Trans. Nonferrous Met. Soc. China 23(2013) 931-941
Corrosion behaviour of thixoformed and heat-treated ZA27 alloys in NaCl solution
Biljana  1, Jelena BAJAT2, Zagorka
1, Jelena BAJAT2, Zagorka  2, Ilija
2, Ilija  3, Bore
3, Bore  1
1
1. Institute ″ ″, University of Belgrade, Milana
″, University of Belgrade, Milana  35, 11000 Belgrade, Serbia;
35, 11000 Belgrade, Serbia;
2. Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, 11120 Belgrade, Serbia;
3. ″ ″ Institute of Nuclear Sciences, University of Belgrade, Mike
″ Institute of Nuclear Sciences, University of Belgrade, Mike  Alasa 12-14, 11001 Belgrade, Serbia
Alasa 12-14, 11001 Belgrade, Serbia
Received 30 May 2012; accepted 22 November 2012
Abstract:
The influence of corrosion on the microstructure of thixoformed and heat-treated ZA27 alloys was investigated. The microstructure of ZA27 alloy was affected by heat treatment. The process of electrochemical corrosion occurs in both ZA27 alloys through the area of η phase. According to the results of immersion test and electrochemical measurements, the corrosion rate of the thixoformed ZA27 alloy is at least 50% lower than that of the thixoformed and thermally processed alloy. This indicates the unfavourable influence of applied heat treatment (T4 regime) on the corrosion resistance of the thixoformed ZA27 alloy.
Key words:
ZA27 alloy; thixoforming; corrosion; heat treatment; microstructure;
1 Introduction
Zinc-based alloy with 27% (mass fraction) aluminium belongs to Zn–Al foundry alloys with relatively high content of aluminium (ZA alloys). The alloy is distinguished by the exceptionally high wear resistance as well as favourable physical, mechanical and technological properties (low density and melting point, high strength and hardness at ambient temperatures, easy machinability, good damping properties, high corrosion resistance) [1,2]. The alloy has been mostly used for pressure die-castings and gravity castings and can be easily cast by sand moulding, shell moulding and permanent moulding [3]. The alloy is shown to be a suitable bearing material for heavy static loads and low load/high speed applications requiring high strength, hardness and wear resistance [4]. Favourable characteristics and low manufacturing cost of ZA27 alloy enable it to compete with other cast metals such as high strength aluminium alloys, aluminium bronzes, grey iron and brass. Typical uses of the alloy include machine tools, presses, internal combustion engines, general engineering industries, transport industry, etc.
The properties of ZA27 alloy can be improved by changes in the microstructure of the alloy. The improvements in the mechanical properties, wear resistance and corrosion resistance were realized at ambient temperatures. This was achieved through the addition of some chemical elements [5,6], by using different heat treatments [7,8] or thermomechanical treatments [9,10]. At last, some special manufacturing techniques such as thixoforming [11,12] and unidirectional solidification [13,14] were applied.
Semi-solid processing has its many advantages over traditional technologies. It has already been applied to industrial production of steels, aluminium alloys and magnesium alloys. Thixoforming is a relatively new technique of metals forming in the semi-solid state [15]. This technique is based on the thixotropic behaviour of alloys with non-dendritic microstructure in the semi-solid state. During thixoforming, vigorous mechanical mixing of the cooling metal melt prevents the formation of normal dendrites and maintains the solid fraction of the melt in the form of rounded primary particles. This technique enables the production of near net-shape components with good mechanical properties at low manufacturing cost [15]. Besides a good combination of strength and ductility, the thixoformed components are heat treatable and weldable. The advantages and drawbacks of thixoforming were described in Refs. [15,16].
ZA27 alloy solidifies in the wide temperature range and is suitable for processing in the semi-solid state. The as-cast alloy is characterised by typical dendritic structure and non-uniform distribution of chemical elements in the alloy phases [17-19]. Traditional casting techniques such as die-casting and permanent mould casting were substituted by thixoforming in order to achieve non-dendritic structure and favourable mechanical properties of the alloy [11,12,20].
The microstructure and properties of ZA27 alloy can also be influenced by the appropriate heat treatment. Ductility [21-23] and sliding wear behaviour of the conventional Zn–Al alloys were improved after thermal treatment [21,24], while their hardness [21,22,24] and tensile strength [21-23] were reduced. It was shown that T4 regime had a beneficial effect on the ductility [25] and tribological characteristics of ZA27 alloy [26,27], although it resulted in minor reduction in hardness and tensile strength [26]. The microstructure of as-cast ZA27 alloy was changed because of the heat treatment. The improved distribution of micro constituents and reduced tendency of forming micro-cracks were achieved [25,27].
The microstructure has a profound influence on the corrosion behaviour of an alloy [28]. It was shown that heat treatment (T4 regime) affected the microstructure and corrosion resistance of as-cast ZA27 alloy [29]. However, there were no results reported so far concerning the effect of T4 regime on the microstructure and corrosion behaviour of the thixoformed ZA27 alloy.
Due to the low melting point [2], ZA27 alloy suffers from deterioration in mechanical properties above 100 °C. It was shown that particulate composites with base ZA27 alloy preserved favourable mechanical characteristics at higher operating temperatures [30]. The composites were obtained by compocasting process through infiltration of ceramic particles into the semi-solid melt of the matrix alloy.
The physical and mechanical characteristics of metal-matrix composites, as well as their corrosion behaviour, are determined by the properties of the matrix alloy [31]. The microstructure of particulate composites with matrix ZA27 alloy is very similar to the microstructure of the thixoformed ZA27 alloy [30].
High corrosion resistance of ZA27 alloy in the natural atmospheres and natural waters is well known [32]. The most common form of corrosion in these environments is general corrosion. The immersion method and electrochemical polarization measurements have been used frequently in corrosion studies to assess the rate of general corrosion [32]. Most corrosion studies were performed in the neutral chloride solutions opened to the atmosphere because chloride ions and dissolved oxygen are present in many corrosive environments and because of great influence of dissolved oxygen on the corrosion mechanism and kinetics of zinc and zinc alloys [33].
In this work, the influence of corrosion on the microstructure of the thixoformed ZA27 alloy was studied. A comparative study was also conducted on the thixoformed and thermally processed ZA27 alloy, to assess the effect of the applied heat treatment on the microstructure and corrosion resistance of the thixoformed ZA27 alloy.
2 Experimental
2.1 Materials
The chemical composition of ZA27 alloy used in this work is given in Table 1. The chemical composition of the alloy is in accordance with the EN standard [34].
Table 1 Chemical composition of ZA27 alloy (mass fraction, %)

The alloy was obtained by conventional melting and casting route in the Department of Materials Science ″ ″ Institute. The alloy was poured at 570 °C into the steel mould preheated up to 100 °C. The as-cast samples with size of 20 mm×30 mm×120 mm were obtained.
″ Institute. The alloy was poured at 570 °C into the steel mould preheated up to 100 °C. The as-cast samples with size of 20 mm×30 mm×120 mm were obtained.
Thixoforming of the ZA27 alloy was performed in order to achieve the permanent transformation of dendritic into the non-dendritic structure. The process was conducted in two phases. In the first phase, the semi-solid melt of the ZA27 alloy was exposed to shear forces (caused by mechanical mixing), to break down the dendritic structure of the as-cast alloy completely. Hot pressing was applied in the second phase in order to reduce the porosity of the thixoformed samples.
The as-cast ZA27 alloy was charged into the crucible of the electro-resistance furnace. It was melted and overheated to 580 °C to clean the slag from the melt surface. The melt was left to cool down to 485 °C at 5 °C/min cooling rate (approximately isothermal regime). The active part of the paddle stirrer was then immersed into the semi-solid melt. Mixing of the melt was performed in the temperature range between liquidus and solidus temperatures with a gradual increase in mixing speed. Stationary mixing regime (i.e. mixing at constant temperature and constant mixing speed) was achieved at 461 °C and 450 r/min. The combination of slow and intensive mixing was applied during thixoforming. Thixoforming at 450 r/min lasted 5 min to break down dendrites in the structure of the as-cast alloy. Mixing speed was then increased to 1000 r/min. This transient mixing speed was applied in very short time (2.5 min). After that, intensive mixing was applied (1500 r/min) during the next 7.5 min until the end of the process. Concerning this, the total mixing time during thixoforming was 15 min. After the termination of mixing, the melt was left to cool down to 450 °C and then it was poured into the steel mould preheated to 300 °C. The samples of the thixoformed ZA27 alloy were obtained with the size of 20 mm×30 mm×120 mm. After cooling, the samples were machine cut into size of 20 mm×30 mm×6 mm and then subjected to hot pressing. Hot pressing was performed in the temperature range of 350-370 °C at pressure of 150 MPa. The samples for microstructure examination and corrosion testing were obtained by machine cutting. A group of the samples were thermally processed according to T4 regime: solutionizing at 370 °C for 3 h followed by water quenching and natural aging.
2.2 Microstructure examination
Optical microscopy (OM) and scanning electron microscopy (SEM) as well as energy dispersive spectroscopy (EDS) were employed for microstructure characterization of the thixoformed and heat-treated ZA27 alloys. Carl Zeiss optical microscope and JEOL JSM–5800 scanning electron microscope coupled with Oxford Link ISIS energy dispersive spectrometer were used.
The microstructures and surface morphology of the thixoformed and heat-treated samples were examined before and after 30 d exposure in the test solution (3.5% (mass fraction) NaCl, pH=6.7). Tests were conducted on the cylindrical samples (5 mm in diameter and 8 mm in height).
The microstructures were examined on the samples that were embedded in the polymethacrylate. The samples were ground and polished. Wet grinding was performed with progressively finer abrasive paper (240, 360, 600 and 800 grit SiC), while polishing was done using polishing cloth and diamond paste (particles size up to 2 μm). The polished samples were used for SEM and EDS analysis, whereas the samples for OM examination were polished and etched in 9% (v/v) nitric acid.
The samples were rinsed with acetone and dried in the air before exposure to the test solution. After finishing of the exposure, the samples were prepared for metallographic examination in the usual way.
2.3 Corrosion rate testing
The immersion test and electrochemical tests were applied to determine the corrosion rates of the thixoformed and heat-treated ZA27 alloys. All tests were performed at room temperature ((23±2) °C), in the approximately neutral NaCl solution (3.5% NaCl, pH=6.7) opened to the atmosphere. The test samples (18 mm × 28 mm × 3 mm) were wet ground using progressively finer SiC abrasive paper (240, 360, 600 and 800 grits). After washing in warm running water and rinsing in acetone, the samples were dried in the air and then they were weighed. After weighing (to an accuracy of 0.0001 g), the samples were stored in the desiccators until the exposure or electrochemical tests.
2.3.1 Immersion test
Preparation of the samples and exposure in the test solution were performed in accordance with ASTM G31 [35]. The samples (in triplicate) were suspended vertically in the quiescent NaCl solution. After 30 d of exposure, the samples were withdrawn from the test solution and rinsed with distilled water. Corrosion products were removed from the surface of the samples by chemical procedure [36]. The samples were then reweighed to determine the mass loss during exposure in the test solution.
2.3.2 Electrochemical tests
The linear polarization method (LPR test) and Tafel extrapolation method were used to assess the corrosion rates of the thixoformed and heat-treated samples of ZA27 alloy. The electrochemical tests were performed at room temperature ((23±2) °C), using the cell for flat samples with 1 cm2 contact area between the test solution and working electrode. Platinum mesh was used as the counter electrode, while the saturated calomel electrode (SCE) was used as the reference electrode. The working electrode (the sample of the thixoformed ZA27 alloy or the thixoformed and thermally processed ZA27 alloy, respectively) was washed by acetone and distilled water before the electrochemical tests. The tests were conducted using Gamry Reference 600 Potentiostat. Three measurements were performed for each sample, with good reproducibility.
The open circuit potential (OCP) of the working electrode was monitored 30-60 min before imposing the electrochemical polarization. After the relatively stable OCP was reached, the working electrode was polarized up to -15 mV (vs OCP). This was immediately followed by a potential scan over 30 mV range (from cathodic towards anodic side) [37] at 0.2 mV/s scan rate. After finishing of the LPR test, the working electrode was allowed to return to OCP.
After each LPR test, the working electrode was polarized potentiodynamically in the potential range ±0.250 V with respect to the OCP and corresponding Tafel graphs were recorded. Potential scan started from cathodic towards anodic side [38] at 0.2 mV/s scan rate.
3 Results and discussion
3.1 Microstructures
Corrosion resistance of both the thixoformed and heat-treated ZA27 alloys is affected by the alloy microstructure, that is, by the chemical composition and distribution of micro constituents (phases) in the alloy. Changes in the microstructures of the thixoformed and heat-treated ZA27 alloys because of corrosion occurrence were presented.
3.1.1 Thixoformed ZA27 alloy
The microstructure of the thixoformed ZA27 alloy is shown in Fig. 1(a), while typical dendritic structure of the as-cast alloy is shown in Fig. 1(b). Because of the shear force action during the mixing of the semi-solid melt, the microstructure of the thixoformed ZA27 alloy is non-dendritic. Large primary particles of irregular oval shape as well as the interdendritic η phase can be seen in Fig. 1(a). In addition, tiny islands of the phase mixture of α+η are visible in the zone of η phase (Fig. 1(a)).

Fig. 1 OM images of ZA27 alloys
Primary particles are complex. They consist of a core C and a periphery P (Fig. 2(a)). The core consists of α phase rich in aluminium (large dark areas in Fig. 2(a)), while the periphery is composed of the phase mixture α+η (light grey areas in Fig. 2(a)). Interdendritic η phase rich in zinc is situated among the primary particles. The phase mixture α+η composed of α and η micro constituents, with clear phase boundaries, is shown in Fig. 2(b).
The chemical composition of the micro constituents in the thixoformed structure is shown in Fig. 3. The line of L in Fig. 2(b) starts in the region of the phase mixture α+η and ends in the η phase. In the region of the phase mixture α+η, the ratio of zinc to aluminium (Zn/Al) corresponds to the nominal composition of the ZA27 alloy. Zinc concentration rises in the area of the η phase, while aluminium concentration in this area decreases rapidly.

Fig. 2 SEM images of thixoformed ZA27 alloy

Fig. 3 Changes in chemical composition of microconstituents in Fig. 2(b)
Distribution of the chemical elements in the microstructure of the thixoformed ZA27 alloy is very similar to the distribution in the microstructure of the as-cast alloy [29]. There are no changes in the chemical composition of the micro constituents during thixoforming. Only regrouping of existing phases takes place. The increase in size of the α phase particles is noticed in the thixoformed structure as well as significant increase in the volume fraction of α phase. In addition, it is observed that the volume fraction of the phase mixture α+η is significantly reduced, while the change in the volume fraction of the η phase is rather small [30]. The radical change in the morphology occurs during thixoforming, in accordance with the scheme proposed by FLEMINGS [39], as evidenced by the elliptical shape and the size of primary particles in the structure of the thixoformed ZA27 alloy. Thixoforming leads to a general coarsening of the microstructure in relation to the microstructure of the as-cast ZA27 alloy [30].
According to FLEMINGS [39], thixoforming of an alloy can be guided towards fragmentation of the microstructure using higher shear rates (during mixing) and higher cooling rates. It is shown that the cooling rates have a much greater influence on the formation of the small-grained structure during thixoforming than the applied shear forces [40].
Agglomeration of the primary particles of α phase takes place during thixoforming. The agglomerates retain elliptical shape due to the influence of shear forces created by mixing [39]. After the termination of mixing, the instantaneous increase in viscosity of the semi-solid melt prevents deagglomeration of the primary particles. With further cooling (after pouring of the melt into the mould), the peritectic reaction takes place (443 °C) between the elliptical agglomerates of α phase and the remains of the melt, so that β phase is formed. This phase is transformed through an eutectoid reaction into the phase mixture α+η.
The influence of corrosion on the microstructure of the thixoformed ZA27 alloy is shown in Fig. 4. Surface appearance of the thixoformed sample that is not exposed in the corrosive environment is shown in Fig. 4(a), for the purpose of comparison. Because of the sample preparation, minor mechanical defects are visible on the edge of the sample, while some inclusions can be noticed on the sample surface.

Fig. 4 Influence of corrosion on thixoformed ZA27 alloy
Surface appearance and microstructures of the thixoformed samples after 30 d exposure in the test solution are shown in Figs. 4(b)-(d). The process of corrosion starts in the area of mechanical damage (on the edge of the sample) and progresses towards the centre of the sample (Fig. 4(b)). Corrosion takes place in the area of η phase as well as in the area of phase mixture α+η (Fig. 4(c)). Elliptical particles of α phase can also be seen on the sample edge (Fig. 4(c)). The process of corrosion has bypassed these particles, creating a relief in the morphology.
The appearance of corrosion damage on the sample edge is shown in Fig. 4(d). Besides the expressed relief, progression of corrosion in the sample depth at micro level can be noticed. Micro-cracks are not observed (Fig. 4(a)) on the thixoformed samples that are not exposed in the test solution. However, their presence is visible near the area that is damaged by corrosion (Figs. 4(b) and (d)).
Initial micro-cracks are possibly formed during hot pressing of the thixoformed samples or during cooling after hot pressing. In the corrosive environment, the growth of these micro-cracks probably occurs. The appearance of micro-cracks in the microstructure of the thixoformed ZA27 alloy should be investigated thoroughly, although this is beyond the scope of this work.
Morphological appearance of the corrosion products on the thixoformed samples is shown in Fig. 4(e). The corrosion products are mainly in the form of rosettes. They appear as spongy deposits on the sample surface.
3.1.2 Thixoformed and heat-treated ZA27 alloy
The microstructure of the thixoformed ZA27 alloy after heat treatment is shown in Fig. 5.

Fig. 5 OM image of thixoformed ZA27 alloy after heat treatment
The incomplete decomposition of the supersaturated phases α and η occurs during solutionizing (3 h at 370 °C), as well as additional rounding of α phase particles and decrease in their size compared to the thixoformed structure (Fig. 1(a)). The continuity in the η phase area is interrupted and this phase appears as a series of separated areas in the microstructure of the thixoformed and heat-treated ZA27 alloy. Quantitative metallographic analysis of the heat-treated samples was performed in order to get an insight into the impact of the applied heat treatment on the microstructure of the thixoformed ZA27 alloy. The results are presented in Table 2.
Table 2 Quantitative metallographic analysis of thixoformed and heat-treated ZA27 alloys

Compared these results with the recently published results in Ref. [30], it can be concluded that the application of T4 regime results in the formation of a relatively small-grained structure. The volume fraction of the phase mixture α+η is significantly increased due to the applied heat treatment, while the volume fractions of the individual phases (α and η) reduce. In addition, there is a decrease of about 30% in the size of primary particles of the α phase with regard to the average size of the largest ferret [41].
The influence of corrosion on the microstructure of the thixoformed and heat-treated samples of the ZA27 alloy is shown in Fig. 6. Surface appearance of the sample before exposure in the test solution is shown in Fig. 6(a). Apart from minor mechanical damage on the edge, the presence of inclusions can be noticed on the surface of the sample. Surface appearance of the samples after 30 d exposure is shown in Figs. 6(b) and (c). Corrosion starts in the area of mechanical damage on the edge of the sample and takes place through the η phase and in the area of the phase mixture α+η, but is limited to the surface of the sample.
In the area affected by corrosion, micro-cracks are formed (Figs. 6(b)-(d)). Some micro-cracks can be noticed on the phase boundaries α+η/η (Fig. 6(c)). It is possible that initial micro-cracks (invisible at the level of applied metallographic examinations) are created during quenching within the applied heat treatment of the thixoformed ZA27 alloy. Probably it comes to their development during exposure of heat-treated samples in the test solution.
From the point of practical application, the appearance of micro-cracks in the thixoformed ZA27 alloy after thermal treatment is undesirable. The influence of T4 regime on the thixoformed microstructure and particularly phenomena that take place during quenching of the thixoformed ZA27 alloy require additional research.
An edge of the thixoformed and heat-treated sample after exposure in the test solution is shown in Fig. 6(d). Corrosion products mainly in the form of tiles and rosettes, as well as some micro-cracks, can be seen on the sample surface. Corrosion products are morphologically very similar to those of the thixoformed ZA27 alloy not subjected to thermal treatment (Fig. 4(e)).

Fig. 6 Influence of corrosion on thixoformed and heat-treated ZA27 alloy
3.2 Corrosion rate
3.2.1 Immersion test
After 30 d of exposure, the corrosion products were removed from the sample surface by chemical procedure [36]. It can be noticed that corrosion occurs uniformly over the surface of the thixoformed and heat-treated samples. The calculation of the average corrosion rate CR is based on the mass loss of the samples Δm (g) during the immersion test:
 (1)
(1)
where K is a constant [35], τ is the exposure time (h), A is the sample surface (cm2) and d is the density of ZA27 alloy (5 g/cm3). The calculated corrosion rates for the thixoformed and heat-treated ZA27 alloys are 0.120 and 0.190 mm/a, respectively. The corrosion rate of the thixoformed alloy is lower than that of the thixoformed and heat-treated ZA27 alloy.
3.2.2 Electrochemical tests
1) LPR test
The polarization curves in the small potential range in the vicinity of the free corrosion potential (±10 mV vs φcorr) were obtained for both the thixoformed and heat-treated ZA27 alloys. Polarization resistance Rp is determined from the slope of each experimental curve (dφ/dJ) at φcorr (Fig. 7).
It can be seen in Fig. 7 that the applied heat treatment (T4 regime) results in the significant decrease of Rp value. The polarization resistance can be converted into corrosion current density using Stern–Geary equation [42]:
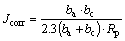 (2)
(2)
where ba and bc denote anodic and cathodic Tafel slope, respectively.

Fig. 7 LPR graphs of ZA27 alloys in NaCl solution
Equation (2) is applicable when both anodic and cathodic reactions are under charge-transfer control. If anodic reaction is under charge-transfer control while cathodic reaction is controlled by diffusion, the modified Stern–Geary equation applies [28]
 (3)
(3)
Corrosion of the thixoformed ZA27 alloy in the NaCl solution (pH=6.7) takes place through anodic dissolution of the alloy. Anodic reaction is under charge-transfer control with anodic Tafel slope ba=40 mV/decade (Fig. 8). The cathodic reaction is controlled by the diffusion of the cathode reactant. In that case, cathodic Tafel slope bc→∞ and Eq. (3) are used to calculate Jcorr.
The calculated values of Jcorr are 9 and 17 μA/cm2, for the thixoformed and heat-treated ZA27 alloy, respectively. This is consistent with the results obtained in the immersion test.
2) Tafel extrapolation method
After each LPR test, the polarization curves were recorded in the potential range of ±0.250 V with respect to φcorr. The curves were corrected because of the ohmic polarization [43,44]. The corresponding Tafel graphs are presented in Fig. 8.

Fig. 8 Tafel graphs of ZA27 alloys in NaCl solution
It may be noted that the anodic polarization curves show significant increase in current density with small increase in polarization, indicating active dissolution of the thixoformed ZA27 alloy. Such behaviour is typical for the dissolution of zinc in NaCl solutions [45]. Anodic curves are linear in the indicated region of current (more than 1.5 current decades). The value of anodic Tafel slope is close to 40 mV/decade, which is consistent with ba values for anodic dissolution of zinc [46].
The cathodic polarization curves in Fig. 8 show highly polarized behaviour. With significant increase in the polarization of the working electrode, a small increase in current density can be seen. This indicates that the cathodic reaction is controlled by diffusion [28]. Due to the low solubility of oxygen in the NaCl solution (about 10-3 mol/L) [28], its transfer to the electrode surface is limited. When a reaction rate is completely under mass transfer control, it no longer depends on potential [47]. In this case, the value of cathodic Tafel slope bc→∞ [47,48].
Extrapolation of the linear region of anodic polarization curve to the intersection with a line, which is determined by φcorr value, was performed. The values of lg Jcorr and Jcorr were obtained. It may be noted that the higher value of corrosion current density is obtained for the heat-treated ZA27 alloy compared to the thixoformed ZA27 alloy. These results are consistent with those based on the polarization resistance measurements in this work. However, somewhat higher values of Jcorr are obtained by extrapolation of anodic Tafel lines. This could be explained by possible irreversible changes on the electrode surface at high values of polarization [49] applied in obtaining Tafel graphs. In addition, the logarithmic dependence between current density and potential in Tafel graphs affects the accuracy of assessing Jcorr.
For the purpose of comparison, the values of Jcorr obtained in the LPR test and by Tafel extrapolation were converted in the penetration rates. The penetration rate PR (mm/a) and corrosion current density Jcorr (μA/cm2) are related through the following expression [50]:
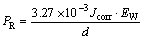 (4)
(4)
where EW denotes the equivalent mass of the ZA27 alloy while d is the same as in Eq. (1). EW is calculated according to the following equation [50]:
 (5)
(5)
where fi is the mass fraction of the i-th element in the alloy, ni is the valence of the i-th element of the alloy and M is the relative atomic mass of the i-th element in the alloy. The equivalent mass of the ZA27 alloy was calculated taking into account the mass fractions of Zn, Al and Cu in the alloy. The calculated value of EW is 19.1.
The corrosion rates (expressed as the penetration rates) obtained in the immersion test and in the electrochemical tests are presented in Table 3.
Table 3 Corrosion rate of thixoformed and heat-treated ZA27 alloys

The results obtained by different methods are in agreement. According to the results of the immersion test and electrochemical polarization measurements, the corrosion rate of the thixoformed ZA27 alloy is at least 50% lower than that of the thixoformed and thermally processed alloy. This indicates that the applied heat treatment results in decreased corrosion resistance of the thixoformed ZA27 alloy. The values of corrosion rate obtained in the immersion test and in the LPR test are in good agreement, while the values obtained by Tafel extrapolation method are somewhat higher. As stated above, this may be due to the irreversible changes of the electrode surface at high anodic polarizations.
By comparing the results obtained with the results reported in Ref. [29], it can be concluded that the corrosion rate of the thixoformed ZA27 alloy is higher than that of the as-cast ZA27 alloy.
Thixoforming influences the microstructure of the ZA27 alloy significantly. During this process, dendritic structure of the as-cast alloy is transformed into the non-dendritic structure. Besides, a significant increase in the volume fraction of α phase and the volume fraction of phase mixture α+η in the microstructure of the thixoformed ZA27 alloy is noticed. The increase in size of α phase particles is also observed. In spite of the radical transformation in morphology during thixoforming, there are no changes in the chemical composition within individual micro constituents [30].
The applied heat treatment (T4 regime) influences the microstructure of thixoformed ZA27 alloy. The volume fraction of α and η phases is reduced by 10% and 6%, respectively, while the volume fraction of phase mixture α+η is increased by 16%.
Besides, micro-cracks appear in the microstructure of thixoformed ZA27 alloy after T4 thermal treatment. According to the microstructural examination in this work, the micro-cracks form on the phases interface α/α+η and α+η/η. The micro-cracks create during quenching in the heat treatment. Solution annealing in the first phase of heat treatment was performed at 370 °C, quite near to the melting point of ZA27 alloy. Under these conditions, the diffusion of zinc atoms from the lattice of metastable α phase (α phase as an individual micro constituent and α phase as a part of α+η phase mixture) is highly intensive. This process is related with an increase in the number of atomic vacancies. Due to the quenching in cold water, the process of diffusion stops immediately and micro-cracks form.
In both the thixoformed and heat-treated ZA27 alloys, the process of electrochemical corrosion takes place in the area of η phase and in the area of the phase mixture α+η.
Lower corrosion resistance of the thixoformed ZA27 alloy subjected to thermal treatment could be explained by progression of corrosion in the micro-cracks, and by an increase in the size of the corroded area.
Considering all the facts presented, it can be concluded that the applied heat treatment (T4 regime) exhibits a negative impact on the corrosion resistance of the thixoformed ZA27 alloy.
4 Conclusions
1) Thixoforming influences the microstructure of ZA27 alloy. Transformation of the dendritic structure into the non-dendritic one occurs without changes in the chemical composition of micro constituents in the thixoformed ZA27 alloy.
2) Heat treatment affects the microstructure of thixoformed ZA27 alloy. A decrease in size of α phase particles is noticed along with significant increase in the volume fraction of α+η phase mixture.
3) In both the thixoformed and heat-treated ZA27 alloys, the process of electrochemical corrosion takes place in the area of η phase and through the area of α+η phase mixture.
4) The corrosion rate of the thixoformed ZA27 alloy is at least 50% lower than that of the thixoformed and thermally processed alloy.
5) Applied heat treatment (T4 regime) results in decreased corrosion resistance of the thixoformed ZA27 alloy.
Acknowledgements
The Ministry of Education and Science of the Republic of Serbia financially supported this work through the projects No. TR 35021 and OI 172005. The authors are gratefully acknowledged to RAR Foundry Batajnica, for providing the master alloy for performance of the research.
Foundry Batajnica, for providing the master alloy for performance of the research.
References
[1] GERVAIS E, BARNHURST R J, LOONG C A. An analysis of selected properties of ZA alloys [J]. Journal of Metals, 1985, 11: 43-47.
[2] Kubel E J. Expanding horizons for ZA alloys [J]. Advanced Materials and Processes, 1987, 132: 51-57.
[3] ASM Handbook. Vol. 15. Casting [M]. Materials Park, Ohio: ASM International, 2008: 1734-1753.
[4] Lyon R. High strength zinc alloys for engineering applications in the motor car [J]. Metals and Materials, 1985, 1: 55-57.
[5] DOMINGUEZ C, MORENO LOPEZ M V, RIOS-JARA D. The influence of manganese on the microstructure and the strength of a ZA27 alloy [J]. Journal of Materials Science, 2002, 37: 5123-5127.
[6] Chen T J, HAO Y, SUN, Y, LI Y D. Effects of Mg and RE additions on the semisolid microstructure of a zinc alloy ZA27 [J]. Science and Technology of Advanced Materials, 2003, 4: 495-502.
[7] CHOUDHURY P, DAS K, DAS S. Evolution of as-cast and heat-treated microstructure of a commercial bearing alloy [J]. Materials Science and Engineering A, 2005, 398: 332-343.
[8] SAVASKAN T, MURPHY S. Mechanical properties and lubricated wear of Zn-25Al-based alloys [J]. Wear, 1987, 116: 211-224.
[9] Aashuri H. Globular structure of ZA27 alloy by thermomechanical and semisolid treatment [J]. Materials Science and Engineering A, 2005, 391:77-85.
[10] CHEN T J, HAO Y, SUN J. Microstructural evolution of previously deformed ZA27 alloy during partial remelting [J]. Materials Science and Engineering A, 2002, 337: 73-81.
[11] CHEN T J, HAO Y, LI Y D. Effects of processing parameters on microstructure of thixoformed ZA27 alloy [J]. Materials and Design, 2007, 28: 1279-1287.
[12] CHEN T J, HAO Y, SUN J, LI Y D. Phenomenological observations on thixoformability of a zinc alloy ZA27 and the resulting microstructures [J]. Materials Science and Engineering A, 2005, 396: 213-222.
[13] SANTOS G A, NETO C M, OSORIO W R, GARCIA A. Design of mechanical properties of a ZA27 alloy based on microstructure dendritic array spacing [J]. Materials and Design, 2007, 28: 2425-2430.
[14] ARES A E, GRASSA L M, GUEIJMAN S F, SCHVEZOV C E. Correlation between thermal parameters, structures, dendritic spacing and corrosion behavior of Zn–Al alloys with columnar to equiaxed transition [J]. Journal of Crystal Growth, 2008, 310: 1355-1361.
[15] HIRT G, KOPP R. Thixoforming [M]. Weinheim: Wiley-VCH Verlag GmbH & CoKgA, 2009: 19.
[16] LOWE A, RIDGWAY K, ATKINSON H V. The pros and cons of semi-solid processing [J]. Materials World, 1999, 9: 541-543.
[17] Durman M. Microstructures and hot tensile properties of pressure-diecast and gravity commercial zinc-based alloys [J]. Zeitschrift fur Metallkunde, 1998, 89: 417-423.
[18] MURPHY S, SAVASKAN T. Metallography of Zn-25%Al based alloys in the as-cast and aged conditions [J]. Practical Metallography, 1987, 24: 204-221.
[19] Lehuy H. Mechanical properties of zinci-aluminium alloys extruded in the liquid-solid state [J]. Journal of Materials Science, 1988, 23: 2943-2950.
[20] KARNI N, BARKAY G B, BAMBERGER M J. Structure and properties of metal-matrix composite [J]. Journal of Materials Science Letters, 1994, 13: 541-544.
[21] Prasad B K. Influence of heat treatment on the physical, mechanical and tribological properties of a zinc-based alloy [J]. Zeitschrift fur Metallkunde, 1997, 87: 226-332.
[22] PRASAD B K, PATWARDHAN A K, YEGNESWARAN A H. Tensile properties of some Zn-27.5 wt% Al alloys as influenced by heat treatment and test conditions [J]. Journal of Materials Science Letters, 1997, 16: 1890-1893.
[23] Prasad B K. Effect of heat treatment on tensile properties of zinc-37.5 mass% aluminium alloy containing nickel or silicon [J]. Materials Transactions, JIM, 1998, 39: 387-390.
[24] Prasad B K. Dry sliding wear response of zinc based alloy: Influence of heat treatment, sliding speed and pressure [J]. Materials Science and Technology, 1997, 13: 928-936.
[25]  I,
I,  M T, ZEC S. Improvement of ductility of a cast Zn-25 alloy [J]. Materials Characterization, 1992, 29: 277-283.
M T, ZEC S. Improvement of ductility of a cast Zn-25 alloy [J]. Materials Characterization, 1992, 29: 277-283.
[26] BABIC M, VENCL A, MITROVIC S, BOBIC I. Influence of T4 heat treatment on tribological behavior of ZA27 alloy under lubricated sliding condition [J]. Tribology Letters, 2009(2): 125-134.
[27] 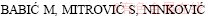 R. Tribological potential of zinc-aluminium alloys improvement [J]. Tribology in Industry, 2009, 31: 15-28.
R. Tribological potential of zinc-aluminium alloys improvement [J]. Tribology in Industry, 2009, 31: 15-28.
[28] SHREIR L L, JARMAN R A, BURSTEIN G T. Corrosion [M]. Oxford: Butterworth-Heinemann, 2000: 36.
[29] BOBIC B, BAJAT J, ACIMOVIC–PAVLOVIC Z, RAKIN M, BOBIC I. The effect of T4 heat treatment on the microstructure and corrosion behaviour of Zn27Al1.5Cu0.02Mg alloy [J]. Corrosion Science, 2011, 53: 409-417.
[30] BOBIC B, BABIC M, MITROVIC S, ILIC N, BOBIC I, JOVANOVIC M T. Microstructure and mechanical properties of Zn25Al3Cu based composites with large Al2O3 particles at room and elevated temperatures [J]. International Journal of Materials Research, 2010, 101: 1524-1531.
[31] ASM Handbook. Vol. 21. Composites [M]. Materials Park, Ohio: ASM International, 2001: 380.
[32] SCHWEITZER P A. Fundamentals of corrosion [M]. New York: CRC Press, Taylor & Francis Group, 2010: 112.
[33] DAFYDD H, WORSLEY D A, MCMURRAY H N. The kinetics and mechanism of cathodic oxygen reduction on zinc and zinc–aluminium alloy galvanized coating [J]. Corrosion Science, 2005, 47: 3006-3018.
[34] BS EN 12844. Zinc and zinc alloys. Castings. Specifications [S]. 1999.
[35] ASTM G31–72. Standard practice for laboratory immersion corrosion testing of metals [S]. 2004.
[36] ASTM G1–03. Standard practice for preparing, cleaning and evaluation of test specimens [S].
[37] BALDWIN K R, ROBINSON M J, SMITH C J E. Corrosion rate measurements of electrodeposited zinc–nickel alloy coatings [J]. Corrosion Science, 1994, 36: 1115-1131.
[38] POORQASEMI E, ABOOTALEBI O, PEIKARI M, HAQDAR F. Investigating accuracy of the Tafel extrapolation method in HCl solutions [J]. Corrosion Science, 2009, 51: 1043-1054.
[39] Flemings M. Behavior of metal alloys in the semi-solid state [J]. Metallurgical Transactions A, 1991, 22: 957-981.
[40] LEHUY H, MASOUNAVE J, BLAIN J. Rheological behaviour and microstructure of stir-casting zinc–aluminium alloys [J]. Journal of Materials Science, 1985, 20: 105-113.
[41] ASM Handbook. Vol. 9. Metallography and microstructures [M]. Materials Park, Ohio: ASM International, 2004: 1005.
[42] STERN M, GEARY A L. Electrochemical polarization I. Theoretical analysis of the shape of polarization curves [J]. Journal of Electrochemical Society, 1957, 104: 56-63.
[43] McCafferty E. Validation of corrosion rates measured by the Tafel extrapolation method [J]. Corrosion Science, 2005, 47: 3202-3215.
[44] FLITT H J, SCHWEINSBERG D P. Evaluation of corrosion rate from polarization curves not exhibiting a Tafel region [J]. Corrosion Science, 2005, 47: 3034-3052.
[45] VAGGE S T, RAJA V S, NARAYANAN R G. Effect of deformation on the electrochemical behavior of hot-dip galvanized steel sheets [J]. Applied Surface Science, 2007, 253: 8415-8421.
[46] van den BOS C, SCHNITGER H C, ZHANG X, HOVESTAD A, TERRYN H, de WIT J H W. Influence of alloying elements on the corrosion resistance of rolled zinc sheet [J]. Corrosion Science, 2006, 48: 1483-1499.
[47] LANDOLT D. Corrosion and surface chemistry of metals [M]. Lausanne: EPFL Press, 2007: 176.
[48] BARRANCO V, FELIU S Jr, FELIU S. EIS study of the corrosion behaviour of zinc-based coatings on steel in quiescent 3% NaCl solution. Part I: Directly exposed coatings [J]. Corrosion Science, 2004, 46: 2203–2220.
[49] Rocchini G. The determination of Tafel slopes by an integral method [J]. Corrosion Science, 1994, 36: 113-125.
[50] ASTM G 102–89. Standard practice for calculation of corrosion rates and related information from electrochemical measurements [S]. 2010.
触变成形和热处理ZA27合金在NaCl溶液中的腐蚀行为
Biljana 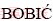 1, Jelena BAJAT2, Zagorka
1, Jelena BAJAT2, Zagorka 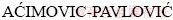 2, Ilija
2, Ilija 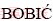 3, Bore
3, Bore  1
1
1. Institute ″ ″, University of Belgrade, Milana
″, University of Belgrade, Milana  35, 11000 Belgrade, Serbia;
35, 11000 Belgrade, Serbia;
2. Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, 11120 Belgrade, Serbia;
3. ″ ″ Institute of Nuclear Sciences, University of Belgrade, Mike
″ Institute of Nuclear Sciences, University of Belgrade, Mike  Alasa 12-14, 11001 Belgrade, Serbia
Alasa 12-14, 11001 Belgrade, Serbia
摘 要:研究触变成形和热处理ZA27合金在NaCl溶液中腐蚀后的显微组织的变化。结果表明,热处理会影响触变成形ZA27合金的组织。对经热处理和未经热处理的触变成形ZA27合金,电化学腐蚀均发生在η相区。浸泡试验和电化学测试结果表明,未经热处理的触变成形ZA27合金的腐蚀速率比经过热处理的触变成形ZA27合金的低50%,表明T4热处理会降低触变成形ZA27合金的耐腐蚀性能。
关键词:ZA27合金;触变成形;腐蚀;热处理;显微组织
(Edited by Sai-qian YUAN)
Corresponding author: Biljana  ; Fax: +381-11-2410 977; E-mail: biljanabobic@gmail.com
; Fax: +381-11-2410 977; E-mail: biljanabobic@gmail.com
DOI: 10.1016/S1003-6326(13)62550-9
Abstract: The influence of corrosion on the microstructure of thixoformed and heat-treated ZA27 alloys was investigated. The microstructure of ZA27 alloy was affected by heat treatment. The process of electrochemical corrosion occurs in both ZA27 alloys through the area of η phase. According to the results of immersion test and electrochemical measurements, the corrosion rate of the thixoformed ZA27 alloy is at least 50% lower than that of the thixoformed and thermally processed alloy. This indicates the unfavourable influence of applied heat treatment (T4 regime) on the corrosion resistance of the thixoformed ZA27 alloy.
[3] ASM Handbook. Vol. 15. Casting [M]. Materials Park, Ohio: ASM International, 2008: 1734-1753.
[15] HIRT G, KOPP R. Thixoforming [M]. Weinheim: Wiley-VCH Verlag GmbH & CoKgA, 2009: 19.
[28] SHREIR L L, JARMAN R A, BURSTEIN G T. Corrosion [M]. Oxford: Butterworth-Heinemann, 2000: 36.
[31] ASM Handbook. Vol. 21. Composites [M]. Materials Park, Ohio: ASM International, 2001: 380.
[34] BS EN 12844. Zinc and zinc alloys. Castings. Specifications [S]. 1999.
[35] ASTM G31–72. Standard practice for laboratory immersion corrosion testing of metals [S]. 2004.
[36] ASTM G1–03. Standard practice for preparing, cleaning and evaluation of test specimens [S].
[47] LANDOLT D. Corrosion and surface chemistry of metals [M]. Lausanne: EPFL Press, 2007: 176.


