文章编号:1004-0609(2008)S1-0310-06
SnO2-Li4Ti5O12复合材料的溶胶-凝胶法制备与表征
熊利芝1, 2,何则强1, 2,尹周澜2,陈启元2
(1. 吉首大学 化学化工学院,吉首 416000;
2. 中南大学 化学化工学院,长沙 410083)
摘 要:以SnCl4·5H2O、CH3COOLi·2H2O、(CH3(CH2)3O)4Ti和NH3·H2O为原料,采用溶胶-凝胶法制备SnO2-Li4Ti5O12复合材料粉末。采用热重和差热分析、X射线衍射、红外光谱、透射电镜和电化学测试等手段对复合材料的结构、表面形貌和电化学性能进行表征。结果表明:采用溶胶-凝胶法制备的复合材料是一种核-壳结构的纳米复合材料。在复合材料中,Li4Ti5O12以无定形态包覆在SnO2颗粒的表面,其包覆层的厚度为20~40 nm。电化学研究表明,0.1C放电时SnO2-Li4Ti5O12复合材料粉末的可逆容量达到688.7 mA?h/g,0.2C放电时经60次循环后复合材料的容量保持率达到93.4%。由于复合材料中Li4Ti5O12能够有效吸收SnO2电极的体积变化,有效阻止循环过程中Sn颗粒的聚集,大大改善了SnO2的循环稳定性。
关键词:
中图分类号:O 614 文献标识码:A
Preparation and characterization of SnO2-Li4Ti5O12 composite by sol-gel technique
XIONG Li-zhi 1, 2, HE Ze-qiang 1, 2, YIN Zhou-lan2, CHEN Qi-yuan2
(1. College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: SnO2-Li4Ti5O12 was prepared by sol-gel method using tin tetrachloride, lithium acetate, tetrabutylorthotitanate and aqueous ammonia as starting materials. The structure, morphology and electrochemical performance of composite were characterized by thermogravimertric (TG) analysis and differential thermal analysis (DTA), X-ray diffractometry (XRD), infrared (IR) spectroscopy, transmission electron microscopy (TEM) and electrochemical tests. The results show that SnO2-Li4Ti5O12 composite derived by sol-gel technique is a nanocomposite with core-shell structure, where the amorphous Li4Ti5O12 layer with 20-40 nm in thickness is coated on the surface of SnO2 particles. Electrochemical tests show that SnO2-Li4Ti5O12 composite delivers a reversible capacity of 688.7 mA?h/g at 0.1C and 93.4% of that is retained after cycling 60 times at 0.2C. The amorphous Li4Ti5O12 in composite can accommodate the volume change of SnO2 electrode and prevent the small and active Sn particles aggregating into larger and inactive Sn clusters during cycling effectively, and enhance the cycling stability of SnO2 electrode significantly.
Key words: SnO2; Li4Ti5O12; composite; sol-gel method; lithium ion batteries
自从1997年日本富士胶片公司[1]宣布将商业化采用SnO2作为锂离子电池负极材料后,SnO2由于其比容量高(为石墨负极材料的两倍以上)而受到广泛关注,被认为将是锂离子电池最好的负极材料[2-7]。SnO2作为锂离子电池负极材料时,Sn是反应的活性组分,其可逆容量是基于锂-锡合金(LiSn,Li7Sn3,Li5Sn2,Li3Sn5,Li7Sn2或Li22Sn5)的可逆生成和分解[8]。在首次循环中,SnO2中的氧与金属锂反应生成无定形Li2O。无定形Li2O能缓冲反应中Sn颗粒之间的团聚作用。Li与SnO2的作用机理可用反应式表示如 下[2, 9-10]:
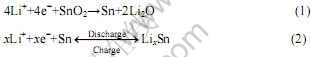
反应(1)中无定形Li2O的形成导致SnO2负极具有高达47.6%首次不可逆容量,这是SnO2作为负极材料的不足之处[2-3]。然而,阻碍SnO2应用于锂离子电池的最致命的问题,就是由于电极循环过程中严重的维度变化(高达358%)导致电极粉化失效[2-3],循环稳定性严重降低。为了降低循环过程中的体积变化,人们采取了许多措施。据LI等[11]报道,SnO2纳米纤维能够有效地吸收电极循环过程中的体积变化,即使在高电流倍率下仍能保持良好的循环稳定性。有人采用SnO2薄膜以降低电极的体积变化[9, 12]。然而,这些电极由于制备条件复杂、生产成本昂贵而导致无法应用于锂离子电池。也有人采用一些金属复合氧化物MxSnyOz(M为Ni、Ca、Fe、Sb、Cu、Mg、Zn等)[13-16],由于金属M对金属Li是非活性的,从而阻止了循环过程中颗粒Sn的聚集成团,改善电极的循环稳定性。但是,这是以牺牲容量为代价的。随着纳米科学技术的发展,许多不同结构和形貌的纳米金属氧化物被研究并用于传感器、磁、电装置和锂离子电池领域。研究表明,通过降低SnO2颗粒的大小,可以有效改善电极的循环稳定性[17-18]。通过对SnO2进行表面修饰,在SnO2表面包覆一层导电物质,如导电石墨、导电聚合物等[19-21]也能改善SnO2电极的循环稳定性。然而,迄今为止,还没有发现通过SnO2表面包覆Li4Ti5O12以改善其循环稳定性的报道。本文作者采用溶胶-凝胶法制备了SnO2-Li4Ti5O12复合材料,研究了其电化学性能。
1 实验
将一定量的SnCl4·5H2O溶解在浓HCl 中,控制反应温度在45 ℃,在不断搅拌下滴加NH3·H2O溶液,维持pH值为3,并持续搅拌,至反应完成,形成白色沉淀。将白色沉淀的母液在室温静止陈化1 h后,用快速定性滤纸进行真空抽滤,所得滤饼先后用蒸馏水和酒精洗涤数次(取上层清液用AgNO3检测,如果没有白色沉淀生成,表示洗涤完全)。 洗涤后所得滤饼置于真空烘箱,于80 ℃下干燥18 h得白色前驱体,再于坩埚电阻炉中在500 ℃下焙烧3 h制得SnO2粉末。
将CH3COOLi·2H2O溶于无水乙醇中(钛酸丁酯与乙醇的体积比为1?5),然后缓慢地向其中加入钛酸四丁酯(溶液中Li与Ti的摩尔比为4?5),并不断搅拌,得到Li4Ti5O12前驱体溶液。然后再加入几滴去离子水并放在干燥箱中100 ℃保持4 h,得到白色或淡黄色的Li4Ti5O12前驱体干凝胶。前驱体干凝胶经500 ℃煅烧4 h,得到Li4Ti5O12粉末。
与上述步骤相同,强烈搅拌下在Li4Ti5O12前驱体溶液中加入SnO2粉末,得到Li4Ti5O12含量不同的Li4Ti5O12-SnO2复合材料。
采用METTLER TOLEDO的TGA/SDTA851e型热分析仪对前驱体进行热分析(测试温度范围为25~900 ℃,升温速度为10 ℃/min,气氛为氩气)。采用日本Rigaku型X射线粉末衍射仪对所得样品进行物相分析(Cu Kα辐射,40 kV,100 mA,步宽0.02?,扫描速度0.5 (?)/min,扫描范围 (2θ)为5?~85?)。采用JEOL公司的JEM2010透射电镜(加速电压为200 kV)对样品的表面形貌进行分析。采用美国Nicolet 205型傅立叶变换红外谱仪对样品进行红外光谱研究(波长为3 600~400 cm-1;分辨率为3 cm-1)。
将活性物质SnO2-Li4Ti5O12粉末、乙炔黑和聚四氟乙烯(PVDF)按质量比75?12.5?12.5在溶剂聚乙烯吡咯烷酮(NMP)中混合均匀,然后将其均匀涂布在集流体铜箔上,在120 ℃真空干燥10 h制成SnO2-Li4Ti5O12电极。以SnO2-Li4Ti5O12为正极,多孔聚丙烯为隔膜,1.0 mol/L LiPF6的EC-DEC(体积比为1?1)溶液为电解液,金属锂为负极,在充满氩气的手套箱中装配成2025型扣式电池。采用PCBT-100-8D型二次电池测试仪进行充放电性能的测试(测试温度为(25±0.1) ℃)。
2 结果与讨论
图1所示为SnO2-Li4Ti5O12前驱体粉末的热重分析(TG)和差热分析(DTA)曲线。从热重曲线可以看出,整个失重过程几乎全部发生在400 ℃以前,包括前驱体表面物理吸附水的挥发、Li4Ti5O12前驱体的分解等步骤,但从热重曲线分不清具体的失重阶段,表明这些失重过程是连续进行的。500 ℃左右热重曲线接近直线,没有热失重的出现,说明前驱体分解完毕。而在差热曲线上400 ℃出现了一个吸热峰,可能是前驱体分解并形成SnO2-Li4Ti5O12复合材料时发生了晶形改变的缘故。
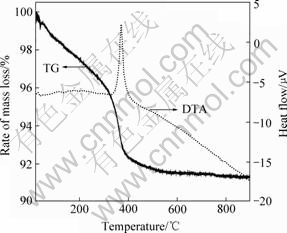
图1 SnO2-Li4Ti5O12前驱体粉末的热分析曲线
Fig.1 Thermal analysis curves of precursor of SnO2-Li4Ti5O12
图2所示为样品粉末的X射线衍射谱。
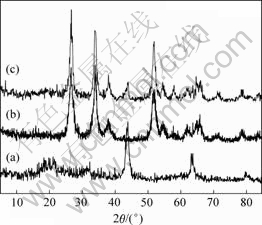
图2 样品粉末的X射线衍射谱
Fig.2 XRD patterns of samples: (a) Li4Ti5O12; (b) SnO2; (c) SnO2-Li4Ti5O12
图2(a)所示X射线衍射谱中所有衍射峰都与JCPDS标准卡片(No.26-1198)一致,表明合成产物是具有面心立方结构(空间群![]() )的Li4Ti5O12,与文献报道一致[22]。图2(b)所示X射线衍射谱中所有衍射峰都与JCPDS标准图谱(卡片号:21-1250)能够很好吻合,说明合成样品为金红石结构的SnO2粉末。在图2(c)所示的SnO2-Li4Ti5O12复合粉末的X射线衍射谱中明显出现了标识SnO2的衍射峰,但标识Li4Ti5O12的衍射峰很弱,可能是因为在SnO2-Li4Ti5O12复合粉末中Li4Ti5O12以无定形态包覆在SnO2的表面。
)的Li4Ti5O12,与文献报道一致[22]。图2(b)所示X射线衍射谱中所有衍射峰都与JCPDS标准图谱(卡片号:21-1250)能够很好吻合,说明合成样品为金红石结构的SnO2粉末。在图2(c)所示的SnO2-Li4Ti5O12复合粉末的X射线衍射谱中明显出现了标识SnO2的衍射峰,但标识Li4Ti5O12的衍射峰很弱,可能是因为在SnO2-Li4Ti5O12复合粉末中Li4Ti5O12以无定形态包覆在SnO2的表面。
图3所示为样品粉末的红外光谱图。由图3(a)可见,Li4Ti5O12样品在687 cm-1、546 cm-1处分别出现了Ti—O键的伸缩振动峰[23]。由3(b)可见,在654 cm-1、509 cm-1处出现了Sn—O键和O—Sn—O键的红外特征吸收峰[24]。这些峰在图3(c)中都有出现,只是峰的位置发生了不同程度的红移或者蓝移。在2 330~2 350 cm-1处都出现了一个吸收峰,可能是由于空气中CO2的干扰峰。三个样品都在1 300~1 500 cm-1以及3 500 cm-1出现O—H和H2O分子缔合的特征峰,可能是样品表面吸附空气中的水分子所致。
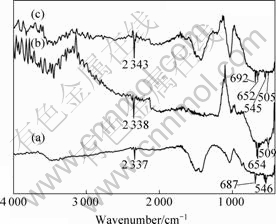
图3 样品粉末的红外光谱图
Fig.3 IR spectra of samples: (a)Li4Ti5O12; (b)SnO2; (c) SnO2- Li4Ti5O12
图4所示为SnO2和SnO2-Li4Ti5O12的透射电镜照片。从图4(a)可以看到,SnO2粉末由比较规则的圆球状颗粒组成,由于颗粒较小,表面活性大,颗粒之间存在一定的团聚。从透射电镜可以大约估计出SnO2粉末的粒度在30~50 nm之间。由图4(b)可见,在SnO2颗粒表面被一层无定形的Li4Ti5O12包裹,形成了一种核-壳结构的纳米复合材料。根据透射电镜结果可以估计,复合材料中无定形Li4Ti5O12层的厚度在20~40 nm之间。
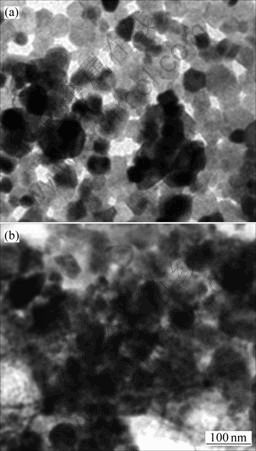
图4 SnO2(a)和SnO2-Li4Ti5O12(b)的透射电镜照片
Fig.4 TEM images of SnO2(a) and SnO2-Li4Ti5O12(b)
图5所示为SnO2和SnO2-Li4Ti5O12电极的首次充放电曲线。为了保证所制备电极(负极)和正极所组成的锂离子电池高电压的特点,充放电制度设定为:电极首先从开路电压放电到0 V,然后在0~1.0 V之间进行充放电。充放电倍率为0.1C(70 mA/g)。从图5可见,SnO2和SnO2-Li4Ti5O12电极的充放电曲线十分相似,表明二者具有相同的电化学机理,Li4Ti5O12的存在并没有对SnO2电极的电化学机理产生影响。SnO2和SnO2-Li4Ti5O12的首次放电容量分别为1 640.5 mA?h/g和1 565.2 mA?h/g;首次充电容量分别为689.8 mA?h/g和688.7 mA?h/g。可见,由于在SnO2表面包覆一层无定形Li4Ti5O12后,复合电极的首次放电容量和充电容量都降低了。这是因为:一方面,由于Li4Ti5O12的理论比容量(168 mA?h/g)比SnO2的理论比容量(782 mA?h/g)低,因此单位质量的SnO2被Li4Ti5O12取代后,复合电极的容量会比SnO2电极的低;另一方面,当充放电截止电压选择在0~1.0 V时,由于Li4Ti5O12的充放电平台电压在1.5V左右[25],因此复合电极中Li4Ti5O12的容量发挥不出来。
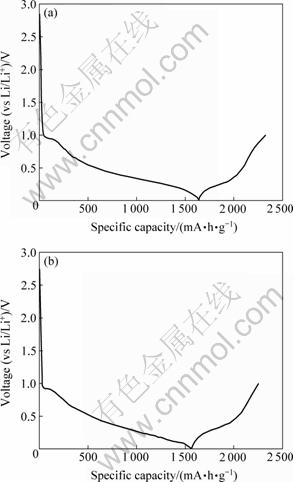
图5 SnO2(a)和SnO2-Li4Ti5O12(b)首次充放电曲线
Fig.5 Initial charge-discharge curves of SnO2(a) and SnO2-Li4Ti5O12(b)
图6所示为SnO2和SnO2-Li4Ti5O12电极0.2C (140 mA/g)充放电时循环性能的比较。根据SnO2电极的放电机理,电极首次放电容量与此后各次循环放电容量明显不同,因此这里只对首次循环后电极的循环性能进行比较。从图6可以看到,0.2C放电时,SnO2电极第2次循环的放电容量为688.1 mA?h/g,60次循环后的容量为587.8 mA?h/g,容量保持率(这里指第60次循环的放电容量占第2次循环放电容量的百分数)为85.4%;相同条件下,SnO2-Li4Ti5O12电极第2次循环的放电容量为687.6 mA?h/g,60次循环后的容量为642.1 mA?h/g,容量保持率93.4%。可见,SnO2表面包覆一层无定形的Li4Ti5O12后得到的SnO2-Li4Ti5O12复合电极的循环性能明显优于SnO2。这可能是由于Li4Ti5O12是一种“零应变”材料,在循环过程中能够有效吸收SnO2电极巨大的体积变化,有效阻止循环过程中Sn颗粒的聚集,改善循环性能。
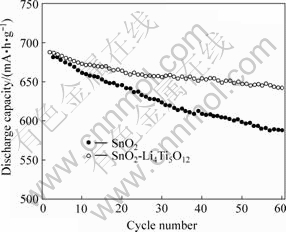
图6 SnO2和SnO2-Li4Ti5O12电极的循环性能
Fig.6 Cycling performance of SnO2 and SnO2-Li4Ti5O12
3 结论
1) 采用溶胶-凝胶法制备了SnO2-Li4Ti5O12复合材料粉末。复合材料中无定形的Li4Ti5O12包裹在SnO2颗粒的表面,形成了一种核-壳结构的纳米复合材料。复合材料中无定形的Li4Ti5O12包裹层的厚度大约在20~40 nm之间。
2) 由于Li4Ti5O12是一种“零应变”材料,SnO2表面包覆一层无定形的Li4Ti5O12后,Li4Ti5O12在循环过程中能够有效吸收SnO2电极巨大的体积变化,有效阻止循环过程中Sn颗粒的聚集,复合材料的循环稳定性明显优于相同条件下SnO2的循环稳定性。
REFERENCES
[1] IDOTA Y, MATSUFUJI A, MAEKAWA Y, NIYASAKA T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material[J]. Science, 1997, 276(5317): 1395-1397.
[2] COURTNEY A, DAHN J R. Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide composites[J]. J Electrochem Soc, 1997, 144(6): 2045-2052.
[3] LIU W F, HUANG X J, WANG Z X, LI H, CHEN L Q. Studies of stannic oxide as an anode material for lithium-ion batteries[J]. J Electrochem Soc, 1998, 145(1): 59-62.
[4] COURTNEY A, DAHN J R. Key factors controlling the reversibility of the reaction of lithium with SnO2 and Sn2BPO6 glass[J]. J Electrochem Soc, 1997, 144(9): 2943-2948.
[5] HE Ze-qiang, LI Xin-hai, WU Xian-ming, HOU Zhao-hui, LIU En-hui, DENG Ling-feng, HU Chuan-yue, TIAN Hui-peng. Preparation and electrochemical properties of nanosized tin dioxide electrode material by sol-gel process[J]. Trans Nonferrous Met Soc China, 2003, 13(4): 998-1002.
[6] 何则强, 李新海, 熊利芝, 刘恩辉, 侯朝辉. 锡基复合氧化物的高能球磨法制备及其电化学性能[J]. 无机化学学报, 2004, 20(1): 102-106.
HE Ze-qiang, LI Xin-hai, XIONG Li-zhi, LIU En-hui, HOU Zhao-hui. Preparation and electrochemical properties of tin-based composite oxide by high-energy ball-milling method[J]. Chinese Journal of Inorganic Chemistry, 2004, 20(1): 102-106.
[7] HE Ze-qiang, LI Xin-hai, XIONG Li-zhi, WU Xian-ming, XIAO Zhuo-bing, MA Ming-you. Wet chemical synthesis of tin oxide-based material for lithium ion battery anodes[J]. Materials Research Bulletin, 2005, 40(5): 861-868.
[8] ANANI A, CROUCH-BAKER S, HUGGINS R A. Kinetic and thermodynamic parameters of several binary lithium alloy negative electrode materials at ambient temperature[J]. J Electrochem Soc, 1987, 134(12): 3098-3102.
[9] RETOUX R, BROUSSE T, SCHLEICH D M. High-resolution electron microscopy investigation of capacity fade in SnO2 electrodes for lithium-ion batteries[J]. J Electrochem Soc, 1999, 146(7): 2472-2476.
[10] BROUSSE T, RETOUX R, HERTERICH U, SCHLEICH D M. Thin-film crystalline SnO2-lithium electrodes[J]. J Electrochem Soc, 1998, 145(1): 1-4.
[11] LI N, MARTIN C R, SCROSATI B. A high-rate, high-capacity, nanostructured tin oxide electrode[J]. Electrochem Solid-State Lett, 2000, 3(7): 316-318.
[12] NAM S C, KIM Y H, CHO W I, CHO B W, CHUN H S, YUN K S. Charge-discharge performance of electron-beam-deposited tin oxide thin-film electrodes[J]. Electrochem Solid-State Lett, 1999, 2(1): 9-13.
[13] BESENHARD J O, YANG J, WINTER M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries?[J]. J Power Sources, 1997, 68(1): 87-90.
[14] CROSNIER O, BROUSSE T, DEVAUX X, FRAGNAUD P, SCHLEICH D M. New anode systems for lithium ion cells[J]. J Power Sources, 2001, 94(2): 169-171.
[15] TIRADO J L. Inorganic materials for the negative electrode of lithium-ion batteries: State-of-the-art and future prospects[J]. Mater Sci Eng R, 2003, 40(3): 103-115.
[16] 袁正勇, 袁良杰, 孙聚堂. 纳米锡锌复合氧化物贮锂材料的合成和性质[J]. 高等学校化学学报, 2006, 27(12): 2252-2255.
YUAN Zheng-yong, YUAN Liang-jie, SUN Ju-tang. Synthesis and properties of nanosized tin-zinc composite oxides as lithium storage materials[J]. Chemical Journal of Chinese Universities, 2006, 27(12): 2252-2255.
[17] GRAETZ J, AHN C C, YAZAMI R, FULTZ B. Highly reversible lithium storage in nanostructured ilicon[J]. Electrochem Solid-State Lett, 2003, 6: A194-A196.
[18] KIM C, NOH M, CHOI M, CHO J, PARK B. Critical size of a nano SnO2 electrode for Li-secondary battery[J]. Chem Mater, 2005, 17(12): 3297-3301.
[19] 麻明友, 何则强, 熊利芝, 李新海, 肖卓炳, 吴显明, 刘文萍. SnO2-石墨复合粉的均匀沉淀法制备与电化学性能[J]. 中国有色金属学报, 2005, 15(5): 793-798.
MA Ming-you, HE Ze-qiang, XIONG Li-zhi, LI Xin-hai, XIAO Zhuo-bing, WU Xian-ming, LIU Wen-ping. Preparation and electrochemical properties of SnO2-graphite composites by homogeneous precipitation technique[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(5): 793-798.
[20] BALAN L, SCHNEIDER R, WILLMANN P, BILLAUD D. Tin-graphite materials prepared by reduction of SnCl4 in organic medium: Synthesis, characterization and electrochemical lithiation[J]. J Power Sources, 2006, 161(1): 587-593.
[21] 何则强, 刘文萍, 熊利芝, 舒 晖, 吴显明, 陈 上, 黄可龙. 锂离子电池用 SnO2-聚苯胺复合材料的制备与表征[J]. 无机化学学报, 2007, 23(5): 813-826.
HE Ze-qiang, LIU Wen-ping, XIONG Li-zhi, SHU Hui, WU Xian-ming, CHEN Shang, HUANG Ke-long. Synthesis and characterization of SnO2-polyaniline composite as anode for lithium ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2007, 23(5): 813-826.
[22] WANG G X, BRADHURST D H, DOU S X, LIU H K. Spinel Li[Li1/3Ti5/3]O4 as an anode material for lithium ion batteries[J]. J Power Sources, 1999, 83(1/2): 156-160.
[23] ALLEN G C, PAUL M. Chemical characterization of transition metal spinel-type oxides by infrared spectroscopy[J]. Appl Spectrosc, 1995, 49(4): 451-458.
[24] HA H W, KIM K, DE BORNIOL M, TOUPANCE T. Fluorine-doped nanocrystalline SnO2 powders prepared via a single molecular precursor method as anode materials for Li-ion batteries[J]. Journal of Solid State Chemistry, 2006, 179(3): 702-707.
[25] FU L J, LIU H, LI C, WU Y P, RAHM E, HOLZE R, WU H Q. Electrode materials for lithium secondary batteries prepared by sol-gel methods[J]. Prog Mater Sci, 2005, 50: 881-887.
基金项目:国家自然科学基金资助项目(20376086);中国博士后科学基金资助项目(2005037700);湖南省自然科学基金资助项目(07JJ3014);湖南省教育厅科学研究重点资助项目(07A058);中南大学博士后科学基金资助项目(2004107)
通讯作者:熊利芝,讲师,博士研究生;电话:13975171695;E-mail: csuhzq@163.com


