文章编号:1004-0609(2015)-09-2471-07
掺氮对WO3薄膜电致变色调制性能的影响
王 伟,李合琴,陶 磊,乔 恺,周 矗,张 静,唐 琼,黄依琴,左 敏,李世伟
(合肥工业大学 材料科学与工程学院,合肥 230009)
摘 要:
采用反应磁控溅射法在ITO玻璃上制备氮掺杂氧化钨(WO3:N)薄膜。采用XRD、XPS、AFM对薄膜的结构、成分、结合键和表面形貌进行表征。将WO3:N薄膜封装制成电致变色器件,并采用直流稳压电源和分光光度计对其进行变色调制性能测试。结果表明:制备的WO3:N薄膜为纳米晶结构,其衍射峰位随着含N量的增加而右移;WO3:N薄膜中W、O分别以W6+和O2-存在,而N以中性价态、WO3中的O位替换以及表面吸附3种状态存在;随着WO3:N薄膜中含N量的升高,表面粗糙度逐渐增大,且有利于器件着色;当掺氮2.80%(摩尔分数)时,电致变色器件调制幅度最大为68.8%,比未掺氮器件的高出7.7%,适用于节能玻璃。
关键词:
中图分类号:TB43 文献标志码:A
Effects of N doping on electrochromic modulation properties of WO3 thin film
WANG Wei, LI He-qin, TAO Lei, QIAO Kai, ZHOU Chu, ZHANG Jing,
TANG Qiong, HUANG Yi-qin, ZUO Min, LI Shi-wei
(School of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China)
Abstract: N doped tungsten oxide (WO3:N) thin films were prepared on ITO coated glass substrate by reactive magnetron sputtering. The structure, composition, chemical bonds and surface morphology of films were characterized by XRD, XPS and AFM, respectively. Furthermore, WO3:N thin films were assembled into electrochromic devices, the modulation properties were investigated by DC regulated power supply and spectrophotometer. The results indicate that WO3:N films are in nanocrystalline structure, whose scattering peaks move right as N content increases. W and O elements exist in W6+ and O2-, respectively, whereas, there are three chemical states for N element, namely, neutral atom, substituting O in W—O bond, and absorption onto film surface during deposition. With N content of WO3:N film increasing, the film surface gets rougher, and this will benefit coloring reaction of the device. The device reaches the highest modulation extent(68.8%, 7.7% higher than that of undoped device) at 2.80%N doping (mole fraction), which is suitable for energy conservation glass.
Key words: WO3:N thin film; reactive magnetron sputtering; electrochromic device; energy conservation
电致变色材料是指在外电场作用下发生可逆颜色变化的一类材料[1]。WO3电致变色薄膜具有着色效率高、稳定性好、无视盲角等优点,但是它存在响应慢、寿命低、调制范围小等问题[2-3],掺杂是改善WO3薄膜性能的有效方法[4]。目前,已有的报道主要是用Ti、Mo等金属元素掺入WO3后替代部分的W原子,进行W位掺杂[5]。PAIPITAK等[6]采用溶胶-凝胶法将Ti掺入WO3薄膜,薄膜结晶度下降,调制幅度提高6.9%。
范芸等[7]采用射频磁控溅射镀制备Mo掺杂WO3薄膜,着色时间缩短,但褪色时间延长。在元素周期表中,N与O同周期,原子序数仅相差1,二者的原子半径相近,电负性、键合性质均较为相似,因此,N是比较理想的可以替换O的元素[8-9]。本文作者采用反应磁控溅射法制备WO3:N薄膜并封装成电致变色器件,通过对WO3的O位N掺杂,改善了WO3的变色调制性能。制备的电致变色器件结构为透明电极层/对电极层/电解质层/电致变色层/透明电极层[10-11],其中透明电极层采用ITO(In2O3:Sn)薄膜;对电极层采用TiO2薄膜[12];电致变色层采用WO3:N薄膜;电解质采用有机凝胶态锂离子电解质[13-14]。
1 实验
用沈科仪FJL560B1型超高真空磁控与离子束联合溅射设备,以纯度为99.99%(质量分数)的金属W为靶材,通入高纯Ar、O2、N2,采用直流反应磁控溅射在ITO玻璃上镀制WO3:N薄膜,通过调节N2的流量来控制掺氮量。用射频反应磁控溅射在ITO玻璃上制备了TiO2对电极薄膜。镀膜前,依次在分析纯级别的丙酮、无水乙醇及去离子水中对衬底进行15 min的超声清洗;真空室本底真空度为2×10-4 Pa,薄膜的制备工艺参数见表1。胶态聚合物电解质制备方法见参考文献[15]。把电解质均匀涂覆于WO3和TiO2薄膜之间,经60 ℃、24 h烘干,制成固态电致变色器件。
用日本理学D/MAX2500VL/PC型X射线衍射仪(Cu Kα射线源)表征薄膜的结构;用ESCALAB250型X射线光电子能谱仪(XPS)检测WO3:N薄膜的表面成分及化学状态;用CSPM4000型原子力显微镜(AFM)测试薄膜的表面形貌特征。器件的变色性能测试采用WYJC-5型直流稳压电源。变色电压选择±3 V,施加负向电压时,Li+从对电极层经电解质层注入电致变色层,电子从透明电极层注入电致变色层,器件变为蓝色,称为着色态;施加正向电压时,Li+和电子脱出,器件恢复无色,称为褪色态[16-17]。以秒表记录着色、褪色响应时间,并以分光光度计(CARY5000)测试器件的着色、褪色态透射谱。
2 结果与讨论
2.1 WO3:N薄膜的结构
WO3:N薄膜的XRD谱如图1所示,其中f(N2)为制备薄膜的N2流量。由图1可知,原位沉积的WO3:N薄膜皆为纳米晶结构。所有薄膜在2θ为24.4°、35.1°、53.1°附近都有宽化的衍射峰;随着N2流量的增加,24.4°的峰位略微右移,当N2流量达到6 mL/min时,衍射峰位右移至24.9°。参照布拉格公式及晶面间距公式,sinθ与点阵常数a成反比,图1表明N的掺入引起WO3点阵畸变,使得点阵常数微量下降。
2.2 WO3:N薄膜的XPS分析
采用X射线光电子能谱仪(XPS)测试WO3:N薄膜的XPS谱如图2所示。各样品均检测到O1s、W4f、C1s峰(C1s为污染所致,但在标定元素时可以作为参照);未通入N2的样品中未探测到N元素,而通入N2的样品均测出了N1s峰,表明向溅射气氛通入N2可以制备掺氮WO3薄膜。表2所列为根据各峰位面积计算出的实际掺氮量。当N2流量为6 mL/min时,薄膜的掺氮量最高为2.80%(摩尔分数),对其全谱中的O1s、W4f、N1s分谱进行分峰拟合,其结果分别如图2(b)、(c)和(d)所示。
O1s拟合出两个峰:530.5 eV、532 eV,分别属于WO3中的O和吸附O[18-19]。W4f出现明显的能级分裂:W4f 7/2和W4f 5/2,二者的结合能分别为35.6 eV和37.7 eV,对应于W6+的4f峰[20-21]。N1s谱经拟合得到3个峰,代表3种化学状态:其中1峰位的W—O(N)键的结合能为398.6 eV,低于中性N原子的1s结合能(399.7 eV),呈现一定的电负性,但又超过WN的N1s结合能(397.3 eV),所以应属于W—O(N)[22];2峰位的399.7 eV为N—N键合,来源于薄膜从外界空气中吸附的N[23];3峰位N—N结合能为401 eV,一般认为是在镀膜过程中,真空室等离子体区的N原子重新结合成N2分子,被薄膜表面捕获,呈现为吸附态[24]。
表1 WO3:N和TiO2薄膜制备工艺参数
Table 1 Preparation parameters of WO3:N and TiO2 thin films
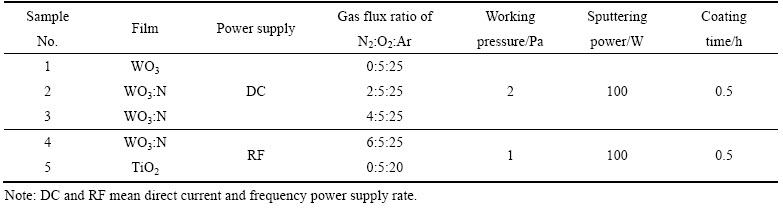
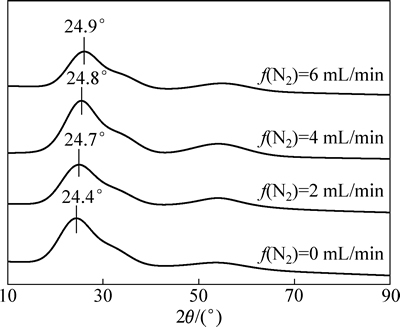
图1 不同N2流量时制备WO3:N薄膜的XRD谱
Fig. 1 XRD patterns of WO3:N thin films prepared under various N2 flux
2.3 WO3:N薄膜的表面形貌(AFM)
图3所示为不同掺氮量的WO3:N薄膜的表面原子力显微形貌。由图3得到薄膜的表面粗糙度列于表3。
从图3和表3可知:薄膜表面的颗粒都较为均匀,未掺氮时,颗粒较小、表面较为平滑;随着掺氮量的增加,颗粒尺寸呈上升趋势,表面粗糙度也逐渐增加。这种变化趋势的成因是:在沉积WO3薄膜时通入N2,由于N替换了部分O,造成了WO3点阵畸变,这个畸变能成为薄膜颗粒长大的驱动力,颗粒间团聚长大的倾向更显著,因此,掺氮WO3薄膜的粗糙度增加。
表2 WO3:N薄膜的N含量
Table 2 N content of WO3:N thin films
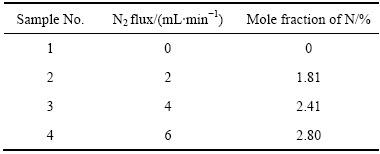
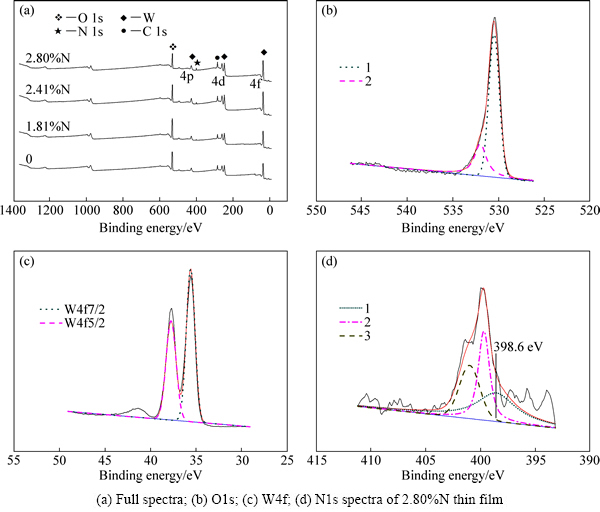
图2 WO3:N薄膜的XPS谱
Fig. 2 XPS spectra of WO3:N thin films
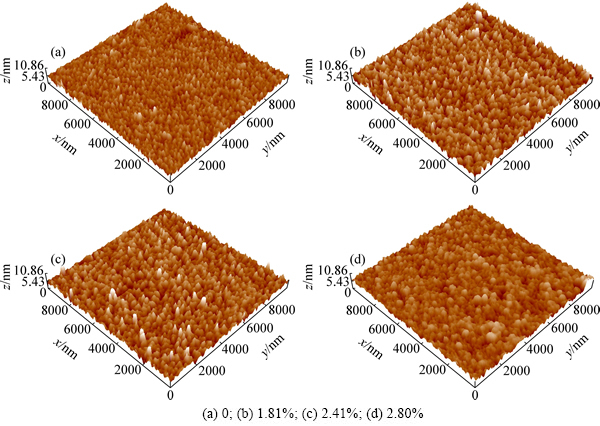
图3 不同掺氮量的WO3:N薄膜的表面AFM形貌
Fig. 3 Surface AFM morphologies of WO3:N thin films with various N contents
表3 WO3:N薄膜表面均方根粗糙度
Table 3 Surface root mean square(RMS) roughness of WO3:N films

2.4 WO3:N电致变色器件的光学调制性能
变色调制是指器件在着色、褪色阶段透过率的差值ΔT (ΔT=Tbleached-Tcolored),ΔT值越大,变色性能越佳。当前,显示装置和节能装置是电致变色器件的两大应用领域;器件对可见光的调制体现在显色的变化,而对近红外区的调制则决定其调节热量的能力[25-26]。将各试样封装成Glass/ITO/TiO2/Li+胶态电解质/WO3:N/ITO/Glass固态器件,施加正、负向外电压,进行着色与褪色测试。图4(a)所示为着色、褪色状态下各样品在390~2000 nm波段的透过率,图4(b)所示为ΔT随入射光波长的变化曲线。表4所列为从图4统计出的透过率定量数据。另外,各器件的变色响应时间也列于表4。
从图4(a)可看出器件经历了两个阶段。
1) 褪色阶段。在约780 nm以下的可见光区,未掺氮器件的透过率最高;在780 nm以上的红外波段,未掺氮以及掺氮1.81%、2.80%器件的透过率相近。变色前后,透明导电层、对电极层、电解质层始终为无色透明,器件的颜色完全由WO3电致变色层所决定。根据图3薄膜表面AFM形貌可知,掺氮导致薄膜粗糙度增加,对入射光的散射增强,使得透过率降低。因此,未掺氮薄膜的颜色更浅、透明度更佳。
2) 着色阶段。在430 nm~800 nm可见光区间,所有器件的透光率均降低;800 nm~1100 nm区间,基本表现为平台;在高于1100 nm的红外波段,器件的透光率均上升。在同一波长下,随着掺氮量的增加,器件透过率下降。目前,关于WO3薄膜电致变色机理的论述,其中化学反应模型(钨青铜模型)指出[27]:着色时,Li+和相等数目的电子注入WO3薄膜,还原成Li原子,然后与WO3生成钨青铜结构,薄膜变蓝;褪色时,发生逆向反应,Li+和电子脱出WO3薄膜,薄膜恢复无色。相比于致密结构,粗糙度越高、表面积越大的薄膜可以容纳更多的Li+和电子,生成的钨青铜数量也越多,透过率更低,外观上体现为颜色更深[28]。

图4 各器件的电致变色透射谱及调制幅度
Fig. 4 Electrochromic transmittance and modulation of each device
表4 器件的透过率和响应时间
Table 4 Transmittance and responding time of devices

掺氮量越高的薄膜,表面越粗糙,变色效果越好。
图4(b)表明:各器件的ΔT在700 nm以下的可见光区均随波长增加呈上升趋势;在红外区均有不同程度的下降,这与SUN等[29]报道的结果相近。表4的数据说明:掺2.80%N的调制幅度ΔT最大,掺1.81%N的次之,二者的ΔT比未掺氮器件分别高7.7%和4.8%。在褪色阶段,掺氮器件在可见光区透过率比未掺氮器件差,但在近红外区与之持平;着色阶段,在整个波长范围,掺氮器件的透过率均明显低于未掺氮器件。
另外,WO3:N 3个器件的响应时间随含N量的增加而缩短,当含N量为2.80%时,响应最快,但仍落后于未掺氮器件。由于掺氮导致WO3点阵畸变,因此,Li+和电子在膜层界面处的扩散受阻,表现为变色响应较慢。
综上所述,合适的掺氮量可以提高器件的调制幅度,特别是在近红外区,这种优势更加显著。因此,WO3:N电致变色器件在对调制时间要求不严格的节能玻璃上具有应用潜力。
图5所示为未掺氮与掺氮器件(2.80%N)的着色、褪色实物效果图。由图5可以看出,掺氮器件褪色态略显浅黄,透过率不及未掺氮器件的;但掺氮器件着色时颜色更深,对比度更大,这与AFM形貌图以及透射谱的变化趋势一致。
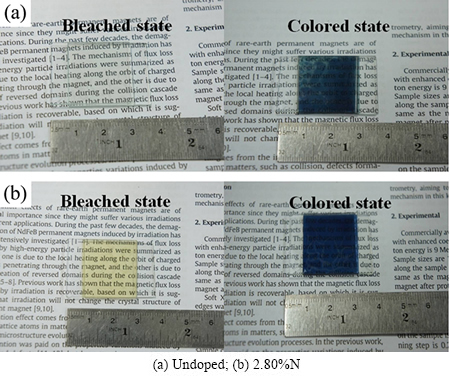
图5 器件的电致变色效果
Fig. 5 Photographs of electrochromic devices in bleached and colored states
3 结论
1) 制备不同掺氮量的WO3:N薄膜皆是纳米晶,掺氮后引起点阵畸变,衍射峰略微右移。
2) 随着制备过程中随N2流量的增加,薄膜的N含量上升,其中2.80%N样品的O1s、W4f、N1s拟合结果表明:W在薄膜中以+6价存在;O主要存在于WO3中;N包括中性价态、代替O与W的键合态、沉积过程中在膜表面形成吸附分子3种状态。
3) 随着N含量的上升,WO3:N薄膜表面粗糙度增加,有利于着色阶段Li+和电子的注入,降低着色态薄膜透光率。
4) N含量为2.80%的器件在测试的全波段调制幅度最大、在近红外区尤为突出,但响应速度不及未掺氮器件,因而,WO3:N电致变色薄膜更加适用于节能玻璃。
REFERENCES
[1] DEB S K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications[J]. Solar Energy Materials & Solar Cells, 2008, 92(2): 245-258.
[2] DYER A L, BULLOCH R H, ZHOU Yin-hua, KIPPELEN B, REYNOLDS J R, ZHANG Feng-ling. A vertical integrated solar-powered electrochromic window for energy efficient buildings[J]. Advanced Materials, 2014, 26(28): 4895-4900.
[3] 董子尧, 李 昕. 电致变色材料、器件及应用研究进展[J]. 材料导报, 2012, 26(7): 50-57.
DONG Zi-yao, LIN Xin. Research progress in electrochromic materials, devices and applications[J]. Materials Review, 2012, 26(7): 50-57.
[4] 胡亚萍, 杨晓红, 张学忠, 马 勇. 掺杂WO3基材料的研究进展[J]. 中国钨业, 2011, 26(3): 41-46.
HU Ya-ping, YANG Xiao-hong, ZHANG Xue-zhong, MA Yong. Research progress on doping WO3-based materials[J]. China Tungsten Industry, 2011, 26(3): 41-46.
[5] CAI Guo-fa, WANG Xiu-li, ZHOU Ding, ZHANG Jia-heng, XIONG Qin-qin, GU Chang-dong, TU Jiang-ping. Hierarchical structure Ti-doped WO3 film with improved electrochromism in visible-infrared region[J]. RSC Advances, 2013, 3(19): 6896-6905.
[6] PAIPITAK K, KAHATTHA C, TECHITDHEERA W, PORNTHEERAPHAT S, PECHARAPA W. Characterization of sol-gel derived Ti-doped tungsten oxide electrochromic thin films[J]. Energy Procedia, 2011, 9: 446-451.
[7] 范 芸, 唐 武, 翁小龙, 邓龙江. 柔性衬底WOx-Mo薄膜电致变色性能研究[J]. 稀有金属材料与工程, 2009, 38(9): 1570-1574.
FAN Yun, TANG Wu, WENG Xiao-long, DENG Long-jiang. Electrochromic properties of WOx-Mo thin films on polyethylene terephthalates soft substrate[J]. Rare Metal Materials and Engineering, 2009, 38(9): 1570-1574.
[8] 赵永红, 孔春阳, 秦国平, 李万俊, 阮海波, 孟祥丹, 卞 萍, 徐 庆, 张 萍. N-In共掺p型ZnO薄膜的结构和电学特性研究[J]. 重庆师范大学学报(自然科学版), 2013, 30(3): 115-120.
ZHAO Yong-hong, KONG Chun-yang, QIN Guo-ping, LI Wan-jun, RUAI Hai-bo, MENG Xiang-dan, BIAN Ping, XU Qing, ZHANG Ping. The investigation on the microstructure and electrical properties of p-type ZnO:In-N films[J]. Journal of Chongqing Normal University (Natural Science), 2013, 30(3): 115-120.
[9] 李 琪, 韩立娟, 刘 刚, 陈作雁, 安兴才. 钒-氮共掺杂TiO2的合成、表征及光催化性能[J]. 环境化学, 2013, 32(6): 1073-1080.
LI Qi, HAN Li-juan, LIU Gang, CHEN Zuo-yan, AN Xing-cai. Synthesis, characterization and degradation performance of V-N-TiO2 nanoparticle photocatalysts[J]. Environmental Chemistry, 2013, 32(6): 1073-1080.
[10] BENEDETTI T M, CARVALHO T, IWAKURA D C, BRAGA F, VIEIRA B R, VIDINHA P, GRUBER J, TORRESI R M. All solid-state electrochromic device consisting of a water soluble viologen dissolved in gelatin-based ionogel[J]. Solar Energy Materials & Solar Cells, 2015, 132: 101-106.
[11] 罗坚义, 周洋洋, 陈润明, 曾庆光, 张 梅. 高质量WO3薄膜的制备及其电致变色数码显示器件[J]. 人工晶体学报, 2013, 42(1): 124-128.
LUO Jian-yi, ZHOU Yang-yang, CHEN Run-ming, ZENG Qing-guang, ZHANG Mei. Preparation of the high quality WO3 thin film and its application in the electrochromic digital display devices[J]. Journal of Synthetic Crystals, 2013, 42(1): 124-128.
[12] SONG Xing-wang, DONG Guo-bo, GAO Fang-yuan, XIAO Yu, LIU Qi-rong, DIAO X G. Properties of NiOx and its influence upon all-thin-film ITO/NiOx/LiTaO3/WO3/ITO electrochromic devices prepared by magnetron sputtering[J]. Vacuum, 2015, 115: 48-54.
[13] SHAPLOV A S, PONKRATOV D O, AUBERT P H, LOZINSKAYA E I, PLESSE C, VIDAL F, VYQODSKII Y S. A first truly all-solid state organic electrochromic device based on polymeric ionic liquids[J]. Chemical Communications, 2014, 50(24): 3191-3193.
[14] KIRISTI M, BOZDUMAN F, GULEC A, TEKE E, OKSUZ L, OKSUZ A U, DELIGOZ H. Complementary all solid state electrochromic devices using carboxymethyl cellulose based electrolytes[J]. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 2014, 51(6): 481-487.
[15] SOUZA F L, BUENO P R, LONGO E, LEITE E R. Sol-gel nonhydrolytic synthesis of a hybrid organic-inorganic electrolyte for application in lithium-ion devices[J]. Solid State Ionics, 2004, 3(7): 83-88.
[16] 张学科, 李合琴, 周 矗, 颜毓雷, 王 伟, 乔 恺. 晶态WO3电致变色薄膜和器件的制备及性能研究[J]. 合肥工业大学学报(自然科学版), 2014, 37(5): 534-537.
ZHANG Xue-ke, LI He-qin, ZHOU Zhu, YAN Yu-lei, WANG Wei, QIAO Kai. Preparation and properties of crystalline WO3 electrochromic thin film[J]. Journal of Hefei University of Technology (Natural Science), 2014, 37(5): 534-537.
[17] 罗 畅, 周白杨, 黄 涛, 吴武地. 制备工艺对氧化钨电致变色薄膜性能的影响[J]. 功能材料与器件学报, 2012, 18(2): 165-171.
LUO Chang, ZHOU Bai-yang, HUANG Tao, WU Wu-di. Impact of preparation technique on the electrochromic properties of tungstic oxide film[J]. Journal of Functional Materials and Devices, 2012, 18(2): 165-171.
[18] LEE B G, CHIO J W, LEE S E, JEONG Y S, OH H J, CHI C S. Formation behavior of anodic TiO2 nanotubes in fluoride containing electrolytes[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(4): 842-845.
[19] YE Wei-chun, MA Chuan-li, WANG Chun-ming, ZHOU Feng. Electroless deposition of W-doped Ag films onto p-Si(100) from diluted HF solution[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(6): 1474-1478.
[20] YANG Li-li, GE Deng-teng, ZHAO Jiu-peng, DING Yan-bo, KONG Xian-ping, LI Yao. Improved electrochromic performance of ordered macroporous tungsten oxide films for IR electrochromic device[J]. Solar Energy Materials & Solar Cells, 2012, 100: 251-257.
[21] WANG Jin-zhong, ELANGOVAN E, FRANCO N, ALVESE A, REGO A, MARTINS R, FORTUNATO E. Influence of oxygen partial pressure on properties of N-doped ZnO films deposited by magnetron sputtering[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(12): 2326-2330.
[22] PARK M H, KIM S H. Temperature coefficient of resistivity of TiAlN films deposited by radio frequency magnetron sputtering[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(2): 433-438.
[23] 石 倩, 任建坤, 王玉萍, 彭盘英, 王维安. N掺杂对钛酸铋复合TiO2催化剂的形貌和性能的影响[J]. 中国有色金属学报, 2013, 23(2): 487-494.
SHI Qian, REN Jian-kun, WANG Yu-ping, PENG Pan-ying, WANG Wei-an. Effect of N doping on morphology and property of bismuth titanate TiO2 composite catalyst[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 487-494.
[24] WANG Chun-kai, LIN Chung-kwei, WU Ching-lin, BRAHMA S, WANG Sheng-chang, HUANG Jow-lay. Characterization of electrochromic tungsten oxide film from electrochemical anodized RF-sputtered tungsten films[J]. Ceramics International, 2013, 39(4): 4293-4298.
[25] 陈 怡, 徐 征, 孙金礼, 邓恒涛, 陈海涛, 赵谡玲. 大面积智能电致变色玻璃的产业化现状及未来[J]. 功能材料, 2013, 44(17): 2441-2446.
CHEN Yi, XU Zheng, SUN Jin-li, DENG Heng-tao, CHEN Hai-tao, ZHAO Su-ling. Commercialization of large area electrochromic smart glasses: Present and future[J]. Journal of Functional Materials, 2013, 44(17): 2441-2446.
[26] 陈中威, 强鹏飞, 杨培华, 麦文杰, 李 玲. 热蒸镀法制备WO3薄膜及其电致变色性能[J]. 暨南大学学报(自然科学与医学版), 2014, 35(2): 196-200.
CHEN Zhong-wei, QIANG Peng-fei, YANG Pei-hua, MAI Wen-jie, LI Ling. Preparation of tungsten trioxide film by thermal evaporation method and its electrochromic performance[J]. Journal of Jinan University (Natural Science & Medical Edition), 2014, 35(2): 196-200.
[27] 牛 微, 毕孝国, 孙旭东. 电致变色机理的研究现状与发展[J]. 材料导报, 2011, 25 (2): 107-110.
NIU Wei, BI Xiao-guo, SUN Xu-dong. Research and development of electrochromic mechanism[J]. Materials Review, 2011, 25 (2): 107-110.
[28] 杨海刚, 宋桂林, 张基东, 王天兴, 常方高. WO3薄膜的电致变色与响应时间机理研究[J]. 人工晶体学报, 2011, 40(5): 1316-1321.
YANG Hai-gang, SONG Gui-lin, ZHANG Ji-dong, WANG Tian-xing, CHANG Fang-gao. Response time and electrochromic mechanism of WO3 films[J]. Journal of Synthetic Crystals, 2011, 40(5): 1316-1321.
[29] SUN Xi-lian, LIU Zhi-min, CAO Hong-tao. Electrochromic properties of N-doped tungsten oxide thin films prepared by reactive DC-pulsed sputtering[J]. Thin Solid Films, 2011, 3(16): 3034.
(编辑 李艳红)
基金项目:安徽省高校自然科学基金资助项目(KJ2009A091,KJ2012A228);中国科学院战略性先导科技专项资助项目(XDA03040000)
收稿日期:2014-12-09;修订日期:2015-07-08
通信作者:李合琴,教授,博士;电话:0551-62901405;E-mail:lhqjs@hfut.edu.cn
摘 要:采用反应磁控溅射法在ITO玻璃上制备氮掺杂氧化钨(WO3:N)薄膜。采用XRD、XPS、AFM对薄膜的结构、成分、结合键和表面形貌进行表征。将WO3:N薄膜封装制成电致变色器件,并采用直流稳压电源和分光光度计对其进行变色调制性能测试。结果表明:制备的WO3:N薄膜为纳米晶结构,其衍射峰位随着含N量的增加而右移;WO3:N薄膜中W、O分别以W6+和O2-存在,而N以中性价态、WO3中的O位替换以及表面吸附3种状态存在;随着WO3:N薄膜中含N量的升高,表面粗糙度逐渐增大,且有利于器件着色;当掺氮2.80%(摩尔分数)时,电致变色器件调制幅度最大为68.8%,比未掺氮器件的高出7.7%,适用于节能玻璃。
[3] 董子尧, 李 昕. 电致变色材料、器件及应用研究进展[J]. 材料导报, 2012, 26(7): 50-57.
[4] 胡亚萍, 杨晓红, 张学忠, 马 勇. 掺杂WO3基材料的研究进展[J]. 中国钨业, 2011, 26(3): 41-46.
[7] 范 芸, 唐 武, 翁小龙, 邓龙江. 柔性衬底WOx-Mo薄膜电致变色性能研究[J]. 稀有金属材料与工程, 2009, 38(9): 1570-1574.
[9] 李 琪, 韩立娟, 刘 刚, 陈作雁, 安兴才. 钒-氮共掺杂TiO2的合成、表征及光催化性能[J]. 环境化学, 2013, 32(6): 1073-1080.
[11] 罗坚义, 周洋洋, 陈润明, 曾庆光, 张 梅. 高质量WO3薄膜的制备及其电致变色数码显示器件[J]. 人工晶体学报, 2013, 42(1): 124-128.
[16] 张学科, 李合琴, 周 矗, 颜毓雷, 王 伟, 乔 恺. 晶态WO3电致变色薄膜和器件的制备及性能研究[J]. 合肥工业大学学报(自然科学版), 2014, 37(5): 534-537.
[17] 罗 畅, 周白杨, 黄 涛, 吴武地. 制备工艺对氧化钨电致变色薄膜性能的影响[J]. 功能材料与器件学报, 2012, 18(2): 165-171.
[23] 石 倩, 任建坤, 王玉萍, 彭盘英, 王维安. N掺杂对钛酸铋复合TiO2催化剂的形貌和性能的影响[J]. 中国有色金属学报, 2013, 23(2): 487-494.
[25] 陈 怡, 徐 征, 孙金礼, 邓恒涛, 陈海涛, 赵谡玲. 大面积智能电致变色玻璃的产业化现状及未来[J]. 功能材料, 2013, 44(17): 2441-2446.
[26] 陈中威, 强鹏飞, 杨培华, 麦文杰, 李 玲. 热蒸镀法制备WO3薄膜及其电致变色性能[J]. 暨南大学学报(自然科学与医学版), 2014, 35(2): 196-200.
[27] 牛 微, 毕孝国, 孙旭东. 电致变色机理的研究现状与发展[J]. 材料导报, 2011, 25 (2): 107-110.
[28] 杨海刚, 宋桂林, 张基东, 王天兴, 常方高. WO3薄膜的电致变色与响应时间机理研究[J]. 人工晶体学报, 2011, 40(5): 1316-1321.


