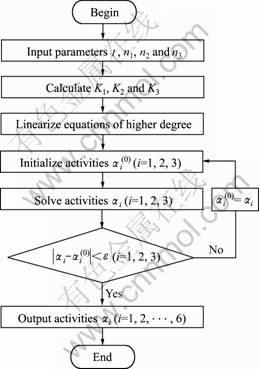
图1 活度计算流程图
Fig.1 Flow chart of activity calculation
基于上述CaO-Cu2O-Fe2O3三元渣系组元活度模型,计算了温度为1 250 ℃时该渣系中Cu2O的活度,其结果如图2所示,其坐标是以摩尔分数计。
由图2可知,理论计算的活度α(Cu2O)与YAZAWA等[14]实测的活度α′(Cu2O)[14]吻合较好,这说明所建的组元活度模型能较正确地反映CaO-Cu2O-Fe2O3渣系结构本质。
图2中的点划线是炉渣碱度B (![]()
![]() )为1.54时的等碱度线。由图2可知,该点划线与各等活度线的交点为α(Cu2O)曲线的顶点,此处活度最大。
)为1.54时的等碱度线。由图2可知,该点划线与各等活度线的交点为α(Cu2O)曲线的顶点,此处活度最大。
图3所示为炉渣中Cu2O摩尔分数为0.3时,碱度B和温度t对![]() 的影响。由图3可知,当B>1.54,α(Cu2O)随碱度B和温度t的升高而降低;当B<1.54趋势正好相反,且受碱度的影响更为显著。
的影响。由图3可知,当B>1.54,α(Cu2O)随碱度B和温度t的升高而降低;当B<1.54趋势正好相反,且受碱度的影响更为显著。
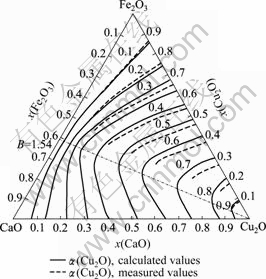
图2 1 250 ℃时Cu2O的等活度曲线
Fig.2 Equal activity curves of Cu2O at 1 250 ℃
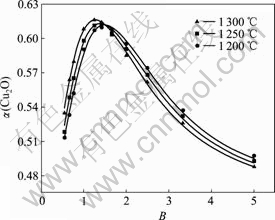
图3 碱度B和温度t对α(Cu2O)的影响
Fig.3 Influence of basicity B and temperature t on α(Cu2O)
由图4和5可以看出,α(Cu2O)呈拉乌尔正偏差,偏差总体上随渣中Cu2O的摩尔分数x(Cu2O)的增大呈先加大后缩小之势。在高碱度(B>1.54)状态下,偏差随B的增大而缩小;在低碱度(B<1.54)状态下,当x(Cu2O)<0.6时,偏差随B的增大而加大,而当 x(Cu2O)>0.6时,偏差随B的增大而缩小,但相差很小。由此可见,对含一定量Cu2O的炉渣,当B=1.54时,将出现α(Cu2O)最大值。
计算了1 200 ℃和1 300 ℃下的渣中CaO的活度,并绘制了等活度线,如图6所示。
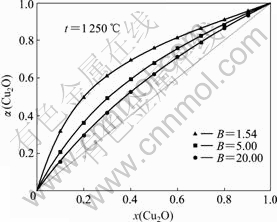
图4 高碱度B时渣含Cu2O对α(Cu2O)的影响
Fig.4 Influence of Cu2O content in slag on α(Cu2O) with high basicity B
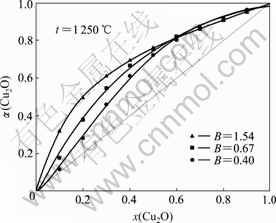
图5 低碱度B时渣含Cu2O对α(Cu2O)的影响
Fig.5 Influence of Cu2O content in slag on α(Cu2O) with low basicity B
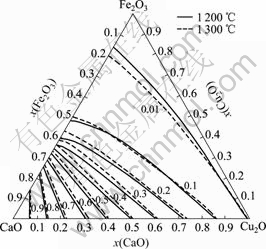
图6 CaO的等活度曲线
Fig.6 Equal activity curves of CaO
由图6可以看出,渣中CaO的活度α(Cu2O)总体上随CaO含量的增加而上升;1 200 ℃和1 300 ℃下的等活度线相距较近,表示α(Cu2O)受温度影响不大。
计算了1 200 ℃和1 300 ℃下的渣中Fe2O3的活度,并绘制了等活度线,如图7所示。
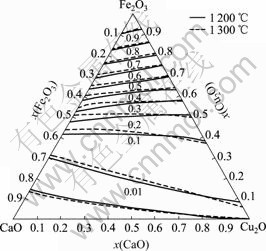
图7 Fe2O3的等活度曲线
Fig.7 Equal activity curves of Fe2O3
由图7可以看出,渣中Fe2O3的活度α(Fe2O3)变化趋势与α(CaO)类似,总体上随Fe2O3含量的增加而上升;1 200 ℃和1 300 ℃下的等活度线相距也较近,表示α(Fe2O3)受温度影响也不大。
图6和7还表明,由于CaO或Cu2O与Fe2O3间的结合力较强,而CaO与Cu2O间的结合力较弱,所以等活度线α(CaO)和α(Fe2O3)沿Fe2O3-CaO边和Fe2O3-Cu2O边的变化较大,而沿CaO-Cu2O边的变化较小。
3 结论
1) 基于熔体结构共存理论,建立了1 200~1 300 ℃下的CaO-Cu2O-Fe2O3三元渣系组元活度计算模型,结果表明,理论计算值α(Cu2O)与实测值α′(Cu2O)吻合较好,说明模型大体可以反映本渣系的结构本质。
2) α(Cu2O)呈拉乌尔正偏差,在B=1.54时具有最大值,当B>1.54时,随碱度和温度的升高而减小;当B<1.54时趋势正好相反。
3) α(CaO)和α(Fe2O3)分别随CaO和Fe2O3在炉渣中含量的升高而增大,受温度的影响都不明显。
4) 根据模型计算结果绘制了CaO、Cu2O和Fe2O3等活度曲线,为采用铁酸钙渣系的炼铜新工艺热力学分析和计算机模拟提供了数据。
REFERENCES
[1] Moskalyk R R, Alfantazi A M. Review of copper pyrometallurgical practice: today and tomorrow[J]. Minerals Engineering, 2003, 16(10): 893-919.
[2] Davenport W G, King M, Schlesinger M, BISWAS A K. Extractive metallurgy of copper[M]. 4th edition. New York: Pergamon Press, 2002: 203-219.
[3] Kongoli F, McBow I, Yazawa A, Takeda Y, Yamaguchi K, Budd R, Llubani S. Liquidus relationships of calcium ferrite and ferrous calcium silicate slag in continuous copper converting[J]. Transactions of the Institutions of Mining and Metallurgy, 2008, 117(2): 67-76.
[4] 鄂加强, 王春华, 龚金科, 李 娟, 彭 雨, 朱 浩. 铜火法冶炼热动力学系统实测数据EMD处理[J]. 中国有色金属学报, 2008, 18(5): 946-951.
E Jia-qiang, WANG Chun-hua, GONG Jin-ke, LI Juan, PENG Yu, ZHU Hao. Process on measurement data from copper pyrometallurgical heat dynamical system by using of EMD method[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(5): 946-951.
[5] GUI Wei-hua, WANG Ling-yun, YANG Chun-hua, XIE Yong-fang, PENG Xiao-bo. Intelligent prediction model of matte grade in copper flash smelting process[J]. Trans Nonferrous Met Soc China, 2007, 17(5): 1075-1081.
[6] 汪金良, 卢 宏, 曾青云, 张传福. 基于遗传算法的铜闪速熔炼过程控制优化[J]. 中国有色金属学报, 2007,17(1): 156-160.
WANG Jin-liang, LU Hong, ZENG Qing-yun, ZHANG Chuan-fu. Control optimization of copper flash smelting process based on genetic algorithms[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(1): 156-160.
[7] Nichita D V, Gomez S, Luna E. Multiphase equilibria calculation by direct minimization of Gibbs free energy with a global optimization method[J]. Computers and Chemical Engineering, 2002, 26(12): 1703-1724.
[8] Ilyushechkin A, Hayes P C, Jak E. Liquidus temperatures in calcium ferrite slags in equilibrium with molten copper[J]. Metallurgical and Materials Transactions B, 2004, 35(2): 203-215.
[9] Sakai T, Ip S W,Toguri J M. Interfacial phenomena in the liquid copper-calcium ferrite slag system[J]. Metallurgical and Materials Transactions B, 1997, 28(3): 401-407.
[10] Florian K, Akira Y. Liquidus surface of FeO-Fe2O3- SiO2-CaO slag containing Al2O3, MgO and Cu2O at intermediate oxygen partial pressures[J]. Metallurgical and Materials Transactions B, 2001, 32(4): 583-592.
[11] SangHL, SeokMM, DongJM, JOD H P. Thermodynamic behavior of nickel in CaO-SiO2-FetO slag[J]. Metallurgical and Materials Transactions B, 2002, 33(1): 55-59.
[12] PavolV, MilanH, VladimírD. Density and surface tension of the systems CaO-FeO-Fe2O3-MgO, CaO-FeO-Fe2O3- ZnO and CaO-Fe2O3-Cu2O[J]. Central European Journal of Chemistry, 2006, 4(1): 174-193.
[13] Fahey N P, Swinbourne D R, Yan S, OSBORNE J M. The solubility of Cr2O3 in calcium ferrite slags at 1 573 K[J]. Metallurgical and Materials Transactions B, 2004, 35(2): 197-202.
[14] YAZAWA A, EGUCHI M, SENDAL J. Equilibrium studies on copper slags used in continuous converting[C]// YANNOPOULOS J, AGARWAL J. An International Symposium: Extractive Metallurgy of Copper. New York: The Metallurgical Society of AIME, 1976: 3-19.
[15] ZHANG Jian. Coexistence theory of slag structure and its application to calculation of oxidizing capability of slag melts[J]. Journal of Iron and Steel Research, 2003, 10(1): 1-10.
[16] ZHANG Jian. Thermodynamic properties and mixing thermodynamic parameters of binary homogeneous metallic melts[J]. Rare metals, 2003, 22(1): 25-32.
[17] Phillips B, Muan A. Phase equilibrium in the system CaO-iron oxide in air and at 1 atm O2 pressure [J]. Journal of the America Ceramic Society, 1958, 41(11): 445-454.
[18] Levin E M, Robbins C R, Mcmurdie H F. Phase diagrams for ceramists[M]. Cleveland: America Ceramic Society, 1969: 17-18.
[19] Takeda Y, Nakazawa S, Yazawa A. Thermodynamics of calcium ferrite slags at 1 200 and 1 300 degree C. [J]. Canadian: Metallurgical Quarterly, 1980, 1(3): 297-305.
[20] 张 鉴. 冶金熔体和溶液的计算热力学[M]. 北京: 冶金工业出版社, 2007: 297-298.
ZHANG Jian. Computational thermodynamics of metallurgical melts and solutions[M]. Beijing: Metallurgical Industry Press, 2007: 297-298.
[21] 梁英教, 车荫昌. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社, 1993: 458.
LIANG Ying-jiao, CHE Yin-chang. Inorganic thermodynamic data Manual[M]. Shenyang: Northeastern University Press,1993: 458.
基金项目: 国家重大产业技术开发资助项目(20051255);江西省科技攻关资助项目(20061B0101100);江西省自然科学基金资助项目(2007GZC0713);江西省教育厅科研资助项目(GJJ09241)
收稿日期:2008-09-26;修订日期:2009-03-09
通讯作者:张传福,教授,博士;电话:0731-8830471;E-mail: cfzhang@mail.csu.edu.cn
(编辑 李向群)


