Trans. Nonferrous Met. Soc. China 26(2016) 2372-2379
Polyoxovanadate (NH4)7[MnV13O38] as cathode material for lithium ion battery and improved electrochemical performance
Wen-liang LI1,2, Er-fu NI2, Xin-hai LI1, Hua-jun GUO1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Highpower International Inc., Shenzhen 518111, China
Received 17 May 2016; accepted 30 August 2016
Abstract:
The polyoxovanadate (NH4)7[MnV13O38] (AMV) was synthesized and characterized by X-ray diffraction pattern, Fourier transform infrared spectra, and field emission scanning electron microscope equipped with energy dispersive X-ray spectroscopy. In order to improve the electrochemical performance of AMV, the particle size of as-prepared AMV is decreased to nanoscale by re-precipitation in the water-ethanol solution. The results of the electrochemical impedance spectra and the 4-pin probe measurements show that the electrical conductivity of AMV is improved by decreasing the particle size. The nanoparticle AMV shows higher initial discharge capacity and energy density than the as-prepared AMV when cycled at 0.5C. On the other hand, the nanoparticle AMV exhibits higher rate capability than the as-prepared AMV.
Key words:
lithium ion battery; cathode material; polyoxovanadate; nanoparticle;
1 Introduction
Lithium ion battery (LIB) as power sources has attracted much attention for a variety of applications, such as portable electronic devices, transportation vehicles, and stationary storage of renewable energy sources like solar and wind. It is generally acknowledged that the cell voltages and capacities of LIB are mainly determined by the cathode materials [1-8]. Currently, the conventional cathode materials mainly focus on the transition metal intercalation oxides, such as the layered compounds LiMO2 (M=Co, Ni, Mn, etc.) [9-18], spinel compounds LiM2O4 (M=Mn, etc.) [19-25], and olivine compounds LiMPO4 (M=Fe, Mn, Ni, Co, etc.) [26-32]. The reversible capacities of the materials largely depend on the stability of crystal structures. For example, LiCoO2 can only deliver capacity of 140 mA·h/g which is half of its theoretical capacity due to the intrinsic structural instability of the material when more than half of the Li ions are extracted. Moreover, the achievable specific capacity of the conventional cathode materials is usually lower than 200 mA·h/g, which is insufficient for meeting the increasing energy demand for large-scale applications, such as hybrid electric vehicles and electric vehicles.
Polyoxometalates (POMs) have been considered as potential candidates for use in lithium ion battery because of the unique molecular cluster ion structures [33-43]. The discharge-charge process in POMs can proceed reversibly by the reaction of lithium ions with the stable molecular cluster ions, rather than depending on the stability of the crystal structures. High capacities are expected to be obtained in a wide voltage window (1.5-4.2 V) due to the available multi-electron redox in the discharge-charge process. Polyoxovanadates K7[MnV13O38] and K7[NiV13O38] have been studied recently as the cathode materials of lithium ion battery [40, 43]. However, the potential high capacities of these materials consisting of the molecular cluster ions [MV13O38]7- (M=Ni, Mn) have not been fully exhibited, because the theoretical capacities of the polyoxovanadates are largely dependent on its equivalent mass. The smaller the equivalent mass of counter cations combined with the molecular cluster ions [MV13O38]7- (M=Ni, Mn) is, the higher the theoretical capacities would be exhibited. Therefore, in this study, the counter cations K+ in K7[MnV13O38] were substituted by NH4+ with smaller equivalent mass to form the polyoxovanadate (NH4)7[MnV13O38] (AMV), which possesses higher theoretical capacity than K7[MnV13O38]. The theoretical capacity of AMV is assumed to be 480 mA·h/g, corresponding to the insertion of 26 lithium ions into the structure, which requires 13 redox couples of V5+/V3+ in one cluster ion unit [MnV13O38]7-. However, the intrinsic low electrical conductivities of POMs have adverse effects on the electrochemical performance of the cell. It has been reported that the electrical conductivities could be improved by decreasing the particle size to nanoscale or employing the carbon additive with high electrical conductivity [39,40,42,43], but the obtained discharge capacities were still far from their theoretical ones, and the rate capabilities were not so high. In this study, the polyoxovanadate AMV was synthesized and the electrochemical performance was investigated.
2 Experimental
The polyoxovanadate AMV was prepared by modifying the reported method [44]. 6.083 g NH4VO3 was dissolved in 180 mL hot water, followed by adding 4 mL 1 mol/L HNO3, 4 mL 1 mol/L MnSO4, and 1.826 g (NH4)2S2O8. The solution was evaporated at 80 °C under magnetic stirring and left about 40 mL after 5 h, then treated with 0.6 g ammonium acetate. Some solid impurities at the end of reaction were removed by filtration, and the filter liquor was cooled down under magnetic stirring for several hours. The yellow crystalline product was collected and washed with 1:1 (volume ratio) ethanol-water. In this study, the preparation of AMV nanoparticles was similar to the reported method [40,43], but the water-soluable organic solvent acetone was substituted by ethanol. For control study, the polyoxovanadate K7[MnV13O38] was prepared according to the reported method [40].
The crystal structure of as-prepared polyoxovanadate was characterized by a powder X-ray diffractometer (Bruker, AXS GMBH) with Cu Kα (40 kV, 40 mA) radiation. The diffraction data were recorded from 10° to 90° in 2θ. Fourier transform infrared (FT-IR) spectra were collected with a spectrometer (Bruker, Alpha) in the range of 400-1500 cm-1. The morphologies were observed by field emission scanning electron microscope (FESEM, Nova NanoSEM 450) equipped with energy dispersive X-ray spectroscopy (EDXs). The particle size distributions were recorded by the particle size analyzer (Malvern Mastersizer 2000). Thermal stability study was carried out by thermogravimetic analysis (TGA) and differential scanning calorimetry (DSC) (Simultaneous TGA-DSC Q600). The TGA-DSC experiment was performed at a heating rate of 10 °C/min up to 800 °C in air.
The water in the as-prepared samples was removed by drying at 120 °C for 1 h before electrodes preparation. The cathode consisted of 3 mg (32%) active material, 6 mg (64%) conductive additive ECP and 0.25 mg (4%) PTFE binder. The area and thickness of cathode were 0.5 cm2 and 1 mm, respectively. The electrochemical performance of cathodes was tested at 25 °C by using CR-2032 coin cells, which were assembled in an argon filled glovebox using metallic lithium as anode, and 1 mol/L LiPF6 in a mixed solvent of ethylene carbonate and diethyl carbonate at a volume ratio of 3:7 as electrolyte. Cycle performance was tested on a NEWARE CT-4008 equipment between 1.5 and 4.2 V (vs Li/Li+) at varied rates (1C=480 mA/g). The cyclic voltammetry (CV) data were obtained with a CHI660C (Shanghai Chenhua) at a scan rate of 1 mV/s between 1.5 and 4.2 V. Electrochemical impedance spectra were performed with a CHI660C (Shanghai Chenhua, China) impedance analyzer in the frequency range of 10-2-105 Hz with an amplitude of 10 mV. The electrical conductivities were measured with the 4-pin probe method on a powder resistivity test equipment (Mitsubishi, MCP-PD51).
3 Results and discussion
The X-ray diffraction pattern of as-prepared polyoxovanadate is shown in Fig. 1, which did not match well with the K7[MnV13O38] in ICSD No. 40961, indicating that the crystal structure of as-prepared polyoxovanadate in this work is different from that of the K7[MnV13O38]. The XRD pattern of the re-precipitated product agrees with the as-prepared polyoxovanadate.
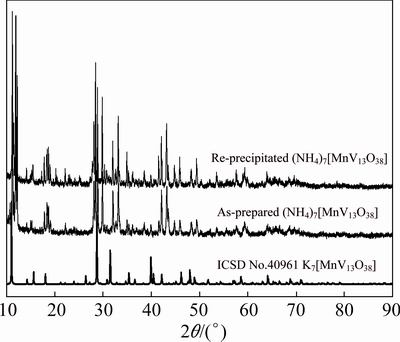
Fig. 1 XRD patterns of as-prepared (NH4)7[MnV13O38], re-precipitated (NH4)7[MnV13O38] and K7[MnV13O38] in ICSD No. 40961
Figure 2 shows the FT-IR spectra of the as-prepared polyoxovanadate. The characteristic absorbance peaks of the as-prepared polyoxovanadate in the wavenumber of 400-1000 cm-1 are consistent with those of K7[MnV13O38], indicating that the molecular cluster ion in the as-prepared polyoxovanadate consisted of [MnV13O38]7-. The characteristic absorbance peak of NH4+ ion observed at 1400 cm-1 indicates that the NH4+ as counter cation should combine with the molecular cluster ion [MnV13O38]7- in the as-prepared polyoxovanadate.
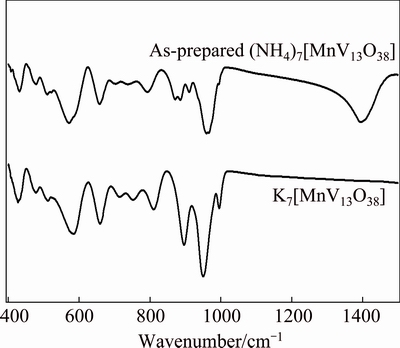
Fig. 2 FT-IR spectra of as-prepared (NH4)7[MnV13O38] and K7[MnV13O38]
The N, Mn and V elements in the as-prepared polyoxovanadate were further confirmed in the elemental distribution maps by EDXs, as shown in Fig. 3. The detected element contents for N, Mn and V are 8.41%, 4.86% and 66.81%, respectively, approximating to the 7:1:13 stoichiometry. This indicates that the polyoxovanadate with the constitution of (NH4)7[MnV13O38] has been prepared in this study.
Figure 4 shows the morphologies of the as-prepared and re-precipitated AMV with different magnifications. It can be seen that the particle size of the as-prepared AMV decreases significantly by re-precipitation with the assistance of ethanol. The particle size distribution is shown in Fig. 5. It is clearly shown that the particle size decreases to nanoscale by re-precipitation. The particle sizes (D50) of as-prepared AMV and re-precipitated AMV are 37.2 and 0.469 μm, respectively. Herein, the re-precipitated AMV is named as nanoparticle.
Figure 6 shows TGA-DSC analysis of the synthesized AMV. The significant water loss associated with endothermal peak exhibited at around 120 °C. The endothermal peak observed at 253 °C might be ascribed to the loss of ammonia. The exothermal peak appeared at 380 °C indicates the complete decomposition of AMV.
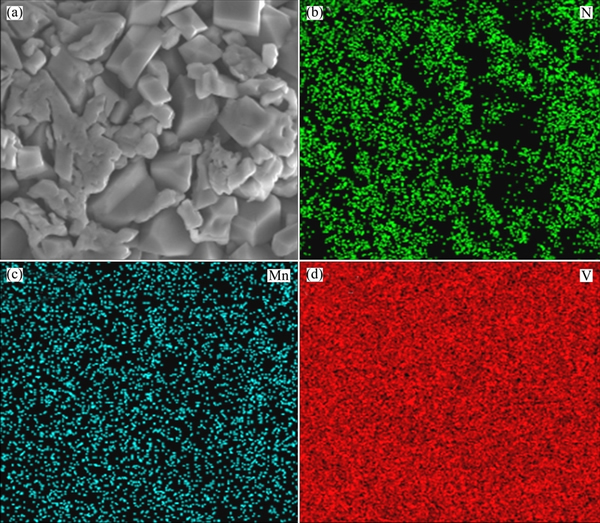
Fig. 3 SEM image (a) of as-prepared (NH4)7[MnV13O38], and corresponding EDXs mappings for N (b), Mn (c) and V (d)
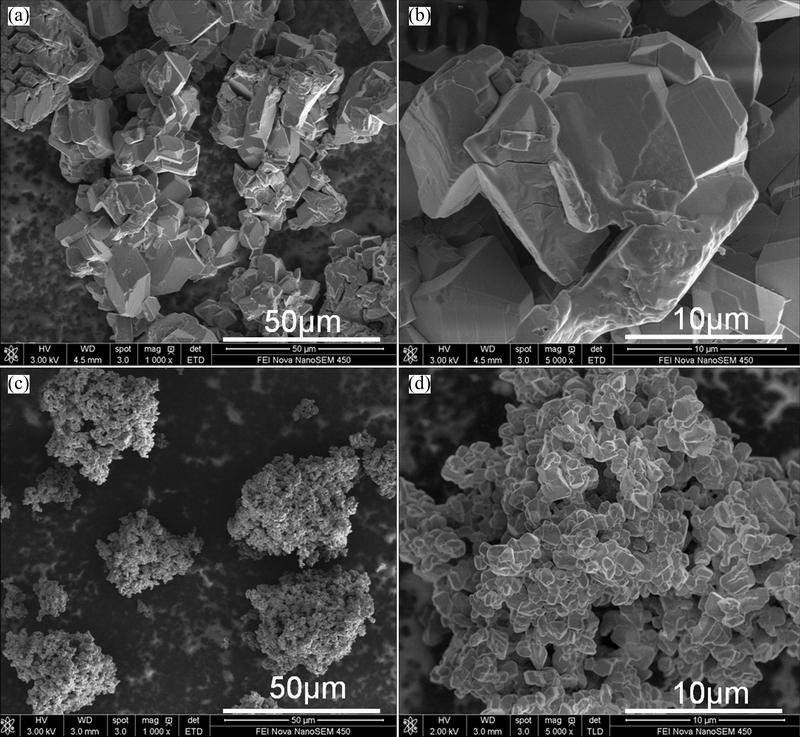
Fig. 4 Morphologies of as-prepared (a, b) and re-precipitated (c, d) (NH4)7[MnV13O38]
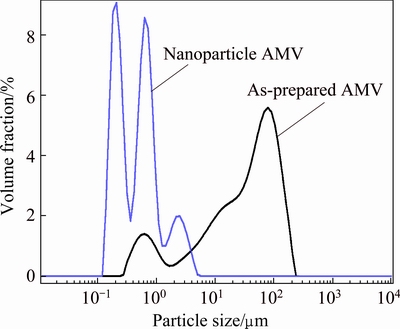
Fig. 5 Particle size distributions of as-prepared (NH4)7[MnV13O38] and nanoparticle (NH4)7[MnV13O38]
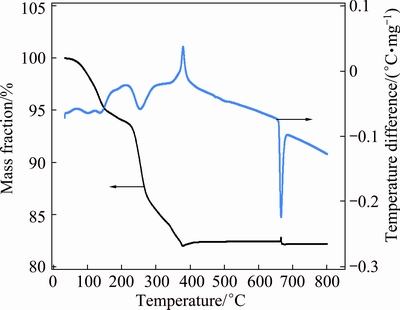
Fig. 6 TGA-DSC curves of as-prepared (NH4)7[MnV13O38]
Figure 7 shows the CV curves of the as-prepared AMV. The electrode exhibited two pairs of broad redox peaks in the voltage range of 1.5-4.2 V. In the first cycle, the cathodic peaks around 2.95 V and 2.05 V and corresponding anodic peak around 3.18 V and 1.8 V are observed. In the following cycles, the redox peaks shifted to the higher voltage, and tended to be stable after the second cycle. This phenomenon should be ascribed to polarization of the electrode. The CV result demonstrates that the reversible redox reaction would proceed in the cathode.
Figure 8(a) shows the initial discharge-charge curves of as-prepared AMV and nanoparticle AMV cycled at 0.5C. The initial discharge capacity of 234 mA·h/g was obtained for the nanoparticle AMV, which is much higher than that of 192 mA·h/g for the as-prepared AMV. Both of the as-prepared AMV and nanoparticle AMV showed large irreversible charge capacity in the following charge process (3.4-4.2 V), which should be contributed to the oxidative decomposition of electrolyte because of the catalysis of POMs [40,43]. Figure 8(b) shows the cycle performance of AMV. The nanoparticle AMV showed gradual capacity increase from the first cycle to the 15th cycle which might be due to the polarization of electrode. Thereafter the capacity decreased gradually, which should be ascribed to the harmful surface film resulted from the decomposed product of electrolyte on the nanoparticle [40]. On the other hand, the as-prepared AMV showed relatively stable cycle performance and higher discharge capacity after long-term cycle. Discharge capacity of 305 mA·h/g was obtained for the as-prepared AMV after 100 cycles, while the nanoparticle AMV maintained discharge capacity of 234 mA·h/g. The energy densities of the two electrodes are shown in Fig. 8(c). The as-prepared AMV and nanoparticle AMV showed energy densities of 722 and 553 mW·h/g after 100 cycles, respectively.
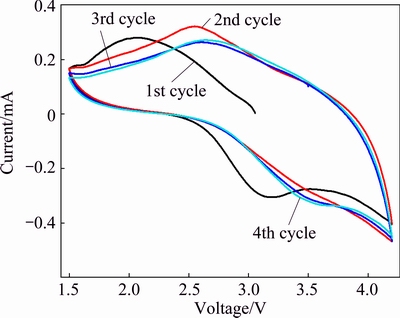
Fig. 7 CV curves of as-prepared (NH4)7[MnV13O38] in voltage range of 1.5-4.2 V
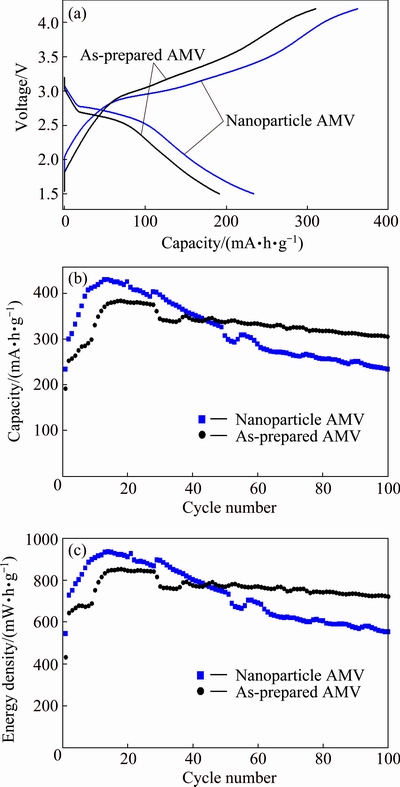
Fig. 8 Initial discharge-charge curves (a), discharge capacity (b) and energy density (c) of as-prepared AMV and nanoparticle AMV cycled at 0.5C
The rate capabilities of as-prepared AMV and nanoparticle AMV after 30 cycles at the varied rates are shown in Fig. 9(a). The discharge capacities of nanoparticle AMV at 1C, 3C and 5C are 330, 238 and 177 mA·h/g, respectively, while those of the as-prepared AMV are 249, 141 and 88 mA·h/g, respectively. The energy densities of the two electrodes at the varied rates are shown in Fig. 9(b). Energy densities of 807, 553 and 395 mW·h/g were obtained for the nanoparticle AMV after 30 cycles at the rates of 1C, 3C and 5C, respectively, while those of 551, 289 and 179 mW·h/g were obtained for the as-prepared AMV.

Fig. 9 Rate capabilities of as-prepared AMV and nanoparticle AMV after 30 cycles at varied rates (a) and energy densities of as-prepared AMV and nanoparticle AMV cycled at varied rates (b)
Figure 10(a) shows the Nyquist plots of as-prepared AMV and nanoparticle AMV after the initial discharge- charge process of the cells at 1C. No obvious difference in both impedance spectra of the two electrodes was observed after one cycle. Both the two impedance spectra showed one semicircle in the high frequency region, which is assigned to the charge transfer resistance, and a line inclined at approximately 45° in the low frequency region represents the Warburg impedance, which is associated with the lithium ion diffusion in the bulk of the active material. The equivalent circuit proposed to fit the Nyquist plots is shown in the inset of Fig. 10(a), where Rs is the electrolyte resistance; Rct is the charge-transfer resistance; CPEct and Zw are the constant phase element and Warburg impedance, respectively. The Rct is 41 and 83 Ω for nanoparticle and as-prepared AMV, respectively. It is clearly shown that the charge-transfer resistance of the electrode was decreased by decreasing the particle size. Figure 10(b) shows the pressure dependence of electrical conductivities of as-prepared AMV and nanoparticle AMV. The nanoparticle AMV exhibited higher electrical conductivity than the as-prepared one. This further demonstrates that the improvement in the electrochemical performance of the electrode should be due to the increase of the electrical conductivity by decreasing the particle size to nanoscale.

Fig. 10 Nyquist plots of as-prepared AMV and nanoparticle AMV after one cycle at 1C (a) and pressure dependence of electrical conductivities of as-prepared AMV and nanoparticle AMV (b)
4 Conclusions
Polyoxovanadate AMV is synthesized with pure phase. The nanoparticle AMV can be prepared by re-precipitation from the water solvent with assistance of ethanol, which shows higher discharge capacity because of the smaller equivalent mass of cation NH4+. The nanoparticle AMV exhibits excellent rate capability at 5C. It should be noted that the available energy density at 0.5C for nanoparticle AMV is twice higher than that of the conventional lithium transition-metal layered oxides (e.g., LiCoO2), suggesting that the POMs with improved electrochemical performance would be a promising alternative as the cathode materials for lithium ion battery.
References
[1] CABANA J, MONCONDUIT L, LARCHER D,  M R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions [J]. Advanced Materials, 2010, 22: E170-E192.
M R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions [J]. Advanced Materials, 2010, 22: E170-E192.
[2] GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries [J]. Chemistry of Materials, 2010, 22: 587-603.
[3] MELOT B C, TARASCON J M. Design and preparation of materials for advanced electrochemical storage [J]. Accounts of Chemical Research, 2013, 46: 1226-1238.
[4] GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: A perspective [J]. Journal of the American Chemical Society, 2013, 135: 1167-1176.
[5] WHITTINGHAM M S. Lithium batteries and cathode materials [J]. Chemical Reviews, 2004, 104: 4271-4301.
[6] ELLIS B L, LEE K T, NAZAR L F. Positive electrode materials for Li-ion and Li-batteries [J]. Chemistry of Materials, 2010, 22: 691-714.
[7] XU B, QIAN D, WANG Z, MENG S Y. Recent progress in cathode materials research for advanced lithium ion batteries [J]. Materials Science and Engineering R, 2012, 73: 51-65.
[8] AURBACH D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries [J]. Journal of Power Sources, 2000, 89: 206-218.
[9] MIZUSHIMA K, JONES P C, WISEMAN P J, GOODENOUGH J B. LixCoO2 (0 [10] REIMERS J N, DAHN J R, Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2 [J]. Journal of the Electrochemical Society, 1992, 139: 2091-2097. [11] OHZUKU T, UEDA A, NAGAYAMA M, IWAKOSHI Y, KOMORI H. Comparative study of LiCoO2, LiNi1/2Co1/2O2 and LiNiO2 for 4 volt secondary lithium cells [J]. Electrochimica Acta, 1993, 38(9): 1159-1167. [12] AURBACH D, GAMOLSKY K, MARKOVSKY B, SALITRA G, GOFER Y, HEIDER U, OESTEN R, SCHMIDT M. The study of surface phenomena related to electrochemical lithium intercalation into LixMOy host materials (M=Ni, Mn) [J]. Journal of the Electrochemical Society, 2000, 147(4): 1322-1331. [13] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiNi1/2Mn1/2O2: A possible alternative to LiCoO2 for advanced lithium-ion batteries [J]. Chemistry Letters, 2001, 30(8): 744-745. [14] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries [J]. Chemistry Letters, 2001, 30(7): 642-643. [15] LI Z, DU F, BIE X, ZHANG D, CAI Y, CUI X, WANG C. Electrochemical kinetics of the Li[Li0.23Co0.3Mn0.47]O2 cathode material studied by GITT and EIS [J]. Journal of Physical Chemistry C, 2010, 114: 22751-22757. [16] LI Z, CHERNOVA N A, ROPPOLO M, UPRETI S, PETERSBURG C, ALAMGIR F M, WHITTINGHAM M S. Comparative study of the capacity and rate capability of LiNiyMnyCo1-2yO2 (y=0.5, 0.45, 0.4, 0.33) [J]. Journal of the Electrochemical Society A, 2011, 158(5): 516-522. [17] PAN C C, ZHU Y R, YANG Y C, HOU H S, JING M J, SONG W X, YANG X M, JI X B. Influences of transition metal on structural and electrochemical properties of Li[NixCoyMnz]O2 (0.6≤x≤0.8) cathode materials for lithium-ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(5): 1396-1402. [18] HUANG Y, WANG Z X, LI X H, GUO H J, WANG J X. Synthesis of Ni0.8Co0.1Mn0.1(OH)2 precursor and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium batteries [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(7): 2253-2259. [19] THACKERAY M M, DAVID W I F, BRUCE P G, GOODENOUGH J B. Lithium insertion into manganese spinels [J]. Materials Research Bulletin, 1983, 18(4): 461-472. [20] TARASCON J M, GUYOMARD D. The Li1+xMn2O4/C rocking- chair system: A review [J]. Electrochimica Acta, 1993, 39(9): 1221-1231. [21] JANG D H, SHIN Y J, OH S M. Dissolution of spinel oxides and capacily losses in 4 V Li/LixMn2O4 cells [J]. Journal of the Electrochemical Society, 1996, 143: 2204-2211. [22] XIA Y, YOSHIO M. An investigation of lithium ion insertion into spinel structure Li-Mn-O compounds [J]. Journal of the Electrochemical Society, 1996, 143(3): 825-833. [23] LIU J, MANTHIRAM A. Understanding the improved electrochemical performances of Fe-substituted 5 V spinel cathode LiMn1.5Ni0.5O4 [J]. Journal of Physical Chemistry C, 2009, 113(33): 15073-15079. [24] YANG M C, XU B, CHENG J H, PAN C J, HWANG B J, MENG Y S. Electronic, structural, and electrochemical properties of LiNixCuyMn2-x-yO4 (0 [25] YI T F, XIE Y, YE M F, JIANG L J, ZHU R S, ZHU Y R. Recent developments in the doping of LiNi0.5Mn1.5O4 cathode material for 5 V lithium-ion batteries [J]. Ionics, 2011, 17(5): 383-389. [26] PADHI A K. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. Journal of the Electrochemical Society, 1997, 144(4): 1188-1194. [27] PADHI A K, NANJUNDASWAMY K S, MASQUELIER C, OKADA S, GOODENOUGH J B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates [J]. Journal of the Electrochemical Society, 1997, 144(5): 1609-1613. [28] CROCE F, EPIFANIO A D, HASSOUN J, DEPTULA A, OLCZAC T, SCROSATI B. A novel concept for the synthesis of an improved LiFePO4 lithium battery cathode [J]. Electrochemical and Solid-State Letters A, 2002, 5(3): 47-50. [29] MORGAN D, VEN A V D, CEDER G. Li conductivity in LixMPO4 (M=Mn, Fe, Co, Ni) olivine materials [J]. Electrochemical and Solid-State Letters A, 2004, 7(2): 30-32. [30] YAMADA A, TAKEI Y,KOIZUMI H, SONOYAMA N, KANNO R, ITOH K, YONEMURA M, KAMIYAMA T. Electrochemical, magnetic, and structural investigation of the Lix(MnyFe1-y)PO4 olivine phases [J]. Chemistry of Materials, 2006, 18(3): 804-813. [31] BRUNETTI G, ROBERT D, BAYLE-GUILLEMAUD P, [32] MARTIN J F, CUISINIER M, DUPRE N, YAMADA A, KANNO R, GUYOMARD D. More on the reactivity of olivine LiFePO4 nano-particles with atmosphere at moderate temperature [J]. Journal of Power Sources, 2011, 196(4): 2155-2163. [33] SONOYAMA N, SUGANUMA Y, KUME T, QUAN Z. Lithium intercalation reaction into the Keggin type polyoxomolybdates [J]. Journal of Power Sources, 2011, 196(16): 6822-6827. [34] UEMATSU S, QUAN Z, SUGANUMA Y, SONOYAMA N. Reversible lithium charge–discharge property of bi-capped Keggin- type polyoxovanadates [J]. Journal of Power Sources, 2012, 217: 13-20. [35] CHEN W, HUANG L J, HU J, LIT F, JIA F F, SONG Y F. Connecting carbon nanotubes to polyoxometalate clusters for engineering high-performance anode materials [J]. Physical Chemistry Chemical Physics, 2014, 16: 19668-19673. [36] KUME K, KAWASAKI N,WANG H, YAMADA T,YOSHIKAWA H, AWAGA K. Enhanced capacitor effects in polyoxometalate/graphene nanohybrid materials: A synergetic approach to high performance energy storage [J]. Journal of Materials Chemistry A, 2014, 2: 3801-3807. [37] NAUMAAN R, KHAN N, MAHMOOD N, LV C L, SIMA G H, ZHANG J, HAO J, HOU Y L, WEI Y G. Pristine organo-imido polyoxometalates as an anode for lithium ion batteries [J]. RSC Advances, 2014, 4: 7374-7379. [38] NI E F, KUME T, UEMATSU S, QUAN Z, SONOYAMA N. Effect of annealing treatment on the electrochemical properties of polyoxomolybdate K4[SiMo12O40] as cathode material of lithium battery [J]. Electrochemistry, 2014, 82: 14-18. [39] NI E F, UEMATSU S, SONOYAMA N. Anderson type polyoxomolybdate as cathode material of lithium ion battery and its reaction mechanism [J]. Journal of Power Sources, 2014, 267: 673-681. [40] NI E F, UEMATSU S, SONOYAMA N. Lithium intercalation into the polyoxovanadate K7MnV13O38 as cathode material of lithium ion battery [J]. Solid State Ionics, 2014, 268: 222-225. [41] WANG H, YAMADA T, HAMANAKA S, YOSHIKAWA H, AWAGA K. Cathode composition dependence of battery performance of polyoxometalate (POM) molecular cluster batteries [J]. Chemistry Letters, 2014, 43: 1067-1069. [42] NI E F, UEMATSU S, TSUKADA T, SONOYAMA N. Lithium intercalation into polyoxomolybdate (NH4)6[NiMo9O32] as the cathode material of lithium battery [J]. Solid State Ionics, 2016, 285: 83-90. [43] NI E F, UEMATSU S, QUAN Z, SONOYAMA N. Improved electrochemical property of nanoparticle polyoxovanadate K7NiV13O38 as cathode material for lithium battery [J]. Journal of Nanoparticle Research, 2013, 15(6): 1-10. [44] FLYNN C M Jr, POPE M T. 1:13 Heteropolyvanadates of manganese (IV) and nickel (IV) [J]. Journal of the American Chemical Society, 1970, 92: 85-90. 李文良1,2,倪尔福2,李新海1,郭华军1 1. 中南大学 冶金与环境学院,长沙 410083; 2. 豪鹏国际集团,深圳 518111 摘 要:合成了聚氧钒酸盐(NH4)7[MnV13O38] (AMV),并采用X射线衍射、傅里叶变换红外光谱及配有能量分布X射线谱的场发射电子扫描显微镜对AMV进行了表征。为了改善AMV的电化学性能,通过在水-乙醇溶剂中再结晶将制备的AMV粒子尺寸降低到纳米级。电化学阻抗谱及4-探针测试结果表明,通过降低粒径尺寸可以提高AMV的电导率。在0.5C下进行充放电循环时,纳米颗粒AMV比制备的AMV表现出更高的初始放电容量及能量密度。另外,纳米颗粒AMV比制备的AMV表现出更高的倍率性能。 关键词:锂离子电池;正极材料;聚氧钒酸盐;纳米粒子 (Edited by Xiang-qun LI) Corresponding author: Er-fu NI; Tel: +86-755-89686522; E-mail: nierfu0733@163.com DOI: 10.1016/S1003-6326(16)64375-3 J L, RAUCH E. F, MARTIN J F, COLIN J F, BERTIN F, CAYRON C. Confirmation of the domino-cascade model by LiFePO4/FePO4 precession electron diffraction [J]. Chemistry of Materials, 2011, 23(20): 4515-4524.
J L, RAUCH E. F, MARTIN J F, COLIN J F, BERTIN F, CAYRON C. Confirmation of the domino-cascade model by LiFePO4/FePO4 precession electron diffraction [J]. Chemistry of Materials, 2011, 23(20): 4515-4524.锂离子电池用聚氧钒酸盐正极材料及其电化学性能改善
Abstract: The polyoxovanadate (NH4)7[MnV13O38] (AMV) was synthesized and characterized by X-ray diffraction pattern, Fourier transform infrared spectra, and field emission scanning electron microscope equipped with energy dispersive X-ray spectroscopy. In order to improve the electrochemical performance of AMV, the particle size of as-prepared AMV is decreased to nanoscale by re-precipitation in the water-ethanol solution. The results of the electrochemical impedance spectra and the 4-pin probe measurements show that the electrical conductivity of AMV is improved by decreasing the particle size. The nanoparticle AMV shows higher initial discharge capacity and energy density than the as-prepared AMV when cycled at 0.5C. On the other hand, the nanoparticle AMV exhibits higher rate capability than the as-prepared AMV.


