
Preparation and characterization of LiFePO4 thin films as cathode materials for lithium ion battery
XIAO Zhuo-bing(肖卓炳), MA Ming-you(麻明友)
College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China
Received 20 April; accepted 30 June 2006
Abstract:
LiFePO4 thin films were prepared by sol-gel technique. The phase and surface morphology were characterized by X-ray diffraction and scanning electron microscopy. The electrochemical properties of the thin films were measured by cyclic voltammetry, galvanostatic charge-discharge experiments and electrochemical impedance spectroscopy in 1 mol/L LiPF6/EC-DMC solution using lithium metal as both counter and reference electrodes. The films prepared by this method are of pure LiFePO4 phase. The capacity of the film annealed at 700 ℃ for 30 min is 145 mA?h/g, and the capacity loss per cycle is 0.06% after being cycled 50 times. The electrochemical impedance spectroscopy shows that the diffusion rate of lithium ion in LiFePO4 thin film is 5.1×10-14cm2/s.
Key words:
thin films; lithium iron phosphate; lithium-ion battery; sol-gel;
1 Introduction
Since the commercialization of the lithium ion-battery LiCoO2/C by Sony in 1991, this kind of battery has drawn a great attention[1-6]. However, compared with other batteries, the battery is relatively expensive due to the scarcity of cobalt. Therefore, a lot of research was conducted for alternative cathode materials with low cost and non-toxicity.
Due to the work of PADHI et al[7], mixed orthophos- phates LiMPO4(M=Mn, Fe, Co) isostructural to olivine have been intensively studied as lithium insertion compounds for lithium batteries[8-14]. Orthorhombic LiFePO4 could be used as a positive electrode for rechargeable lithium batteries. This compound, having a theoretical capacity of 170 mA?h/g, is environmentally benign and inexpensive, and shows good cycle stability due to the structural similarity in the charged and discharged states. These properties make it an attractive candidate for large batteries that are to be required for electric and hybrid electric vehicles.
2 Experimental
The stoichiometric amount of LiH2PO4 and Fe(NO3)3·9H2O were dissolved in a small amount of deionized water. Then ethanol was added to adjust the viscosity and wetting property of the solution. The wet films were obtained by spin coating LiFePO4 precursor solution onto the substrate and then heated at 300 ℃ in air for 10 min to remove solvents and other organic substances at a heating rate of 10 ℃/min. The deposition and heat treatment procedures were repeated to prepare a desired thickness of films. The multilayered films were finally annealed at high temperature for 30 min under a atmosphere of V(N2):V(H2)=9:1 to make them crystalline. Fig.1 shows the flow chart of the preparation of LiFePO4 thin films by sol-gel technique.
The thermal decomposition behavior of the dried LiFePO4 precursor gel, which was derived from heating the precursor solution at 120 ℃ for 40 min, was examined with a METTLER TOLEDO TGA/SDT851e instrument by thermogravimetric analysis(TGA) and differential thermal analysis(DTA) at a heating rate of 10 ℃/min.
The phase identification and surface morphology studies were conducted with an X-ray diffractometer (XRD) and scanning electron microscopy(SEM). The transmittance spectra in the range of 200-1 500 cm-1 were obtained by a Perkin-Elmer PE983 spectrometer. For electrochemical measurements, LiMn2O4 thin films deposited on Pt-coated substrate were placed in an open beaker cell which contained 1 mol/L LiPF6 dissolved in

Fig.1 Flow chart showing preparation of LiFePO4 thin films by sol-gel technique
ethylene carbonate(EC) and dimethyl carbonate(DMC) (1:1, volumetric ratio). Lithium metal was used as both counter and reference electrodes. The entire cell was assembled in an argon-filled glove box. The galvanostatic charge-discharge experiments were conducted between the cut-off voltages of 3.9 V and 3.1 V under a constant current density of 100 mA/cm2. The cyclic voltammograms were performed at 1 mV/min. For the measurement of chemical diffusion coefficients, it was done with the technique of electrochemical impedance spectroscopy.
3 Results and discussion
The TGA and DTA results of the dried LiFePO4 precursor gel are shown in Fig.2. As seen in the figure, the mass loss of the gel terminates at 550 ℃. The mass loss below the temperature of 130 ℃ can be ascribed to the evaporation of the absorbed water in the air, which corresponds to an endothermic peak in the DTA curve. The mass loss in the temperature range 130-550 ℃ is associated with the decomposition and combustion of constituents in the powder, which correspond to several exothermic peaks in the curve of DTA.
The XRD patterns of LiFePO4 thin films are shown in Fig.3. As seen in the figure, more than 20 reflections can be indexed in the Pnmb space group with the lattice parameters a=10.317 9 ?, b=6.003 4 ?, c=4.691 1 ?, in perfect agreement with triphylite LiFePO4 (JCPDS card No. 42-0580). No impurities are detected with the XRD results, indicating that the synthesized thin film is of pure LiFePO4.
Table 1 lists the wavenumber of infrared spectra of LiFePO4 prepared under different conditions. As shown

Fig.2 TGA and DTA results of dried LiFePO4 precursor gel

Fig.3 XRD patterns of LiFePO4 thin film annealed at various temperatures for 30 min
Table 1 Infrared peaks of LiFePO4 prepared under different conditions (cm-1)
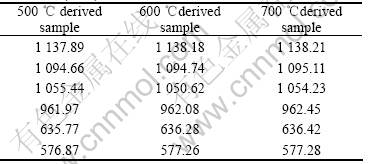
in the table, except the peak at about 1 050 cm-1, the peaks of the samples shift slightly to high wavenumber. This means that the P—O link becomes stronger, which may weaken the Li—O link and make the deinter- calation of lithium ion become much easier.
The cyclic voltammogram of the LiFePO4 thin film annealed at 700 ℃ is presented in Fig.4. As observed in the figure, one reduction and oxidation process appear, characterized by the corresponding cathodic and anodic peak potentials respectively. The two peaks are symmetrical, indicating that the film possesses high coulombic efficiency.
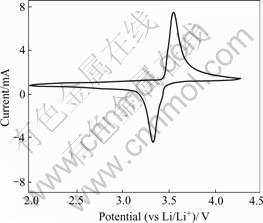
Fig.4 Cyclic voltammogram of LiFePO4 thin film annealed at 700℃
The charge-discharge curves of LiFePO4 thin films are displayed in Fig.5. As observed in the figure, the discharge curves show a voltage plateau at about 3.5 V. The discharge capacity of the film increases from 134 mA?h/g to 145 mA?h/g as annealing temperature increases from 500 ℃ to 700 ℃, which may be caused by the improvement of crystallinity of the films.
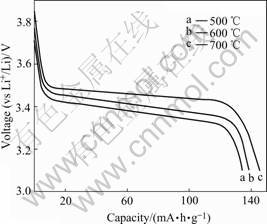
Fig.5 Discharge curves of LiFePO4 thin films annealed at different temperatures for 30 min
Table 2 presents the variation of capacity as a function of cycle number for LiFePO4 thin films annealed under different conditions. The cycling behavior becomes better as the annealing temperature increases from 500 ℃ to 700 ℃. The capacity fading of the thin films annealed at 700 ℃ is very small. The capacity loss per cycle after being cycled 50 times is only about 0.06%, suggesting that the film has good rechargeability.
Fig.6 shows the electrochemical impedance spectro- scopy of LiFePO4 thin film annealed at 700 ℃ for 30
Table 2 Cycling behavior of LiFePO4 thin films annealed at various temperatures for 30 min

Fig.6 Electrochemical impedance spectroscopy of LiFePO4 thin-film electrode at open circuit voltage of 3.52 V
min. The measurement was performed at 3.56 V after preliminary potentiostatic polarization at the same potential for over 1 h to achieve equilibrium. The impedance spectroscopy consists of a semicircle in a high frequency, a line inclined at 45? to the real axis in the low frequency range, and at very low frequency, the 45? begins to give way to a more or less vertical line corresponding to finite space diffusion process. This is because of the finite thickness of thin film, which limits the extent of the diffusion behavior at the very low frequency, and results in a ‘redox capacitance’. The semicircle in the high frequency range is due to the ‘charge transfer reactions’ at the interface of the electrolyte/oxide electrode, and the inclined line in the low frequency range is attributable to ‘Warburg impedance’ that is associated with lithium diffusion through the oxide electrode.
In the equivalent circuit (Fig.7), Re is the electrolyte

Fig.7 Equivalent circuit
resistance, Rct the charge-transfer resistance, Cdl the double layer capacitor, Zw the Warburg impedance, and CL the intercalation capacitance.
Under semi-infinite conditions (v?2DLi/h2), the Warburg impedance can be expressed as[15]
![]() (1)
(1)
![]() (2)
(2)
Therefore
![]() (3)
(3)
where s is the Warburg coefficient, which can be obtained from the slope of Z? versus v-1/2 or Z? versus v-1/2, n is the number of electrons per molecule oxidized or reduced, VM is the molecular volume, dE/dx is the slope of the coulometric titration curve versus mobile ion concentration x at each x value. The insertion capacitance CL as a function of dE/dx is expressed as [16]:
![]() (4)
(4)
where mf is the mass of the film, dQ/dE is the differential specific capacity of LiFePO4 at each potential, and Q1 is the specific capacity of LiFePO4 (here we used the theoretical value as Q1=612 C?g-1). In addition,
![]() (5)
(5)
wherre Vf is the volume of the film, and Mw is the relative molecular mass of LiFePO4. Combining Eqns.(1), (4) and (5), the following equation can be obtained.
![]() (6)
(6)
where h is the maximum length of the diffusion pathway (the film thickness). CL can be obtained directly from the imaginary part of the impedance in the finite diffusion range by using Eqn.(3).
According to the above equations, the diffusion coefficient at different open circuit voltage can be calculated. The average chemical diffusion coefficient of lithium ion in the thin film is about 5.1?10-14 cm2/s.
4 ConclusionsLiFePO4 thin films were successfully prepared by sol-gel method. As the annealing temperature increases from 600 ℃ to 700 ℃, the capacity of the films increases from 134 mA?h/g to 145 mA?h/g. The film annealed at 700 ℃ for 30 min possesses the best cycling behavior with a capacity loss per cycle of 0.06% after being cycled 50 times. The electrochemical impedance spectroscopy shows that the diffusion of lithium ion in LiFePO4 thin film is 5.1?10-14cm2/s.
References[1] MOLENDA J, PA?UBIAK D, MARZEC J. Transport and electrochemical properties of the LiyCrxMn2-xO4 (0
[2] HAN C J, EOM W S, LEE S M, CHO W I, JANG H. Study of the electrochemical properties of Ga-doped LiNi0.8Co0.2O2 synthesized by a sol-gel method [J]. J Power Sources, 2005, 144: 214-219.
[3] SURESH P, RODRIGUES S, SHUKLA A K, VASAN H N, MUNICHANDRAIAH N. Synthesis of LiCo1-xMnxO2 from a low-temperature route and characterization as cathode materials in Li-ion cells [J]. Solid State Ionics, 2005, 176: 281-290.
[4] HONG Y S, PARK Y J, RYU K S, CHANG S H. Charge/discharge behavior of Li[Ni0.20Li0.20Mn0.60]O2 and Li[Co0.20Li0.27Mn0.53]O2 cathode materials in lithium secondary batteries [J]. Solid State Ionics, 2005, 176: 1035-1042.
[5] TANIGUCHI I. Powder properties of partially substituted LiMxMn2-xO4 (M=Al, Cr, Fe and Co) synthesized by ultrasonic spray pyrolysis [J]. Mater Chem Phy, 2005, 92: 172-179.
[6] SURESH P, SHUKLA A K, MUNICHANDRAIAH N. Capacity stabilization of layered Li0.9Mn0.9Ni0.1O2 cathode material by employing ZnO coating[J]. Mater Lett, 2005, 59: 953-958.
[7] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144: 1188-1192.
[8] YAMADA A, CHUNG S C, HINOKUMA K. Optimized LiFePO4 for lithium battery cathodes [J]. J Electrochem Soc, 2001, 148: A224-229.
[9] TAKEUCHI T, TABUCHI M, NAKASHIMA A, NAKAMURA T, MIWA Y, KAGEYAMA H, TATSUMI K. Preparation of dense LiFePO4/C composite positive electrodes using spark-plasma- sintering process [J]. J Power Sources, 2005, 146: 575-579.
[10] KIM C W, LEE M H, JEONG W T, LEE K S. Synthesis of olivine LiFePO4 cathode materials by mechanical alloying using iron(Ⅲ) raw material [J]. J Power Sources, 2005, 146: 534-538.
[11] SHIRAISHI K, DOKKO K, KANAMURA K. Formation of impurities on phospho-olivine LiFePO4 during hydrothermal synthesis [J]. J Power Sources, 2005, 146: 555-558.
[12] OSORIO-GUILLEN J M, HOLM B, AHUJA R, JOHANSSON B. A theoretical study of olivine LiMPO4 cathodes [J]. Solid State Ionics, 2004, 167: 221-227.
[13] TAJIMI S, IKEDA Y, UEMATSU K, TODA K, SATO M. Enhanced electrochemical performance of LiFePO4 prepared by hydrothermal reaction [J]. Solid State Ionics, 2004, 175: 287-290.
[14] TAKAHASHI M, TOBISHIMA S, TAKEI K, SAKURAI Y. Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries [J]. Solid State Ionics, 2002, 148: 283-289.
[15] THOMAS M G S R, BRUCE P G, GOODENOUGH J B. AC impedance of the Li(1-x)CoO2 electrode [J]. Solid State Ionics, 1986, 18-19: 599-1235.
[16] FUNABIKI A, INABA M, OGUMI Z, YUASA S, OTSUJI J, TASAKA A. Impedance study on the electrochemical lithium intercalation into natural graphite powder [J]. J Electrochem Soc, 1998, 145: 172-178.
Corresponding author: XIAO Zhuo-bing; Tel: +86-743-8563911; E-mail: xiaoyddd@163.com


