
Kinetic and thermodynamic studies on biosorption of Cu(Ⅱ) by chemically modified orange peel
FENG Ning-chuan(冯宁川)1, 2, GUO Xue-yi(郭学益)1, LIANG Sha(梁 莎)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Basic Medical Science, Ningxia Medical College, Yinchuan 750004, China
Received 30 June 2008; accepted 27 March 2009
Abstract:
Cu(Ⅱ) biosorption by orange peel that was chemically modified with sodium hydroxide and calcium chloride was investigated. The effects of temperature, contact time, initial concentration of metal ions and pH on the biosorption of Cu(Ⅱ) ions were assessed. Thermodynamic parameters including change of free energy![]() enthalpy
enthalpy![]() and entropy
and entropy![]() during the biosorption were determined. The results show that the biosorption process of Cu(Ⅱ) ions by chemically treated orange peel is feasible, spontaneous and exothermic under studied conditions. Equilibrium is well described by Langmuir equation with the maximum biosorption capacity(qm) for Cu(Ⅱ) as 72.73 mg/g and kinetics is found to fit pseudo-second order type biosorption kinetics. As the temperature increases from 16 ℃ to 60 ℃, copper biosorption decreases. The loaded biosorbent is regenerated using HCl solution for repeatedly use for five times with little loss of biosorption capacity.
during the biosorption were determined. The results show that the biosorption process of Cu(Ⅱ) ions by chemically treated orange peel is feasible, spontaneous and exothermic under studied conditions. Equilibrium is well described by Langmuir equation with the maximum biosorption capacity(qm) for Cu(Ⅱ) as 72.73 mg/g and kinetics is found to fit pseudo-second order type biosorption kinetics. As the temperature increases from 16 ℃ to 60 ℃, copper biosorption decreases. The loaded biosorbent is regenerated using HCl solution for repeatedly use for five times with little loss of biosorption capacity.
Key words:
orange peel; copper; biosorption; isotherms; kinetics; thermodynamics;
1 Introduction
The removal of heavy metal ions by biosorption has been widely studied in the last decade due to its potential application, particularly in wastewater treatment. Biosorption is a process that utilizes inexpensive biomass to sequester toxic heavy metals and is particularly useful for the removal of trace level of contaminants from industrial effluents[1]. Compared with conventional methods, such as chemical oxidation and reduction, precipitation and membrane separation, biosorption process has several advantages, including low operating cost, minimization of the volume of chemical and/or biological sludge to be disposed of, high efficiency in detoxifying extremely dilute effluents, and no nutrient requirements[1-2].
Different types of biomass have been investigated for the biosorption of copper ions, including yeast[3], algae[4], fungi[5], bacteria[6], and agricultural by-products (inexpensive plant material) such as dried leaves[7], wheat bran[8], sawdust[9], banana pith[10], sugar-beet-pulp[11], apple wastes[12]. Among these materials, agricultural by-products are relatively cheap and show quite high biosorption potential. However, the direct use of the raw plant wastes as adsorbents has been found to be limited due to leaching of organic substances such as lignin, tannin, pectin and cellulose into the solution[13]. To resolve these problems, chemical modification on solid adsorbents has been used as a technique to improve their physical, chemical properties and biosorption capacity[14-18].
The use of orange peel as a biosorbent material presents strong potential due to its main components of cellulose, pectin (galacturonic acid), hemicellulose and lignin. These components bear various polar functional groups including carboxylic and phenolic acid groups to be involved in metal binding[19-20] and are biopolymers admittedly associated to the removal of heavy metals[21-22]. As a low cost biosorbent, orange peel is an attractive option for the biosorption removal of dissolved metals. AJMAL et al[23] and ANNADURAI et al[24] employed raw orange peel for removal of metal ions from simulated wastewater. The aim of this work is to study the biosorption capacity of orange peel that was chemically modified with sodium hydroxide and calcium chloride for biosorption of Cu(Ⅱ) ions from aqueous solutions. The effects of temperature, contact time, initial concentration of metal ions and pH on the biosorption of Cu(Ⅱ) ions were investigated. Finally, elution-reuse of the biosorbent was evaluated.
2 Experimental
2.1 Chemicals
All chemicals used in the present work were of analytical purity. The stock solution of Cu(Ⅱ) was prepared in 1.0 g/L concentration using CuSO4·5H2O and then diluted to appropriate concentrations for test. 0.1 mol/L HCl and 0.1 mol/L NaOH were used for pH value adjustment.
2.2 Chemical treatment of orange peel
Orange peel(OP) was used as the raw material for the preparation of the chemically modified biosorbent. The OP collected from a local plantation was cut into small pieces, washed several times with distilled water and dried at 80 ℃. The product was crushed and sieved to obtain an average particle size lower than 0.45 mm and then treated with sodium hydroxide and calcium chloride solutions to improve the capacity of biosorption to metal ions. For this purpose, 100 g of dried OP was soaked in solution containing 500 mL ethanol, 250 mL NaOH (0.5 mol/L) and 250 mL CaCl2 (1.5 mol/L) for 24 h. After repeating decantation and filtration, the modified biomass (SCOP) was washed with deionized water until pH value of the solutions reached 7.0, then dried for the test use.
2.3 Instruments used for biosorbent characterizations
A pH meter (PHS-3C, made in China) was used to measure pH of the suspensions. FTIR spectroscopy was used to identify the chemical groups in the biosorbent. FTIR spectra of the biosorbent before and after adsorbing Cu(Ⅱ) were recorded in 400-4 000 cm-1 using a JASCO-410 model FTIR spectrometer with KBr discs.
2.4 Biosorption tests
Batch biosorption experiments were conducted by mixing biosorbent with Cu(Ⅱ) ion solutions with desired concentrations in 100 mL sealed conical flask using a shaking thermostat machine at a speed of 120 r/min. The effect of pH on the equilibrium biosorption of Cu(Ⅱ) was investigated by mixing 0.100 g of SCOP with 25 mL 50 mg/L of Cu(Ⅱ) ion solutions between pH 2.0 and 6.5. In kinetic experiments, the biosorption time was varied from 0 to 120 min. In isotherm experiments, 0.100 g of biosorbent was mixed with 25 mL of Cu(Ⅱ) ion solutions at various concentrations (25-800 mg/L). After the pre-set contact time was reached, the samples were withdrawn and centrifuged at 4 000 r/min for 5 min, and then the supernatant fluid was analyzed for the Cu(Ⅱ) ion concentrations by using Rayleigh WFX-130B atomic absorption spectrophotometer. The amount of biosorptions(q) was calculated by the following equation:
![]() (1)
(1)
where ρ0 and ρe are the initial and equilibrium Cu(Ⅱ) ion concentrations, respectively (mg/L); V is the volume of the solutions (L) and m is the amount of adsorbent used (g). All the biosorption experiments were conducted in duplicate, and the mean values were calculated.
2.5 Desorption tests
To investigate the possibility of repeated use of the biosorbent, desorption and regeneration experiments were also conducted. After biosorption experiments of 50 mg/L of Cu(Ⅱ) ion solution with 0.2 g of adsorbent in 50 mL conical flask, the copper-loaded biosorbent was then transferred to another conical flask and agitated with 50 mL of 0.05 mol/L HCl solutions for 30 min. It was again filtered, and then Cu(Ⅱ) ions desorbed in the filtrate were determined. The eluted biosorbent was washed several times with distilled water to remove excess acid. The biosorbent regenerated was then used in next biosorption processes.
3 Results and discussion
3.1 Effect of biomass treatment on biosorption
BAIG et al[25] studied on the binding of Pb(Ⅱ), Cu(Ⅱ), Ni(Ⅱ), Cd(Ⅱ), Zn(Ⅱ), Cr(Ⅲ) and Cr(Ⅵ) to the inactivated biomass of Solanum elaeagnifolium and suggested that carboxyl groups (—COOH) are responsible to some extent for the binding of metal ions. This means that metal binding can be enhanced by increasing the number of carboxylate ligands in the biomass. The major constituents of orange peel, cellulose, pectin, hemicellulose and lignin, contain methyl esters that do not bind metal ions significantly. However, these methyl esters can be converted to carboxylate ligands by treating the biomass with a base such as sodium hydroxide, thereby increasing the metal-binding ability of the biomass. The hydrolysis reaction of the methyl esters is as follows:
R—COOCH3+NaOH=R—COO-+CH3OH+Na+
The addition of calcium chloride makes the pectin acid precipitated, which reduces its solubility in solution. Therefore, chemically modifying the biomass increases the number of carboxylate ligands, which would enhance the binding ability of the biomass. The metal-binding capacities of the biomass before and after treatment by NaOH-CaCl2 were compared. An increase of approximately 30% of Cu(Ⅱ) ions was obtained for 50 mg/L Cu(Ⅱ) solution.
3.2 FTIR analysis
The FTIR spectra of OP and SCOP are shown in Fig.1. The broad and intense absorption peaks at 3 400 cm-1 correspond to O—H stretching vibrations of cellulose, pectin, absorbed water, hemicellulose, and lignin. The peaks at 2 924 cm-1 can be attributed to C—H stretching vibrations of methyl, methylene and methoxy groups. The presence of the peaks at 1 744 cm-1 and 1 638 cm-1 in the OP spectrum indicates the stretching bands of ester carbonyl (C=O) groups and carboxylate ion (COO-), respectively[26]. The vibrations in 1 430- 1 455 cm-1 can be due to aliphatic and aromatic (C—H) groups in the plane deformation vibrations of methyl, methylene and methoxy groups. The bands in 1 300- 1 000 cm-1 can be assigned to C—O stretching vibrations of carboxylic acids and alcohols. For FTIR spectrum of the SCOP, weakened intensity of the peak at 1 744 cm-1 indicates that the methyl esters are hydrolyzed with NaOH and ester group is converted to carboxylate ions.
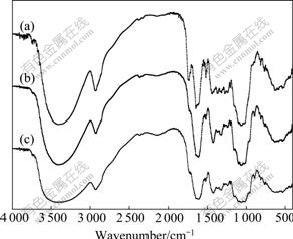
Fig.1 FTIR spectra of OP(a) and SCOP before (b) and after Cu binding(c)
Different biosorption mechanisms including complexation, ion exchange and electrostatic attraction may be involved in the biosorption process. After Cu(Ⅱ) biosorption, OH stretching vibration shifts to 3 449 cm-1. The significant shifts of these specific peaks to the lower wavenumber after adsorbing metal ions suggest that chemical interactions between the metal ions and the hydroxyl (—OH) groups occur on the biomass surface. The carboxyl (C=O) peak occurs at 1 609 cm-1 for Cu(Ⅱ)-loaded biomass. In addition, after loading metal ion, the peak of C—O groups shifts to 1 046 cm-1. These results indicate that the functional groups mentioned above are mainly involved in the biosorption of Cu(Ⅱ) onto the biomass[27].
3.3 Effect of pH on biosorption
It is well known that pH can affect the protonation of the functional groups on the biomass as well as the metal chemistry[28]. The effect of pH on biosorption capacity of SCOP to Cu(Ⅱ) ions is shown in Fig.2. At lower pH, the amount of biosorption to Cu(Ⅱ) is small. Biosorption to Cu(Ⅱ) increases with the increase of pH from 2.5 to 4.5. The highest biosorption efficiency is observed in the pH range of 4.5-6.0. These observations can be explained by the fact that at lower pH values, the surface charge of the biomass is positive, which is not favorable to cations biosorption. Meanwhile, hydrogen ions compete strongly with metal ions at the active sites, resulting in less biosorption. With increasing pH, electrostatic repulsions between cations and surface sites and the competing effect of hydrogen ions decrease. Consequently, the metal biosorption increases[29].
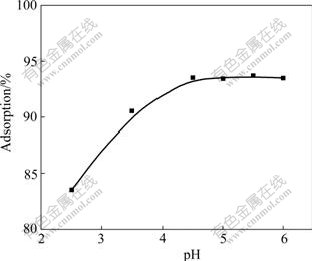
Fig.2 Effect of pH on biosorption to Cu(Ⅱ) under conditions: 50 mg/L of Cu(Ⅱ); biosorbent of 0.100 g, biosorption medium of 25 mL, contact time of 2 h, and temperature of 25 ℃
3.4 Biosorption kinetics
Biosorption capacity to Cu(Ⅱ) was determined as a function against time to obtain the optimum contact time for the biosorption of SCOP to Cu(Ⅱ) ions. Fig.3 shows the results of the biosorption equilibrium of Cu(Ⅱ) ions on SCOP against time. It can be seen from Fig.3, the biosorption process is very fast and reaches equilibrium at about 10 min. Therefore, 2 h of contact time was chosen as the biosorption time for the experimental test to ensure that equilibrium was achieved.
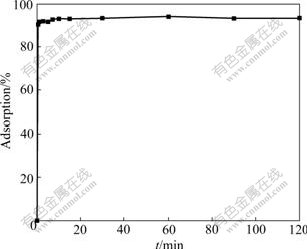
Fig.3 Biosorption kinetics of Cu(Ⅱ) ions under conditions: pH of 5.3, Cu(Ⅱ) concentration of 50 mg/L, biosorbent of 0.100 g, biosorption medium of 25 mL, temperature of 25 ℃, and pH of 5.3
The kinetics of Cu(Ⅱ) ion biosorption on SCOP were analyzed using pseudo-second order[30]:
![]() (2)
(2)
where k2 is the constant of pseudo-second-order rate; qe is the biosorption capacity at equilibrium; and qt is the biosorption capacity at time t. The equilibrium biosorption capacity and the pseudo-second-order rate constant can be experimentally determined from the slope and the intercept of the plot t/qt against t. The graphical interpretation of the data for the second-order-kinetic model is given in Fig.4. A good fit (R2=0.999 8) is obtained and the theoretical value of qe also agrees very well with the experimental value, indicating that the biosorption conforms to the pseudo-second-order mechanism and the biosorption rate is controlled by chemical biosorption. k2 and qe obtained from the plot are 2.01 g/(mg×min) and 11.67 mg/g, respectively.
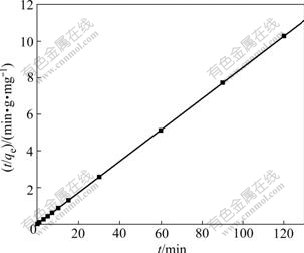
Fig.4 Pseudo-second-order plot of biosorption kinetics of Cu(Ⅱ) ions
3.5 Biosorption isotherms
Fig.5 presents the experimental biosorption isotherm for Cu(Ⅱ) on SCOP with the initial concentrations of Cu(Ⅱ) varied from 25 to 800 mg/L at 25 ℃. Two isotherm models, Langmuir and Freundlich, were used to describe the equilibrium data. The amount of Cu(Ⅱ) adsorbed (qe) at equilibrium metal concentrations of ρe can be defined by the Langmuir equations as
![]() (3)
(3)
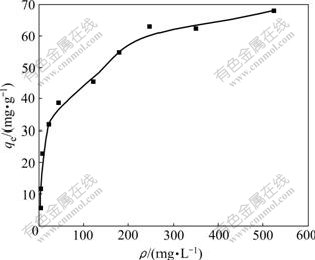
Fig.5 Biosorption isotherms of Cu(Ⅱ) under conditions: pH 5.3, contact time of 2 h, biosorbent of 0.100 g, biosorption medium of 25 mL, temperature of 25 ℃, and pH of 5.3
where qm is the maximum monolayer capacity of the biosorbent, and b is the biosorption constant. The plot of ρe/qe vs ρe should be a straight line with slope of 1/qm and intercept of 1/(qmb) when the biosorption follows the Langmuir equation.
The Freundlich equation can be expressed as:
![]() (4)
(4)
where KF and 1/n are Freundlich isotherm constants related to biosorption capacity and intensity of biosorption, respectively. Using Eq.(4), a plot of lg qe vs lg ρe will give a straight line with slope of 1/n and intercept of lg KF.
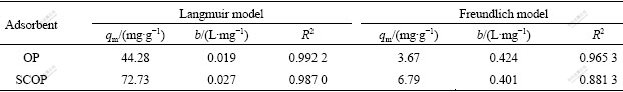
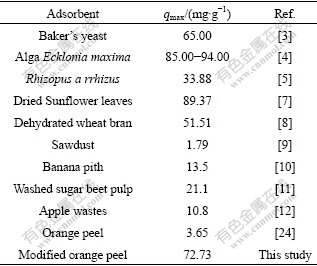
The Langmuir and Freundlich biosorption constants evaluated from the isotherms with the correlation coefficients are listed in Table 1. Equilibrium data agree well with the Langmuir model, illustrating that the biosorption on the surface of SCOP is a monolayer biosorption. Also, the maximum biosorption capacity of SCOP is significantly higher than that of OP. A comparison of biosorption capacities (qm) of SCOP with some other biosorbents is listed in Table 2. The biosorption capacity of SCOP for Cu(Ⅱ) is the highest among those biomasses mentioned. Therefore, it can be noteworthy that the SCOP has an important potential for the removal of Cu(Ⅱ) ions from aqueous solutions.
Table 1 Langmuir and Freundlich isotherm constants for Cu(Ⅱ) biosorption on OP and OPAA
 \
\
Table 2 Adsorbents available for biosorption of Cu(Ⅱ)
3.6 Effect of temperature on biosorption
The effect of the temperature on the biosorption of Cu(Ⅱ) ions was studied in the temperature range of 16-60 ℃. The result shows that temperature has a negative effect on the biosorption efficiency for copper biosorption.
In order to describe thermodynamic behavior of the biosorption of Cu(Ⅱ) ions on SCOP from aqueous solution, thermodynamic parameters including the changes in free energy![]() enthalpy
enthalpy![]() and entropy
and entropy![]() are calculated by following equations:
are calculated by following equations:
![]() -RTlnKD (5)
-RTlnKD (5)
where R is the gas constant R=8.314 J/(mol×K); T is the thermodynamic temperature (K) and KD is the distribution coefficient.
KD can be calculated using the following equation [31]:
![]() (6)
(6)
where qe and ρe are the equilibrium biosorption capacities of metal ions on adsorbent (mg/g) and the equilibrium concentration of metal ions in the solution (mg/L), respectively.
The enthalpy![]() and entropy
and entropy![]() are estimated from the following equation:
are estimated from the following equation:
![]() (7)
(7)
According to Eq.(7), ![]() and
and ![]() can be calculated from the slope and the intercept of the plot of ln KD versus 1/T, respectively (Fig.6).
can be calculated from the slope and the intercept of the plot of ln KD versus 1/T, respectively (Fig.6).
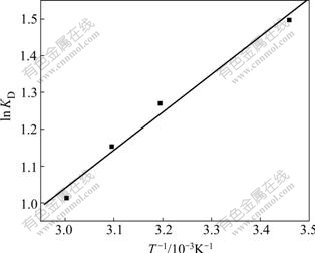
Fig.6 Plot of ln KD vs 1/T for estimation of thermodynamic parameters for biosorption of Cu(Ⅱ) on SCOP
Gibbs free energy change![]() was calculated to be -3.58, -3.30, -3.09 and -2.79 kJ/mol for Cu(Ⅱ) biosorption at 16, 40, 50 and 60 ℃, respectively. These negative
was calculated to be -3.58, -3.30, -3.09 and -2.79 kJ/mol for Cu(Ⅱ) biosorption at 16, 40, 50 and 60 ℃, respectively. These negative![]() values indicate thermodynamically feasible and spontaneous nature of the Cu(Ⅱ) biosorption on SCOP. The increase in
values indicate thermodynamically feasible and spontaneous nature of the Cu(Ⅱ) biosorption on SCOP. The increase in ![]() with increase in tempera- ture shows a decrease in feasibility of biosorption at higher temperatures.
with increase in tempera- ture shows a decrease in feasibility of biosorption at higher temperatures. ![]() and
and ![]() are found to be -8.55 and -17.00 J/(mol×K) for Cu(Ⅱ) biosorption. The negative
are found to be -8.55 and -17.00 J/(mol×K) for Cu(Ⅱ) biosorption. The negative ![]() indicates the exothermic nature at 16- 60 ℃. The negative
indicates the exothermic nature at 16- 60 ℃. The negative ![]() value suggests a decrease in the randomness at solid/solution interface during the biosorption of Cu(Ⅱ) on SCOP.
value suggests a decrease in the randomness at solid/solution interface during the biosorption of Cu(Ⅱ) on SCOP.
3.7 Desorption study
After biosorption of Cu(Ⅱ) ions, the biosorbent was regenerated with hydrochloric acid (0.05 mol/L), and rinsed with deionized water, and then subsequent experiments were repeated. After the fifth biosorption- desorption cycle, the biosorption efficiency is decreased from 94.8% to 90.8%. The initial amount of SCOP employed in the first cycle is 0.2 g and it is reduced to 0.146 g after the fifth cycle.
4 Conclusions
1) The biosorption of copper(Ⅱ) ions from aquatic system was carried out using chemically modified orange peel(SCOP).
2) The selected biosorbent exhibits high biosorp- tion capacity. The copper biosorption performance on SCOP is significantly affected by pH and metal concentrations. For the solution of 50 mg/L Cu(Ⅱ) ion, the maximum biosorption efficiency for Cu(Ⅱ) is 94.8% at 25 ℃ and pH 5.3.
3) Biosorption kinetic is found to be best fit pseudo-second-order equation. The biosorption equilibrium data fit well to the Langmuir isotherm. The maximum biosorption capacity is found to be 72.73 mg/g. The thermodynamic calculation indicates the feasibility, exothermic and spontaneous nature of the biosorption of Cu(Ⅱ) ion on SCOP at 16-60 ℃. The interactions between Cu(Ⅱ) ions and functional groups on the surface of the biosorbent are confirmed by FTIR analysis and the spectra show that carboxyl and hydroxyl groups are involved in Cu(Ⅱ) ion binding to the SCOP.
References
[1] VOLESKY B. Sorption and biosorption [M]. St. Lambert, Québec: BV Sorbex, 2003.
[2] Kaewsarn P. Biosorption of copper(Ⅱ) from aqueous solutions by pre-treated biomass of marine algae Padina sp. [J]. Chemosphere, 2002, 47: 1081-1085.
[3] Yu Jun-xia, Tong Mi, Sun Xiao-mei, Li Bu-hai. Enhanced and selective adsorption of Pb2+ and Cu2+ by EDTAD-modified biomass of baker’s yeast [J]. Bioresour Technol, 2008, 99: 2588-2593.
[4] Feng D, Aldrich C. Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima [J]. Hydrometallurgy, 2004, 73: 1-10.
[5] Sag Y, Kaya A, Kutsal T. The simultaneous biosorption of Cu(Ⅱ) and Zn(Ⅱ) on Rhizopus arrhizus: Application of the adsorption models [J]. Hydrometallurgy, 1998, 50: 297-314.
[6] Pagnanelli F, Esposito A, Toro L, Veglio F. Metal speciation and pH effect on Pb, Cu, Zn and Cd biosorption onto Sphaerotilus natans: Langmuir-type empirical model [J]. Water Res, 2003, 37: 627-633.
[7] Bena?ssa H, Elouchdi M A. Removal of copper ions from aqueous solutions by dried sunflower leaves [J]. Chem Eng Process, 2007, 46(7): 614-622.
[8] ?zer A, ?zer D, ?zer A. The adsorption of copper(Ⅱ) ions on to dehydrated wheat bran(DWB): Determination of the equilibrium and thermodynamic parameters [J]. Process Biochem, 2004, 39: 2183-2191.
[9] YU B, ZHANG Y, SHUKLA A, SHUKLA S S, DORRIS K L. The removal of heavy metals from aqueous solution by sawdust adsorption-removal of copper [J]. J Hazard Mater, 2000, B80: 33-42.
[10] LOW K S, LEE C K, LEO A C. Removal of metals from electroplating wastes using banana pith [J]. Bioresour Technol, 1995, 51(2/3): 227-231.
[11] REDDAD Z, GERENTE C, ANDRES Y, le CLOIREC P. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and equilibrium studies [J]. Environ Sci Technol, 2002, 36: 2067-2073.
[12] LEE S H, YANG J W. Removal of copper in aqueous solution by apple wastes [J]. Sep Sci Technol, 1997, 32(8): 1371-1387.
[13] NOELINE B F, MANOHAR D M, ANIRUDHAN T S. Kinetic and equilibrium modelling of lead(Ⅱ) sorption from water and wastewater by polymerized banana stem in a batch reactor [J]. Sep Purif Technol, 2005, 45: 131-140.
[14] SIMKOVIC I, LASZLO J. Preparation of ion exchangers from bagasse by cross-linking with epichlorohydrin NH4OH or epichlorohydrin-imidazole [J]. J Appl Polym Sci, 1997, 64: 2561-2566.
[15] UNNITHAN M R, ANIRUDHAN T S. The kinetics and thermodynamics of sorption of chromium(Ⅵ) onto iron(Ⅲ) complex of carboxylated polyacrylamide-grafted awdust [J]. Ind Eng Chem Res, 2001, 40: 2693-2699.
[16] SREEDHAR M K, ANIRUDHAN T S. Preparation of an adsorbent by graft polymerization of acrylamide onto coconut husk for mercury(Ⅱ) removal from aqueous solution and chloralkaliindustry wastewater [J]. J Appl Polym Sci, 2000, 75: 1261-1269.
[17] ANIRUDHAN T S, NOELINE B F, MANOHAR D M. Phosphate removal from wastewaters using a weak anion exchanger prepared from a lignocellulosic residue [J]. Environ Sci Technol, 2006, 40: 2740-2745.
[18] FENG N, GUO X, LIANG S. Adsorption study of copper(Ⅱ) by chemically modified orange peel [J]. J Hazard Mater, 2009, 164: 1286-1292.
[19] MATHEICKAL J T, YU Q, WOODBURN G M. Biosorption of cadmium(Ⅱ) from aqueous solutions by pre-treated biomass of marine alga DurvillAea potatorum [J]. Water Res, 1999, 33: 335-342.
[20] TING Y P, PRINCE I G, LAWSON F. Uptake of cadmium and zinc by the alga Chlorella vulgaris (II): Multi-ion situation [J]. Biotechnol Bioeng, 1991, 37: 445-455.
[21] GANALLAH I, GEY D, KILBERTUS G, THAURONT J. Decontamination of industrial effluents for environment protection and recycling of metals [J]. Resources, Conservation and Recycling, 1994, 10: 97-106.
[22] GABALLAH I, GEY D, ALLAIN E, KILBERTUS G, THAURONT J. Recovery of copper through decontamination of synthetic solutions using modified barks [J]. Metall Mater Trans B, 1997, 28B: 13-23.
[23] AJMAL M, RAO R A K, AHMAD R, AHMAD J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(Ⅱ) from electroplating wastewater [J]. J Hazard Mater, 2000, B79: 117-131.
[24] ANNADURAI A, JUANG R S, LEE D J. Adsorption of heavy metals from water using banana and orange peels [J]. Water Sci Technol, 2002, 47: 185-190.
[25] BAIG T H, GARCIA A E, TIEMANN K J, GARDEA-TORRESDEY J L. Adsorption of heavy metal ions by the biomass of Solanum elaeagnifolium (Silverleaf night-shade) [C]// ERICKSON L E. Proceedings of the 10th Annual EPA Conference on Hazardous Waste Research. Washington DC: US Environmental Protection Agency, 1999: 131-139.
[26] GNANASAMBANDAM R, PROTOR A. Determination of pectin degree of esterification by diffuse reflectance Fourier Transform Infrared Spectroscopy [J]. Food Chem, 2008, 68: 327-332.
[27] SAR A, TUZEN M, UIU?ZL? ? D, SOYLAK M. Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass [J]. Biochem Eng J, 2007, 37: 151-158.
[28] YANG J, VOLESKY B. Biosorptions of uranium on Sargassum biomass [J]. Water Res, 1999, 33: 3357-3363.
[29] AKSU Z. Equilibrium and kinetic modelling of cadmium(Ⅱ) biosorptions by C. vulgaris in a batch system: Effect of temperature [J]. Sep Purif Technol, 2001, 21: 285-294.
[30] HO Y S, MCKAY G. The kinetics of sorptions of basic dyes from aqueous solutions by sphagnum moss peat [J]. Can J Chem Eng, 1998, 76: 822-827.
[31] SALTAL? K, SAR? A, AYD?N M. Removal of ammonium ion from aqueous solution by natural Turkish (Y?ld?zeli) zeolite for environmental quality [J]. J Hazard Mater, 2007, B141: 258-263.
Foundation item: Project(50774100) supported by the National Natural Science Foundation of China
Corresponding author: GUO Xue-yi; Tel/Fax: +86-731-88836207; E-mail: xyguo@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60451-3
(Edited by YANG Hua)


