
Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi ilmenite concentration
TAN Ping1, HU Hui-ping2, ZHANG Li2
1. Department of Biological and Chemical Engineering,
Hunan Mechanical and Technical Polytechnic, Changsha 410151, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 4 November 2010; accepted 14 April 2011
Abstract:
The effects of oxidation-reduction treatment and mechanical activation on the hydrochloric acid leaching performance of Panxi ilmenite concentration were investigated. The results show that both of oxidation-reduction treatment and mechanical activation significantly accelerate the extraction of Fe, Ca and Mg from Panxi ilmenite concentration; however, the CaO and MgO contents of the calcined residues obtained from oxidized-reduced ilmenite concentration are higher than the standard values required by chlorination process. The Ca and Mg in oxidized-reduced ilmenite concentration can be leached much faster after mechanical activation, yielding a synthetic rutile which meets the requirements of chlorination process containing 90.50% TiO2 and 1.37% total iron as well as combined CaO and MgO of 1.00%. The optimum oxidation and reduction conditions are as follows: oxidization at 900 °C in the presence of oxygen for 15 min and reduction at 750 °C by hydrogen for 30 min.
Key words:
ilmenite concentration; mechanical activation; oxidation; reduction; hydrochloric acid leaching;
1 Introduction
China is endowed with large reserves of ilmenite estimated at around 965 million tons amounting to 38.85 % of the total world reserves, and more than 90% of them are found in the Panxi area, Southwest China. Unlike the sand ilmenite, the Panxi ilmenite concentration which is a complex polymetallic rock ore has the following characteristics [1]: 1) the iron oxides in the ilmenite concentration are mainly present in ferrous forms leading to a high molar ratio of FeO/Fe2O3; 2) it presents a compact structure of crystal with high content of gangue (w(MgO+CaO)=5%-8%) which is difficult to be separated by conventional beneficiation procedures; 3) the content of SiO2 is very high ranging from 1.7% to 3.8%. And those characteristics render Panxi ilmenite concentration undesirable for use in industrial processes without some degree of purification.
At present, the chlorination process is gradually replacing the sulfate process and becoming the dominant process among the processes of upgrading ilmenite concentration. But the major drawback of the chloride route is the requirements of superior quality feedstocks containing not less than 80% TiO2 for titanium slag, 90% for synthetic rutile, and with a much less amount of the impurities such as magnesium and calcium, which form high-boiling-point chloride, leading to the malfunctioning of fluidized bed chlorinator [2]. Hence, the feedstock to the chlorinator is restricted to a combined impurity content of both CaO and MgO not more than 1.50% [3]. At present, the means of producing these feedstocks from ilmenite concentration include smelting process, the Becher process, hydrochloric acid leaching process, and so on [4-6]. The hydrochloric acid leaching process presents advantages of fast leaching, excellent impurities removal and acid regeneration technology [7], so it is considered to be the optimum process to prepare synthetic rutile from Panxi ilmenite concentration. However, previous studies demonstrated that there is a solid/liquid separation problem in the direct hydrochloric acid leaching of Panxi ilmenite concentration. According to the characteristics of Panxi ilmenite concentration, YE et al [8] introduced oxidation- reduction pretreatment to treat Panxi ilmenite concen- tration to solve the solid/liquid separation problem, and the magnetic separations were performed after the calcination of leaching residues to remove the gangue, and finally a synthetic rutile containing more than 90% TiO2 was obtained. If no magnetic separations were performed after the calcination of leaching residues, the contents of MgO and CaO in the product are higher than the standard values required by chlorination process.
In previous studies [9-11], hydrochloric acid leaching behavior of mechanically activated plagioclase, titanaugite and ilmenite monomineral, which were beneficiated from Panxi ilmenite concentrate, were studied. It was found that the mechanical activation significantly accelerated the dissolution of Fe, Ca and Mg from plagioclase, titanaugite and ilmenite monomineral. In addition, our previous study [11] demonstrated that a large amount of Mg in ilmenite monomineral from Panxi area isomorphously displace Fe in the structure of ilmenite, while minorities associate with gangue minerals, and Ca more likely exists in gangue minerals. Therefore, we can infer that the mechanical activation could enhance the leaching of Fe, Ca and Mg from Panxi ilmenite concentration.
In the present work, the oxidation and reduction of Panxi ilmenite concentration were investigated, and the effects of mechanical activation on hydrochloric acid leaching behavior of untreated ilmenite concentration and oxidized-reduced ilmenite concentration were also investigated. The objective of this study was to upgrade the Panxi ilmenite concentration into synthetic rutile which meets the requirements for the chlorination process.
2 Experimental
2.1 Materials
The ilmenite concentration sample used in the present work was provided by Titanium Company of Panzhihua Steel & Iron (Group) Corporation (Sichuan Province, China) and had a range of particle size from 10 to 150 ?m, and the median diameter of the particles was about 60 ?m. XRD analysis shows that the major mineral constitution was hexagonal structure FeTiO3 with a purity of about 90%. The impurity elements of calcium, aluminum, manganese and so on mostly exist in silicate minerals such as plagioclase and titanaugite in the Panxi ilmenite concentration [9-10], whose chemical composition is summarized in Table 1. The Panxi natural ilmenite concentration was dried at 120 °C for 24 h to get untreated ilmenite concentration. All the other chemicals used for leaching and chemical analysis were of at least analytical grade.
Table 1 Chemical composition of ilmenite concentration (mass fraction, %)
2.2 Experimental procedure
2.2.1 Oxidation
Oxidation of ilmenite concentration was carried out in a horizontal tube furnace (SK–6-10, Changcheng Furnace Plant, Changsha, China) with oxygen continuously blown into the furnace at a flow rate of 0.2 L/min. The samples were heated to different temperatures at a constant ramping rate of 10 °C/min. The charge was allowed to cooling to room temperature in nitrogen atmosphere, and then the phase composition and the FeO content of oxidized ilmenite concentration were analyzed.
2.2.2 Reduction
The reduction operation of oxidized ilmenite concentration took place in box type controlled atmosphere furnace (SX–10-13Q, Changcheng Furnace Plant, Changsha, China), in the presence of hydrogen. After placing in the furnace, the furnace lid was tightly affixed, and the system fluxed with nitrogen for 10 min before the reduction was started. At a heating rate of 10 °C/min, the furnace was heated to the desired temperature, then the flow was changed from nitrogen to hydrogen. After a period of time, the reduction was stopped by cutting the power supply and then the oxidized-reduced ilmenite concentration was cooled in a flow of nitrogen in order to prevent reoxidation. And contents of Fe, FeO as well as the total iron content of the oxidized-reduced ilmenite concentration were determined. Sample 3 and Sample 4 were the reduction products by reducing Sample 1 and Sample 2 at 750 °C for 30 min, respectively.
2.2.3 Mechanical activation
Ball milling experiments were conducted at room temperature in a planetary ball mill (QM-ISP Planetary mill, Nanjing University Instrument Plant, Nanjing, China). The mill has a rotation platform, over which two vessels are fixed. Mechanically activated ilmenite concentration was prepared as follows: the untreated ilmenite concentration (25 g) was added into a PTFE vessel with 250 g agate balls (50 g balls with 20 mm in diameter, 100 g balls with 10 mm in diameter and 100 g balls with 6 mm in diameter) with a ball/ore mass ratio of 10:1, then the mechanical activation was carried out in air with a rotation speed of 300 r/min. Mechanically activated ilmenite concentration was obtained after milling for 2 h.
The oxidized-reduced-mechanical activation ilmenite concentration was prepared as follows: a sample of the oxidized-reduced ilmenite concentration weighing 25 g was placed into a stainless steel vessel with 250 g agate balls (50 g balls with 20 mm in diameter, 100 g balls with 10 mm in diameter and 100 g balls with 6 mm in diameter). A vacuum with a residual pressure ≤1 Pa was then pulled on the vessel, followed by pouring highly pure nitrogen through an inlet of this vessel for 5 min. This operation was performed five times. Then the mechanical activation was carried out under a rotation speed of 300 r/min. The oxidation-reduction-mechanical activation ilmenite concentration was obtained after milling for 2 h.
2.2.4 Leaching experiments
When ilmenite concentration was leached by hydrochloric acid, the iron, and other metal elements were selectively leached to leave a titanium and silicon-enriched rutile phase. The main chemical reaction during ilmenite concentration leaching in hydrochloric acid can be represented by following reactions [12] and gangue minerals containing calcium, magnesium, aluminum, manganese etc are similarly dissolved.
![]() =
=![]() (1)
(1)
![]() =
=![]() (2)
(2)
The leaching experiments were carried out in a 500 mL three-necked glass reactor fitted with an agitator at a stirring rate of 250 r/min and a reflux condenser to minimize volatilization of hydrochloric acid. The third opening was used to feed reactants and withdraw the solution samples. In each experiment, 210 mL of 20% hydrochloric acid solution was preheated to the desired temperature 105 °C in a thermostatically controlled oil bath before 60 g sample was added (with a liquid/solid ratio of 3.5 mL/g). After appropriate leaching time, a certain volume of suspension was withdrawn and then centrifuged to obtain a solution, the contents of titanium and iron in the solution were determined. The extractions of Fe, Ca and Mg from the ilmenite concentration are calculated by Eq. 3:
![]() (3)
(3)
where ηi stands for the extraction of Fe, Ca or Mg from the ilmenite concentration; ci (g/L) stands for the content of Fe, Ca or Mg in the leaching solution; V (mL) stands for the volume of the leaching solution; m (g) stands for the mass of ilmenite concentration used in the leaching experiment; and wi (%) stands for the mass fraction of Fe, Ca or Mg in ilmenite concentration.
2.2.5 Preparation of calcined residues
At the end of each leaching experiment, the hot liquor was filtered to get the filtrate and leaching residue. The leaching residue was washed with 2% HCl, and then washed with distilled water, finally dried at 120 °C and calcined at 900 °C for 2 h to remove residual hydrochloric acid and water. The calcined residue was then analyzed for the contents of TiO2, total iron, CaO, MgO and SiO2.
2.3 Analysis and characterization
Titanium contents of untreated ilmenite concentration and calcined residues were directly determined by redox titration with ammonium ferric sulfate. Total iron and ferrous contents were determined using standard potassium dichromate titration. Metallic iron content was determined by the potassium dichromate method in the presence of the ferric chloride solution. Both calcium and magnesium were determined by complexometric titration against EDTA using calcium indicator and Eriochrome-Black T indicators. Silica content was determined spectrophotometrically using ammonium molybdate as complexing agent at 660 nm [13].
X-ray diffraction (XRD) characterization was carried out with a diffractometer D/max 2550 X (Rigaku, Japan), using Cu Kα radiation (λ=0.154 056 nm, voltage 40 kV, and current 300 mA), with a step size of 0.02° and a recorded range from 10° to 80°.
The morphology analysis was performed using JSM-5600Lv scanning electron microscope (JEOL, Japan) equipped with an EDX-GENESIS 60S X-ray energy dispersive spectrometer (EDAX, USA).
3 Results and discussion
3.1 Oxidation of untreated ilmenite concentration
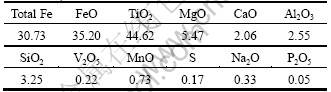
To study the oxidation mechanism of Panxi ilmenite concentration, a series of experiments in which untreated ilmenite concentration was oxidized in the flowing oxygen in the temperature range of 700-900 °C for 2 h were carried out. The XRD patterns and the FeO content of the oxidation products are given in Fig. 1 and Table 2, respectively.
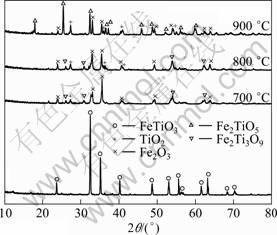
Fig. 1 XRD patterns of oxidized ilmenite concentrations at different temperatures for 2 h
Table 2 FeO content of oxidized ilmenite concentrations at different temperatures for 2 h
![]()
From the results plotted in Fig. 1, it is evident that, with the increase of oxidation temperature, the Panxi ilmenite concentration was first oxidized to hematite, rutile and pseudorutile (Fe2Ti3O9), and then the pseudorutile decomposed to hematite and rutile which subsequently combined to form pseudobrookite (Fe2TiO5). The oxidation temperature has an impact on the oxidation mechanism of ilmenite concentration from Panxi area [14-16]. The oxidation of untreated ilmenite concentration in oxygen gave a mixture of hematite, rutile and pseudorutile in the range of 700-800 °C, while at 900 °C, a mixture of pseudobrookite, hematite and rutile was obtained. As presented in Table 2, the FeO content of oxidized ilmenite concentrations at 700, 800 and 900 °C for 2 h are 7.26%, 0.24% and 0.086%, respectively. Taking into account that oxidation has been reported to be very slow in the later stages[17], the untreated ilmenite concentration can be practically considered fully oxidized when the FeO content is less than 0.50%. Therefore, effects of oxidation time on the FeO content of untreated ilmenite concentration oxidized at 800 °C and 900 °C were studied. And the results are shown in Fig. 2.
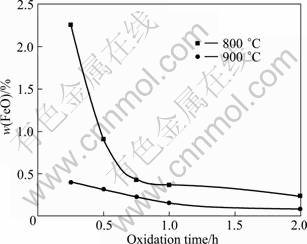
Fig. 2 Effect of oxidation time on oxidation of ilmenite concentrations at 800 °C and 900 °C
It can be seen from Fig. 2 that the FeO mass fraction sharply decreases to 0.43% in the initial 45 min with oxidation at 800 °C, and then reduces slightly with the increase of oxidation time. While at an oxidation temperature of 900 °C for 15 min, the FeO mass fraction reduces to 0.40%, and then prolonged oxidation of untreated ilmenite concentrations at 900 ℃ induces no substantial change in the FeO content. Therefore, the oxidized ilmenite concentration which was oxidized at 800 °C for 45 min (Sample 1) and at 900 °C for 15 min (Sample 2) were chosen to be studied in the following experiments. The XRD pattern demonstrates that the phases of Sample 1 are Fe2O3, TiO2 and Fe2Ti3O9, while the phases of Sample 2 are Fe2O3, TiO2 and Fe2TiO5.
Semi-quantitative EDS analysis (Table 3) revealed 62.80% Fe and 17.45% Ti on the surface of Sample 2, while the contents of Fe and Ti were 41.65% and 34.84%, respectively, on the surface of untreated ilmenite concentration. Thus, these results suggest that Fe migrated to the surface of mineral particles during the oxidation.
Table 3 EDS analysis of untreated ilmenite concentration and Sample 2

3.2 Reduction of oxidized ilmenite concentration
Sample 1 was reduced at 600, 700 and 800 °C for 2 h by using hydrogen as the reducing agent to get oxidized–reduced ilmenite concentration, and the total content of Fe2+ and Fe (abbreviated as (total FAI) of the oxidized-reduced ilmenite concentrations were determined (Table 4).
Table 4 Total content of Fe2+ and Fe in reduced ilmenite concentrations at different temperatures for 2 h

The total iron of untreated ilmenite concentration used in this work is 30.73% (Table 1), and the total FAI contents in reduced ilmenite concentrations of Sample 1, which were reduced at 600 °C and 700 °C for 2 h, are 17.69% and 28.62% (Table 4), respectively. So there is still some ferric iron in reduced ilmenite concentrations of Sample 1. However, when the reduction was operated at 800 °C for 2 h, the total FAI content in reduced ilmenite concentration of Sample 1 reached 40.29%, and exceeded the total iron (30.73%) of untreated ilmenite concentration. This indicates that titanium in Sample 1 may be partially reduced by hydrogen at the temperature of 800 °C, then the low-valence titanium was oxidized to Ti4+, consuming some volume of potassium dichromate during the titratration of ferrous iron with potassium dichromate, which made the total FAI content in reduced ilmenite concentration of Sample 1 higher than the total iron(30.73%). So it is relatively suitable for the reduction of oxidized ilmenite concentration at 750 °C. Therefore, the reduction of Sample 1 and Sample 2 at 750 °C was investigated. The results are shown in Fig. 3.

Fig. 3 Effect of reduction time on reduction of Sample 1 and Sample 2 at 750 °C
As noted in Fig. 3, the total FAI contents in reduced ilmenite concentrations of Samples 1 and 2 increased continuously with increasing reduction time. When reduced for 30 min, the total FAI contents in the reduction products of Samples 1 and 2 were closed to the total iron in untreated ilmenite concentration; but the titanium in Samples 1 and 2 might be reduced gradually beyond 30 min of treatment, which made the total FAI content in reduced ilmenite concentrations higher than the total iron in untreated ilmenite concentration. The reduction investigations on synthetic ilmenite with hydrogen by ZHAO and SHADMAN [18] indicated that the reduction of titanium did not occur to any significant extent as long as iron metalization was not completed. However, as iron metalization approaches completion, more and more titanium is reduced by hydrogen. So the production of metal iron must be strictly controlled in the reduction operation. Therefore, the reduction operation is chosen to be at 750 °C for 30 min. And the analysis results show that the contents of iron in Samples 3 and 4, which were obtained by reducing Samples 1 and 2 at 750 °C for 30 min, were 7.40% and 5.90%, respectively.
The micrographs of Sample 3 and Sample 4 are shown in Fig. 4. The SEM images of ilmenite grains show the presence of microcracks in the oxidized-reduced samples. Sample 3 is seen to be very dense, if the microcracks are not considered; Sample 4 exhibits a porous structure, which is contributed to a faster leaching. Thus it can be seen that the morphology of Panxi ilmenite concentration can change apparently with the change of oxidation temperature during the oxidation–reduction treatment.
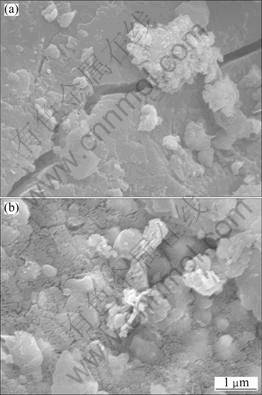
Fig. 4 SEM images of Sample 3 (a) and Sample 4 (b)
Table 5 lists the EDS analysis of Sample 4. The contents of Fe and Ti remained almost constant during the reduction treatment, while the oxygen content decreased significantly because of the formation of water vapour. Therefore, the new surface enriched in Fe will be beneficial to the removal of Fe during the leaching process.
Table 5 EDS analysis of Sample 4 (mass fraction, %)
![]()
3.3 Hydrochloric acid leaching behaviour of ilmenite concentration
3.3.1 Leaching of untreated ilmenite concentration and mechanically activated ilmenite concentration
Mechanical activation is one of the effective and attractive methods of accelerating leaching which is widely applied to the mineral leaching process in hydrometallurgical industry. In recent two decades, the effect of mechanical activation on the dissolution of ilmenite concentration from different regions were investigated [19-20], and found that the enhancement of leaching activity of mechanically activated ilmenite concentration is mostly consistent with the increase of lattice disorder resulted from mechanical activation. Our previous work [11] reveals that the lattice distortion increased apparently in the initial 2 h of ball-milling and then increased slightly under this ball-milling condition (Section 2.2.3). Therefore, the mechanical activation time is chosen to be 2 h in the following experiments.
Figure 5 illustrates the variation of Fe extraction rate of untreated ilmenite concentration and mechanically activated ilmenite concentration with the increase of leaching time when the samples were leached in 20% hydrochloric acid solution at 105 °C.
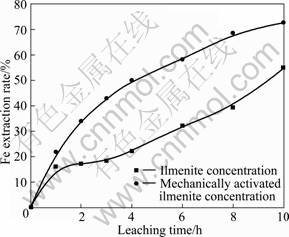
Fig. 5 Extraction rate of Fe from ilmenite concentration and mechanically activated ilmenite concentration (20% HCl, 105 °C)
As shown in Fig. 5, for the untreated ilmenite concentration, the Fe extraction rates with leaching time of 1, 2 and 3 h were only 16.15%, 17.16% and 18.34%, respectively, presenting a slow change region. After leaching for 3 h, the Fe extraction rate increased rapidly with increasing leaching time again, and reached 54.83% after leaching for 10 h. In the case of mechanically activated ilmenite concentration, it is evident that the mechanical activation significantly affected the extraction rate of Fe (Fig. 4). The Fe extraction rate of mechanically activated ilmenite concentration increased continuously with the prolonging leaching time, and up to 72.64% after leaching for 10 h.
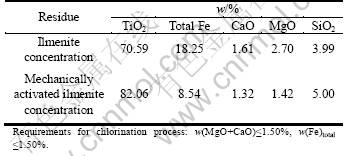
Following the leaching process, the 10 h-leached residues of untreated ilmenite concentration and mechanically activated ilmenite were subjected to wash, dry and calcine, then the calcined residues obtained were analyzed for the contents of TiO2, total iron, CaO, MgO and SiO2. It is clearly seen from Table 6 that these residues do not meet the requirements of the chlorination process for producing high grade synthetic rutile, because the contents of total iron and (MgO+CaO) are significantly higher than the standard value (≤1.5%). Therefore, further treatments on Panxi ilmenite concentration will be necessary.
Table 6 Chemical composition of calcined residue obtained from ilmenite concentration and mechanically activated ilmenite (mass fraction, %)
3.3.2 Leaching of oxidized-reduced ilmenite concentra- tions
The leaching behaviours of oxidized-reduced ilmenite concentrations (Samples 3 and 4) in 20% hydrochloric acid solution at 105 °C were studied. Figure 6 illustrates the extraction rate of Fe from Samples 3 and 4 as a function of leaching time.
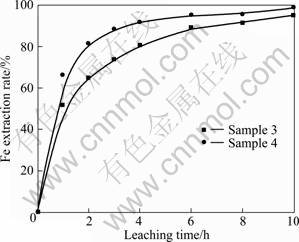
Fig. 6 Fe extraction rate from Sample 3 and Sample 4 (20% HCl, 105 °C)
As can be seen in Fig. 6, the Fe extraction rate of Samples 3 and 4 increased apparently with the increase of the leaching time, and then increased slightly after 6 h leaching. At the same time, it is noteworthy from Fig. 6 that with the same leaching time, the Fe extraction rate of Sample 4 was always higher than that of Sample 3. This is because that oxidation destroys the natural ilmenite crystalline structure (such as Sample 2, its crystalline structure was destroyed completely by oxidizing at 900 °C for 15 min) or at least increases the imperfections in the ilmenite crystalline structure (such as Sample 1, its crystalline structure was destroyed partially and only the interplaner spacing decreased), which will in turn facilitate the subsequent leaching of the ilmenite concentration. Therefore, from the viewpoint of both the product quality and effective utilization of resources, the formation of pseudobrookite by oxidizing at 900 °C is more favorable to upgrade Panxi ilmenite concentration to synthetic rutile. Therefore, the optimum operational conditions for the oxidation-reduction pretreatment were obtained as follows: oxidized at 900 °C in the presence of oxygen for 15 min, reduced at 750 °C in hydrogen for 30 min. All oxidized-reduced ilmenite concentrations mentioned in the following experiments were prepared in the optimum conditions.
Table 7 lists the chemical composition of the calcined residue obtained from Sample 4. The residue compositions are 88.31% TiO2, 1.79% total iron, 1.27% CaO, 0.94% MgO and 6.45% SiO2. Of course, the contents of total iron and (MgO+CaO) are higher than the standard values required by chlorination process.
Table 7 Chemical composition of calcined residue obtained from oxidized-reduced ilmenite concentration (mass fraction, %)

3.3.3 Leaching of oxidized-reduced-mechanically activated ilmenite concentration
In order to prevent the oxidation of oxidized- reduced-mechanically activated ilmenite concentration, the oxidized-reduced ilmenite concentration was immediately leached in 20% hydrochloric acid solution at 105 °C after ball-milling for 2 h. After appropriate leaching time, a certain volume of suspension was withdrawn and then centrifuged to analyze the contents of Fe, Ca and Mg in the solution for calculating their extraction rate. And the leaching results are illustrated in Fig. 7.

Fig. 7 Extraction rates of Fe, Ca and Mg from oxidized- reduced-2h mechanically activated ilmenite concentration (20% HCl, 105 °C)
It can be found from Fig. 7 that the mechanical activation has a positive influence on the hydrochloric acid leaching of oxidized-reduced ilmenite concentration, especially during the initial leaching stage. More than 90% Fe amenable to be leached from the ilmenite concentration mineral grains is solubilized within 1 h of leaching, thereafter, Fe extraction became negligible. After leaching in 20% HCl at 105 °C for 10 h, the Fe extraction rate achieved 98.93%. And Fig. 7 also shows 80.50% Ca and 88.33% Mg of extraction rate from oxidized-reduced-mechanically activated ilmenite concentration after 10 h leaching , whereas only 40.52% Ca and 81.97% Mg were achieved from oxidized- reduced ilmenite concentration.
Table 8 lists composition of the synthetic rutile, prepared by the process of “oxidation-reduction- mechanical activation-hydrochloric acid leaching”. It contains 90.50% TiO2, 1.37% total iron, 0.40% CaO, 0.60% MgO and 6.66% SiO2. The contents of total iron and (MgO+CaO) are low enough to meet the requirements of chlorination process.
Table 8 Chemical composition of calcined residue obtained from oxidized-reduced-mechanically activated ilmenite concentration (mass fraction, %)

4 Conclusions
1) Both of oxidation-reduction treatment and mechanical activation significantly accelerate the extraction of Fe, Ca and Mg from Panxi ilmenite concentration.
2) Fe in Panxi ilmenite concentration migrates to the surface of mineral particles during the oxidation. And the formation of pseudobrookite by oxidizing at 900 °C is more favorable to upgrade Panxi ilmenite concentration to synthetic rutile due to the complete destruction of the ilmenite crystalline structure.
3) The optimum oxidation and reduction conditions are as follows: oxidation at 900 °C in the presence of oxygen for 15 min, reduction at 750 °C in hydrogen for 30 min.
4) The synthetic rutile which prepared by the process of “oxidation-reduction-mechanical activation- hydrochloric acid leaching” contains 90.50% TiO2 and 1.37% total iron as well as combined CaO and MgO of 1.00%. The contents of total iron and (MgO+CaO) are low enough to meet the requirements of chlorination process.
References
[1] QIU Dao-chang, DUAN Chao-yu. Preparation of synthetic rutile by hydrochloric acid direct leaching of Panzhihua ilmenite [J]. Iron Steel Vanadium Titanium, 1982(4): 50-58. (in Chinese)
[2] CARDARELLI F. Materials handbook: A concise desktop reference [M]. London: Springer, 2008: 289.
[3] LI Chun, LIANG Bin, WANG Hai-yu. Preparation of synthetic rutile by hydrochloric acid leaching of mechanically activated Panzhihua ilmenite [J]. Hydrometallurgy, 2008, 91: 121-129.
[4] FARROW J B, RITCHIE I M. The reaction between reduced ilmenite and oxygen in ammonium chloride solutions [J]. Hydrometallurgy, 1987, 18(1): 21-38.
[5] LASHEEN T. Chemical beneficiation of Rosetta ilmenite by direct reduction leaching [J]. Hydrometallurgy, 2005, 76: 123-129.
[6] NAYL A A, ALY H F. Acid leaching of ilmenite decomposed by KOH [J]. Hydrometallurgy, 2009, 97: 86-93.
[7] WALPOLE E A. Acid Regeneration: US, 5635152 [P]. 1997-06-03.
[8] YE En-dong, CHEN De-ming, ZHANG Ji-dong. Method for the production of synthetic rutile: CN, 101244841A [P]. 2008-08-20. (in Chinese)
[9] WEI Liang-ping, HU Hui-ping, CHEN Qi-yuan, TAN Jun. Effects of mechanical activation on the HCl leaching behaviour of plagioclase, ilmenite and their mixtures [J]. Hydrometallurgy, 2009, 99: 39-44.
[10] ZHANG Li, HU Hui-ping, WEI Liang-ping, CHEN Qi-yuan, TAN Jun. Effects of mechanical activation on the HCl leaching behavior of titanaugite, ilmenite and their mixtures [J]. Metallurgical and Materials Transactions B, 2010, 41(6): 1158-1165.
[11] ZHANG Li, HU Hui-ping, WEI Liang-ping, CHEN Qi-yuan, TAN Jun. Hydrochloric acid leaching behaviour of mechanically activated panxi ilmenite (FeTiO3) [J]. Separation and Purification Technology, 2010, 73(2): 173-178.
[12] OLANIPEKUN E. A kinetic study of the leaching of a Nigerian ilmenite ore by hydrochloric acid [J]. Hydrometallurgy, 1999, 53: 1-10.
[13] The Analysis Center of Beijing Research Institute of Mining & Metallurgy. Handbook of mines and non-ferrous metals analysis [M]. Beijing: Metallurgical Industry Press, 1990. (in Chinese)
[14] TEUFER G, TEMPLE A K. Pseudorutile-a new mineral intermediate between ilmenite and rutile in the alteration of ilmenite [J]. Nature, 1966, 211(5045): 179-181.
[15] RAO D B, RIGAUD M. Kinetics of the oxidation of ilmenite [J]. Oxidation of Metals, 1975, 9(1): 99-116.
[16] WANG Yu-ming, YUAN Zhang-fu, GUO Zhan-cheng, TAN Qiang-qiang, LI Zhao-yi, JIANG Wei-zhong. Reduction mechanism of natural ilmenite with graphite [J]. Trans Nonferrous Met Soc China, 2008, 18: 962-968.
[17] KARKHANAVALA M D, MOMIN A C. The alteration of ilmenite [J]. Economic Geology, 1959, 54(6): 1095-1102.
[18] ZHAO Y, SHADMAN F. Reduction of ilmenite with hydrogen [J]. Industrial & Engineering Chemistry Research, 1991, 30: 2080-2087.
[19] WELHAM N J, LLEWELLYN D J. Mechanical enhancement of the dissolution of ilmenite [J]. Minerals Engineering, 1998, 11(9): 827-841.
[20] LI Chun, LIANG Bin, GUO Ling-hong. Dissolution of mechanically activated Panzhihua ilmenites in dilute solutions of sulphuric acid [J]. Hydrometallurgy, 2007, 89: 1-10.
机械活化和氧化-还原处理对
攀西钛铁矿精矿盐酸浸出的影响
谭 平1, 胡慧萍2, 张 黎2
1. 湖南机电职业技术学院 生物化工系,长沙 410151;
2. 中南大学 化学化工学院,长沙410083
摘 要:研究机械活化和氧化-还原处理对攀西钛铁矿精矿盐酸浸出过程的影响。结果表明:机械活化和氧化-还原处理均可明显提高钛铁矿精矿中铁、钙和镁的浸出;氧化-还原处理的钛铁矿经盐酸浸出后得到的人造金红石,其钙镁含量过高,不能满足沸腾氯化法的要求;经机械活化处理的氧化-还原钛铁矿,能进一步降低盐酸浸出渣的钙镁含量,所得到的人造金红石含TiO2 90.50%、全铁1.37%、钙镁总量1.00%,完全满足沸腾氯化法的生产要求。最佳的氧化还原处理条件为:在900 °C氧气气氛中氧化15 min,在750 °C氢气气氛中还原30 min。
关键词:钛铁矿精矿;机械活化;氧化;还原;盐酸浸出
(Edited by LI Xiang-qun)
Foundation item: Project (2009FJ3082) supported by Research Project of Science and Technology in Hunan Province, China; Project (2007CB613606) supported by the National Basic Research Program of China
Corresponding author: HU Hui-ping; Tel: +86-731-88876034; E-mail: phuhuiping@126.com
DOI: 10.1016/S1003-6326(11)60875-3


