DOI:10.19476/j.ysxb.1004.0609.2019.04.18
类铝冶金的废铅膏低温还原清洁炼铅的技术思路
刘伟锋,张坤坤,邓循博,张杜超,陈 霖,杨天足
(中南大学 冶金与环境学院,长沙 410083)
摘 要:
废铅酸蓄电池是最重要的再生铅资源,通常先拆解再分别回收利用,由于废铅膏中存在大量硫酸铅,使其成为废铅酸蓄电池资源化利用的瓶颈。废铅膏处理传统火法工艺和湿法工艺均是以阴极铅为目标产物,其中火法工艺获得了广泛应用,近些年材料冶金思路则是绕开废铅膏制备阴极铅的过程,用废铅膏直接制备铅酸蓄电池用的铅粉。借鉴铝冶炼工业由铝土矿到氧化铝再到金属铝的工艺思路,本文提出一种基于水热还原转化的废铅膏低温还原清洁炼铅工艺:首先,废铅膏通过硫酸浸煮脱除杂质;其次,浸煮渣在碱和还原剂同时存在下水热处理,使硫酸铅和二氧化铅均转化为氧化铅;最后,低温熔盐中用淀粉还原氧化铅产出金属铅。该工艺为废铅膏处理的工艺改革提供一种新的思路。
关键词:
文章编号:1004-0609(2019)-04-0810-11 中图分类号:TF812;TM912.1 文献标志码:A
铅是银白色重有色金属,基于其特殊的物理化学性质被广泛应用于铅蓄电池、化学防腐、焊料等行业,其中铅酸蓄电池是最主要的消费领域,占全球总铅消耗量的80.0%以上[1]。铅酸蓄电池具有性价比高、技术成熟和安全性能好等优点,被广泛应用于汽车、储能和电动车等诸多国民经济重要领域[2-5],铅酸蓄电池使用量占全球二次电池市场份额的70.0%以上。近些年我国汽车行业的快速崛起促进了铅酸蓄电池行业的持续发展,目前我国已经成为世界上最大的铅酸蓄电池制造国、消费国和出口国[6-7],铅酸蓄电池的大量使用势必会产生相当数量的废铅酸蓄电池,使得废铅酸蓄电池已经变成最重要的再生铅资源[8]。
根据国家统计局和中国有色金属工业协会数据,2014年中国精炼铅产量达到421.8万t,其中再生铅产量为160.0万t,占全年铅产量的37.9%[9],随着铅酸蓄电池使用量和报废量的迅速增加,再生铅所占比例将进一步增加。利用再生资源每生产1 t金属铅,其生产成本和能耗比从矿产铅生产分别降低约38%和33%[10]。所以,发展再生铅产业,使铅进入生产-消费-再生的系统内良性循环,不但可以缓解铅资源日益枯竭的局面,同时可以降低生产成本并减少环境污染,对于促进我国铅工业的可持续发展具有战略意义。
铅酸蓄电池在长期的充放电过程,由于极板硫酸盐化、板栅腐蚀和活性物质软化脱落等原因,导致其无法正常进行充放电作业,即报废产出废铅酸蓄电池。由于铅酸蓄电池使用过程是全密闭式,在使用过程中是相对清洁的,因此铅酸蓄电池导致的铅污染主要来源于铅酸蓄电池生产和回收环节。为了减少铅酸蓄电池对环境的污染,最重要的是开发清洁高效的回收铅技术,以减少回收过程对环境的二次污染[11]。
本文在充分对比废铅酸蓄电池铅膏的不同处理工艺的基础上,针对废铅膏还原熔炼存在的问题,借鉴铝冶炼工业由铝土矿到氧化铝再到金属铝的工艺思路,提出一种类铝冶金的废铅膏低温还原清洁炼铅的技术路线,拟实现废铅膏间接低温还原熔炼的清洁生产目标,为废铅膏资源化利用提供新的发展方向。
1 废铅酸蓄电池拆解
废铅酸蓄电池各部分成分不同且性质各异,通常先破碎拆解,再经分选后分别回收利用。不同企业的破碎分选系统所使用的设备有所不同,应用较多是意大利Engitec公司的CX破碎分选系统[12]和美国MA公司的MA破碎分选系统[13]。破碎分选工艺过程首先是粗碎废电池使其中的电解液流出,然后破碎至一定尺寸的碎片,再在滚筒中用水冲洗,最后根据密度和粒度差别在水中分选产出板栅和塑料,料浆经过压滤出废铅膏。
废铅酸蓄电池拆解产物及废铅膏处理工艺见图1,拆解产物有四种:一是废电解液,其成分为硫酸溶液,其质量占蓄电池总质量的10%~20%,通常返回使用或送废水处理;二是板栅与连接头,主要成分为铅锑合金,其质量占蓄电池总质量的20%~30%,通常经过重新熔化后再铸成合金板栅使用;三是有机物,包括壳体和隔板,其材质主要为塑料或橡胶,其质量占总质量的10%~15%,通常制成颗粒后资源化利用;四是铅膏,主要是铅的氧化物和硫酸盐,其质量占总质量的35%~50%。铅膏是正负极板上活性物质经过充放电后形成的浆状物质,由于电池生产厂家不同和报废程度差异,各组分质量分数有所波动,通常为PbSO4(40%~60%)、PbO2(25%~35%)、PbO(5%~10%)和Pb(1%~5%)及少量杂质[14-17]。由于废铅膏中存在大量的硫酸铅与各种价态的铅氧化物,使其成为废铅酸蓄电池资源化利用的瓶颈。
由图1可以看出,废铅膏处理分为火法工艺和湿法工艺,这两种工艺通常都是以阴极铅为目标产物,各有利弊,其中火法工艺获得了广泛应用。而近些年材料冶金思路被提出,即绕开废铅膏制备阴极铅的过程,直接用废铅膏制备铅酸蓄电池用的铅粉,仍处于研究阶段。
2 废铅膏火法处理工艺
自21世纪90年代后,以熔池熔炼为主的火法炼铅技术获得较大发展,即铅精矿经过氧化熔炼和还原熔炼过程产出粗铅,粗铅电解精炼产出阴极铅。随着矿产铅冶炼技术的进步,废铅膏火法处理工艺沿用了该技术过程,即废铅膏还原熔炼产出粗铅后再电解精炼得到阴极铅,火法处理工艺具有技术成熟、处理量大、经济性好等优点,目前仍占据铅膏处理工艺的主导地位。铅膏的火法处理工艺分为单独熔炼工艺和混合熔炼工艺。
2.1 单独熔炼工艺
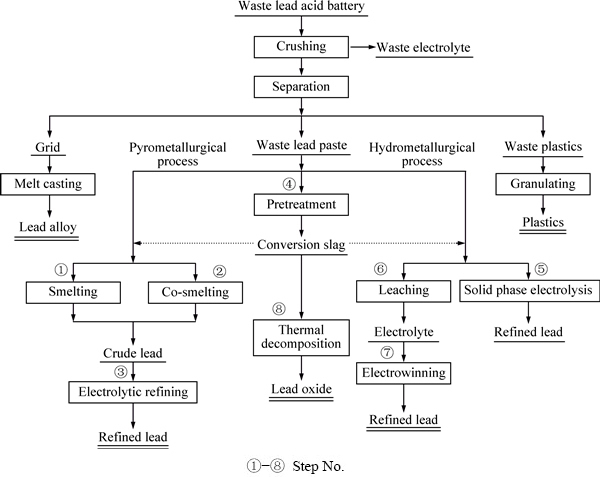
图1 废铅酸电池拆解产物及废铅膏处理工艺示意图
Fig. 1 Flow sheet of disposing spent lead acid battery and extracting lead from waste lead paste
单独熔炼工艺指废铅膏经过还原熔炼产出粗铅,分为直接和和间接两种方式,直接单独熔炼方法是指废铅膏在反射炉、鼓风炉、短窑和富氧侧吹炉[11,18-21]等熔炼炉中用焦炭或煤直接还原熔炼产出粗铅,如图1中步骤①+③所示。该方法具有流程短的优点,但是存在熔炼温度高和烟气处理难度大等缺点,普遍认为是废铅膏中的硫酸铅引起的[22]。
为了消除硫酸铅的不良影响,研究人员提出间接单独熔炼方法,即废铅膏首先经过预处理以脱除硫酸根,然后脱硫产物再用焦炭或粉煤还原熔炼产出粗铅[23-24],如图1中步骤④+①+③所示。预处理过程虽然有效地改善了后续熔炼过程,但是存在的脱硫不彻底和生产成本高等缺点往往限制了间接单独熔炼方法的推广。另外,为了简化预处理脱硫过程,有研究人员将废铅膏与纯碱混合后还原熔炼[25],虽然缩短了处理工艺流程,同时也改善了还原熔炼过程的操作条件,但是却带来炉砖侵蚀和熔炼渣后续处理等新的难题。
2.2 混合熔炼工艺
混合熔炼方法是将废铅膏与铅精矿搭配后熔炼,利用铅精矿的化学反应热,使两者同步熔炼产出粗铅,熔炼烟气用于制备硫酸,如图1中步骤②+③所示。具有代表性的是河南豫光金铅公司采用氧气底吹炉熔炼系统处理铅精矿和铅膏混合物料[26],混合物料首先在氧化底吹炉熔炼并产出含铅50%以上的高铅渣,然后高铅渣在还原底吹炉产出粗铅,其中混合物料中铅膏的配入量已经达到30%~40%。该方法在原生铅冶炼厂获得了广泛应用。
虽然混合熔炼工艺表面上解决了能耗高和环境污染严重的问题,但是存在两方面问题,一是将杂质含量低的高品位铅膏与铅精矿混合熔炼,相当于铅膏中相对纯净的铅被铅精矿或熔剂中的杂质元素“污染”,必须电解精炼提纯。二是铅膏的配入,不仅导致原生铅冶炼系统炉况恶化,如炉内热平衡紊乱和熔炼渣铅含量升高等,而且硫酸铅主要分解产物SO3在烟气制酸的淋洗环节又以污酸形式产出,即废铅膏中的硫酸根经过混合熔炼后又以硫酸根形式产出,所以,混合熔炼过工艺只是巧妙地将问题隐藏了起来。
3 废铅膏湿法处理工艺
为了克服废铅膏火法处理工艺的缺点,国内外的研究人员开发了铅膏湿法回收工艺,即废铅膏经过预处理后再电解或电积产出阴极铅,预处理过程则是采用化学方法改变废铅膏中铅的存在物相,为后续工序提取铅创造有利条件,目前典型的铅膏湿法处理工艺主要有固相电解法、浸出-电积法和转化-浸出-电积法共三种类别。
3.1 预处理方法
废铅膏预处理方法主要完成脱硫和还原两个目的,如图1中步骤④所示。第一个目的是脱除废铅膏中PbSO4的硫酸根,同时使硫酸铅转化为碳酸铅或氢氧化铅;脱硫转化过程使用的转化剂主要有Na2CO3、K2CO3、(NH4)2CO3、NH4HCO3、NaOH和KOH[14,27-28]等,可以看出,脱硫过程通常在碱性体系中进行,存在的典型问题有脱硫率低和脱硫副产物硫酸钠开路困难;第二个目的是将废铅膏中PbO2还原为PbO,还原过程采用的还原剂有Na2SO3、H2O2、FeSO4、Na2S2O3和H2C2O4[29-32]等,而还原过程在酸性体系中还原,存在的典型问题是还原率低和试剂消耗量大,这两个过程必须分开进行。
理论上该预处理方法是非常完美的,但在实际操作中硫酸根的脱除率和二氧化铅的还原率均较低,主要原因是在充放电过程铅酸蓄电池中的硫酸铅和二氧化铅相互包裹,试剂很难与被包裹的物相发生反应。脱硫不彻底和还原效率低严重影响了预处理方法的应用效果,研究人员提出采用超重力[7]、球磨[33]和超声波[25]等手段强化转化脱硫过程,但仍然达不到期望的效果,所以,采用何种方法能实现完全转化脱硫和还原转化两个目的则是废铅膏处理的关键。
3.2 固相电解工艺
固相电解工艺是将废铅膏涂布于阴极板,以不溶金属板作为阳极,在碱性体系电解产出阴极铅,如图1中步骤⑤所示,该方法的最大优点是工艺流程短[34],但由于废铅膏中不同含铅组分在电解过程放电条件不一致,存在电解效果差且电流效率低的缺点。于是研究人员提出采用焙烧[35]和湿法氧化[36-37]等强化方式,将废铅膏全部转化为硫酸铅,以提高电解效率,焙烧法是将废铅膏和电解液混合焙烧使铅全部转化为PbSO4,而湿法氧化法是在电解液中用HNO3或H2O2铅膏中的铅物相全部转化为PbSO4,大大改善了固相电解过程。
3.3 浸出-电积工艺
为了克服固相电解过程的缺点,开发了浸出-电积工艺,如图1中步骤⑥+⑦所示,即将废铅膏浸出进入溶液后再电解沉积产出阴极铅。该工艺最大的不同是用试剂溶解废铅膏,使铅化合物溶解进入溶液,其中氯盐和碱性溶液是常用的湿法浸出铅的体系[38-39],HCl-NaCl体系使铅以PbCln2-n配合离子进入溶液[40],但同时SO42-也会进入溶液,而添加CaCl2的HCl-NaCl-CaCl2体系则较好地解决了这个问题[41-42]。碱性溶液中加入丙三醇、山梨醇、酒石酸和木糖醇等有机物不仅提高了铅膏中铅的溶解效率[43],而且大幅度降低了溶液中氢氧化钠浓度,但是含铅溶液电解过程有机物会氧化消耗,增加了处理成本。
3.4 转化-浸出-电积工艺
由于废铅膏中各种铅化合物溶解效率不同,为了提高铅的浸出率,又提出转化-浸出-电积工艺,如图1中步骤④+⑥+⑦所示,即废铅膏预处理转化后再经过浸出并电积产出阴极铅,该工艺更加注重废铅膏中铅的提取效率和溶液再生等方面,通常浸出在HBF4或H2SiF6体系进行,不同工艺的转化阶段略有不同。
经典的RSR工艺[44-45]则是用(NH4)2CO3脱除废铅膏中的硫酸根并使其转化为碳酸铅,然后用亚硫酸钠和二氧化硫还原铅膏中的二氧化铅,转化渣用HBF4或H2SiF6浸出,含铅溶液最后电积产出阴极铅。USBM工艺[46]略有区别的是采用铅粉作为还原剂,而CX-EW工艺[47]则采用碳酸钠作为脱硫剂和双氧水作为还原剂。CX-EWS工艺[48]则首先用细菌将废铅膏中的铅化合物转化为硫化铅后再用氟硼酸高铁溶液氧化浸出,浸出液在阴极室电积阴极铅,阳极室实现氟硼酸高铁溶液的再生。转化-浸出-电积工艺较好地解决了火法工艺存在的问题,但是仍然存在试剂消耗大和能耗高的缺点,尤其是电积过程难以抑制阳极PbO2的析出,使得铅回收率低。
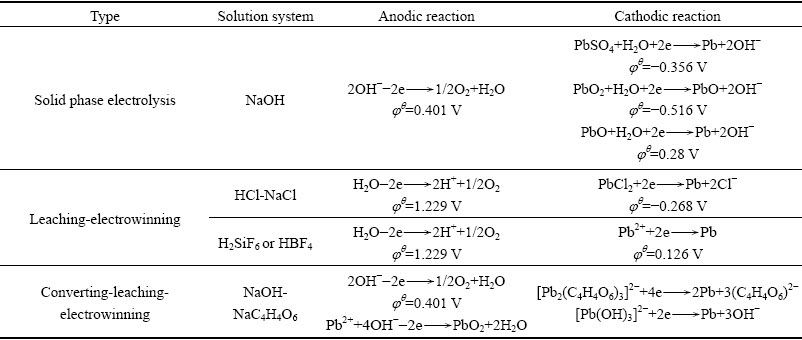
各种废铅膏湿法处理工艺均采用电沉积的提取方式,电解过程的阴阳极反应见表1,可以看出,无论是何种体系阳极反应主要是析氧反应,阴极反应则主要是二价铅析出反应,由于体系不同相对应的电位也不同。
3.5 材料冶金工艺
目前废铅膏火法和湿法回收工艺的目的都是将废铅膏制备成金属铅,然后再采用球磨法或气相氧化法制备出铅酸蓄电池制造用铅粉,为了减少能源消耗,研究人员提出用废铅膏直接制备铅酸蓄电池用氧化铅粉的材料冶金工艺,如图1中步骤④+⑧所示,即废铅膏经过预处理产出中间前驱体,中间前驱体再焙烧产出铅粉,研究较多的有柠檬酸铅[49-51]、碳酸铅[15]和草酸铅[9]等中间产物,最为典型是柠檬酸法。
剑桥大学KUMAR等[52-53]提出用柠檬酸处理废铅膏并制备PbO粉新工艺,首先在柠檬酸水溶液中用H2O2作为还原剂,使废铅膏中PbO2还原生成柠檬酸铅;其次是废铅膏中PbSO4与Na3C6H5O7·2H2O和C6H8O7·H2O共同作用生成类柠檬酸铅前驱体,最后柠檬酸铅前驱体在温度300~500 ℃焙烧得到Pb和PbO混合粉末,该工艺为废铅膏的回收利用提供一种更绿色环保回收途径,但是昂贵的试剂价格和较慢的反应速度限制了该工艺的产业化应用。华中科技大学杨家宽等[49]对该工艺进行了深入研究,并提出向柠檬酸钠溶液中加入醋酸溶液的强化反应过程的方案,有效地提高铅的溶解速度并适当降低了生产成本。
北京化工大学潘军青等[54-55]提出了原子经济法,即废铅膏首先在NaOH溶液中加入铅粉做还原剂使铅溶解进入溶液,固液分离后冷却析出PbO晶体和析铅后液,PbO晶体再次溶解于NaOH溶液中重结晶产出纯净PbO固体,PbO纯度可达到99.99%以上;析铅后液加入NaOH并冷却除去Na2SO4,溶液继续返回循环使用。该方法利用氢氧化钠与铅的配合作用,虽然可以实现氧化铅的提纯,但是副产物硫酸钠中氢氧化钠夹杂损失严重。
表1 废铅膏湿法处理工艺电解过程的阴阳极反应
Table 1 Electrode reactions of electrolysis process of waste lead paste by hydrometallurgy
4 废铅膏低温还原清洁炼铅的技术思路
4.1 新技术思路的产生
为了进一步规范铅酸蓄电池的回收体系和处理技术,近些年我国相继出台了一系列文件以保证铅酸蓄电池回收的有序发展。2009年环保部发布《废铅酸蓄电池处理污染控制技术规范》(2009年第71号),对废铅酸蓄电池的收集、运输、贮存和工艺过程污染控制都进行了详细规定。2011年环保部发文《关于加强铅酸蓄电池极再生铅行业污染防治工作的通知》,切实加强了铅蓄电池生产与回收以及再生铅行业的污染防治工作管理。2012年8月,工信部与环保部联合制定了《再生铅行业准入条件》,对废铅膏处理工艺提出了更加明确的要求,“对分选出的铅膏必须进行脱硫预处理或送硫化铅精矿冶炼厂合并处理,脱硫母液必须进行处理并回收副产品……,再生铅企业应采用密闭熔炼、低温连续熔炼、新型节能环保熔炼炉等先进工艺及设备”。
废铅膏回收工艺正在向可持续、环境友好、低消耗和高质量的方向发展,借鉴铝冶炼工业由铝土矿到氧化铝再到金属铝的工艺思路,本文提出一种基于水热还原转化的废铅膏低温还原清洁炼铅工艺,即废铅膏首先通过硫酸浸煮脱除杂质,然后浸煮渣在碱和还原剂同时存在下水热处理,使硫酸铅和二氧化铅均转化为氧化铅,最后在低温熔盐中用淀粉还原产出金属铅,加热烟气直接排放,熔炼烟气冷却收尘后达标排放,工艺流程如图2所示。
4.2 原料
实验原料是废旧铅酸蓄电池拆解产物废铅膏,首先用水洗涤至pH=6.8,然后于110 ℃烘干后磨细以保证其粒径小于80 μm,其主要化学成分见表2。

图2 类铝冶金的废铅膏低温还原清洁炼铅工艺流程图
Fig. 2 Flow sheet of lead clean smelting from waste lead paste at low temperature referred to aluminum metallurgy
表2 废铅膏的主要化学成分
Table 2 Main composition of waste lead paste (mass fraction, %)

表3 废铅膏中铅物相组成占比
Table 3 Proportion of lead phase in waste lead paste(mass fraction, %)

由表2可以看出,废铅膏中主要含有铅和硫两种元素,结合化学物相分析检测(见表3)可以看出,废铅膏中主要有PbSO4、PbO2和PbO三种物相,其占比分别为59.21%、28.46%和10.14%,另外,废旧铅蓄电池拆解过程少量板栅的金属铅进入废铅膏,其占比为2.19%。
4.3 废铅膏脱除杂质
废旧铅酸蓄电池拆解时,易混入废铅膏中的杂质有Sb(来源为板栅的Pb-Sb合金)、Ca(来源为板栅中的Pb-Ca合金)、SiO2(来源为玻璃元素纤维隔膜)、Cu(来源为导电柱)和Fe(来源为设备材质)等,于是提出采用硫酸浸煮方式脱除废铅膏中的杂质,在硫酸溶液中用PbO2将锑、铜和铁等杂质氧化溶解,而SiO2和Ca则在后续低温还原熔炼过程脱除。硫酸浸煮过程发生的化学反应如下:
Cu+2H2SO4+PbO2=CuSO4+PbSO4↓+2H2O (1)
Fe+2H2SO4 +PbO2=FeSO4+PbSO4↓+2H2O (2)
2Sb+4H2SO4 +3PbO2= (SbO)2SO4+3PbSO4↓+4H2O (3)
PbO+H2SO4=PbSO4↓+H2O (4)
Pb +2H2SO4+PbO2=2PbSO4↓+2H2O (5)
上述废铅膏用4 mol/L硫酸溶液在温度95 ℃浸煮1.0 h,液固分离后用水洗涤至pH=6.8,硫酸浸煮渣中的Cu、Fe和Sb含量均降至0.001%以下;硫酸浸煮时废铅膏中的部分PbO和Pb均会与硫酸反应生成PbSO4沉淀,硫酸浸煮渣中铅物相分析结果见表4。
由表4可以看出,废铅膏经过硫酸浸煮后,浸煮渣中硫酸铅的含量有所增加,而二氧化铅、氧化铅和金属铅的含量有所降低,与化学反应式基本一致。
表4 硫酸浸煮渣中铅物相组成占比
Table 4 Proportion of lead phase in sulfuric acid leaching residue(mass fraction, %)

4.4 水热还原双重转化
由于常规脱硫时往往存在试剂消耗大和脱硫效率低的缺点,刘伟锋等[56]在铜阳极泥碱性加压氧化浸出研究中发现浸出渣中铅全部转化为氧化铅,而铅酸蓄电池充放电过程与粗铜电解精炼过程类似,因此,本文提出采用水热方式强化废铅膏转化脱硫过程的设想。
水热法作为一种强化手段,在高温高压水溶液中采实现常温常压难以实现的目的,广泛应用于冶金和先进材料制备领域[57-58]。为了打破废铅膏中硫酸铅和二氧化铅的相互包裹现象,考虑在在水热条件下加入合适的还原剂使 PbO2还原转化为 PbO,可以选择的还原剂众多,相比较而言,多糖类淀粉和纤维素等高分子物质不仅来源广泛,不引入有害杂质,而且还原能力强,所以本文选择以NaOH为脱硫剂,以淀粉作为还原剂,采用水热还原方式同时实现废铅膏的脱硫转化和还原转化两个目的。水热还原双重转化过程的主要反应如下:
PbSO4+2NaOH=PbO+Na2SO4+H2O (6)
(C6H10O5)n+12PbO2=12PbO+6CO2↑+5H2O (7)
硫酸浸煮渣水热转化条件:氢氧化钠用量为理论量的1.05倍、淀粉用量为理论量的1.2倍、温度175 ℃和时间2 h,结果表明硫酸根的脱除率和二氧化铅的还原率均达到99.5%以上,且暗红色的废铅膏变为灰黄色的氧化铅,氧化铅的物相分析结果见表5。
表5 氧化铅中铅物相组成占比
Table 5 Proportion of lead phase in lead oxide (mass fraction, %)

由表5可以看出,废铅膏硫酸浸煮渣经过水热还原转化后,硫酸铅和二氧化铅全部转化为氧化铅,实现了双重转化的目标。
4.5 低温还原清洁炼铅
废铅膏硫酸浸煮渣采用水热还原双重转化后,使得废铅膏中各种形态的铅化合物均转化为为氧化铅,但是仍然含有少量二氧化硅和钙等杂质,无法直接用于制造铅酸蓄电池,一方面为了继续脱除氧化铅中的杂质,另一方面为了便于后续球磨制备铅粉系统使用,只能将氧化铅还原熔炼成金属铅。但传统的火法还原熔炼过程均使用燃煤或焦炭作为还原剂[59],它们燃烧灰分中含有大量的SiO2和Fe2O3等造渣组分,所以毫无例外地均需要造FeO-SiO2-CaO三元系高熔点渣(熔点为1150~1200 ℃),这不仅需要更高的熔炼温度,而且燃煤灰分和造渣组分中的杂质金属进入金属铅,粗铅必须经过电解精炼进一步提纯。
为了降低熔炼温度并提高铅产品品质,能否借鉴冶铝炼工业由铝土矿到氧化铝再到金属铝的思路,在低温熔盐中将氧化铅还原为金属铅?该假设能否成立取决于选择合适的低温熔盐和还原剂。首先,要找到一种对氧化铅溶解度大的低温熔盐。其实,早在中国西汉时期出现的铅釉陶器[60]上,古代工匠就使用了以氧化铅为助熔剂的低温铅釉。该铅釉在700℃左右即可熔融,该类以硅酸盐为主的铅釉中PbO的含量可以达到46.89%。据此类推,可以使用主要成分为B2O3、PbO和Na2O的铅釉型低温熔盐作为还原载体。其次,要找到一种自身灰分低的还原剂。在贵金属冶金过程中,通常用火法试金法测定样品中贵金属含量[61],为了避免还原煤燃烧灰分等因素干扰测定结果,该方法用淀粉作为还原剂还原氧化铅产出粗铅,故可以选用淀粉作为还原剂。
因此,氧化铅低温熔盐还原清洁炼铅的工艺过程如下:首先将高氧化铅釉型熔盐在熔炼锅内熔化,然后加入氧化铅和淀粉混合物低温熔炼产出精铅,氧化铅中少量的二氧化硅和钙等进入低温熔盐除去。低温还原熔炼过程主要反应如下:
12PbO+(C6H10O5)n=12Pb+6CO2↑+5H2O (8)
首先在外加热的熔炼锅内将低温熔盐700 ℃熔化并保温1 h后,然后向低温熔盐中加入氧化铅和淀粉混合物,反应1.5 h 后倒出底铅。结果表明,低温还原熔炼过程中金属铅的直收率可达 96.0%以上,金属铅纯度达到99.95%及以上。
5 结论与展望
1) 废铅酸蓄电池是最重要的再生铅资源,通常先拆解再分别回收利用。由于废铅膏中存在大量硫酸铅,使其成为废铅酸蓄电池资源化利用的瓶颈。废铅膏处理传统火法工艺和湿法工艺均是以阴极铅为目标产物,其中火法工艺获得了广泛应用。近些年材料冶金思路被提出,即绕开废铅膏制备阴极铅的过程,用废铅膏直接制备铅酸蓄电池用的铅粉。
2) 废铅膏火法处理工艺是废铅膏经过高温还原熔炼产出粗铅,然后再电解精炼得到阴极铅。与单独熔炼方法相比,混合熔炼方法充分利用铅精矿的化学反应热,该方法在原生铅冶炼厂获得了广泛应用。废铅膏湿法回收工艺是废铅膏经过预处理后再电解或电积产出阴极铅,典型的废铅膏湿法处理工艺主要有固相电解法、浸出-电积法和转化-浸出-电积法共三种类别,预处理过程通常是采用化学方法改变废铅膏中铅的存在状态,预处理过程的实施效果对工艺的稳定运行影响较大。
3) 借鉴铝冶炼工业由铝土矿到氧化铝再到金属铝的工艺思路,本文提出一种基于水热还原转化的废铅膏低温还原清洁炼铅工艺:首先,废铅膏首先通过硫酸浸煮脱除杂质;其次,浸煮渣在碱和还原剂同时存在下水热处理,使硫酸铅和二氧化铅均转化为氧化铅;最后,低温熔盐中用淀粉还原氧化铅产出金属铅,铅的直收率可达 96.0%以上,金属铅纯度达到99.95%及以上。该工艺具有环境友好和工艺流程短等优点,为铅膏处理的研究和工艺改革提供一种新的思路。
REFERENCES
[1] 朱新锋, 刘万超, 杨海玉, 李 磊, 杨家宽. 以废铅酸电池铅膏制备超细氧化铅粉末[J]. 中国有色金属学报, 2010, 20(1): 132-136.
ZHU Xin-feng, LIU Wan-chao, YANG Hai-yu, LI Lei, YANG Jia-kuan. Preparation of ultrafine PbO powders from lead paste in spent lead acid battery[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(1): 132-136.
[2] PAN Jun-qing, ZHANG Chao, SUN Yan-zhi, WANG Zi-hao, YANG Yu-sheng. A new process of lead recovery from waste lead-acid batteries by electrolysis of alkaline lead oxide solution[J]. Electrochemistry Communications, 2012, 19: 70-72.
[3] REZAEI B, MALLAKPOUR S, TAKI M. Application of ionic liquids as an electrolyte additive on the electrochemical behavior of lead acid battery[J]. Journal of Power Sources, 2009, 187(2): 605-612.
[4] MAO Jian-su, MA Lan, LIANG Jing. Changes in functions, forms, and locations of lead during its anthropogenic flows to provide services[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 233-242.
[5] KARAMI H,KARIMI M A, HAGHDAR S, SADEGHI A, MIR-GHASEMI R, MAHDI-KHANI S. Synthesis of lead oxide nanoparticles by sonochemical method and its application as cathode and anode of lead-acid batteries[J]. Materials Research Bulletin, 2008, 108(2/3): 337-344.
[6] TIAN Xi, GONG Yu, WU Yu-feng, AGYEIWAA A, ZUO Tie-yong. Management of used lead acid battery in China: Secondary lead industry progress, policies and problems[J]. Resources, Conservation and Recycling, 2014, 93: 75-84.
[7] NING Peng, PAN Jun-qing, LI Xue, ZHOU Yue, CHEN Jian-feng, WANG Jie-xin. Accelerated desulphurization of waste lead battery paste in a high-gravity rotating packed bed[J]. Chemical Engineering and Processing: Process Intensification, 2016, 104: 148-153.
[8] LIANG Jing, MAO Jian-su. A dynamic analysis of environmental losses from anthropogenic lead flow and their accumulation in China[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1125-1133.
[9] MA Cheng, SHU Yue-hong, CHEN Hong-yu. Recycling lead from spent lead pastes using oxalate and sodium oxalate and preparation of novel lead oxide for lead-acid batteries[J]. RSC Advances, 2015, 5: 94895-94902.
[10] 王升东, 王道藩, 唐忠诚, 唐文彬. 废铅蓄电池回收铅与开发黄丹、红丹以及净化铅蒸汽新工艺研究[J]. 再生资源与循环经济, 2004(2): 24-28.
WANG Sheng-dong, WANG Dao-pan, TANG Zhong-cheng, TANG Wen-bin. Research on the new process of recovery of lead and producing yellow lead or red lead and purifying lead steam by recycling waste lead acid battery[J]. Renewable Resources and Recycling Economy, 2004(2): 24-28.
[11] 潘军青, 边亚茹. 铅酸蓄电池回收铅技术的发展现状[J]. 北京化工大学学报(自然科学版), 2014, 41(3): 1-14.
PAN Jun-qing, BIAN Ya-ru. Development and current situation of the recovery technology for lead acid batteries[J]. Journal of Beijing University of Chemical Technology (Natural science), 2014, 41(3): 1-14.
[12] 李卫锋, 蒋丽华, 湛 晶, 张传福. 废铅酸蓄电池铅再生技术现状及进展[J]. 中国有色冶金, 2011, 40(6): 53-56.
LI Wei-feng, JIANG Li-hua, ZHAN Jing, ZHANG Chuan-fu. Status and progress of recycling technology of waste lead-acid battery[J]. China Nonferrous Metallurgy, 2011, 40(6): 53-56.
[13] 宋剑飞, 李丹, 陈昭宜. 废铅蓄电池的处理及资源化——黄丹红丹生产新工艺[J]. 环境工程, 2003, 21(5): 48-50.
SONG Jian-fei, LI Dan, CHEN Zhao-yi. Treatment and resource recovery of spent lead battery—A new process of producing yellow lead and red lead[J]. Environmental Engineering, 2003, 21(5): 48-50.
[14] LYAKOV N K, ATANASOVA D A, VASSILEV V S, HARALAMPIEV G A. Desulphurization of damped battery paste by sodium carbonate and sodium hydroxide[J]. Journal of Power Sources, 2007, 171(2): 960-965.
[15] ZHU Xin-feng, YANG Jia-kuan, GAO Lin-xia, LIU Jian-wen, YANG Dan-ni, SUN Xiao-juan, ZHANG Wei, WANG Qin, LI Lei, HE Dong-sheng, KUMAR R V. Preparation of lead carbonate from spent lead paste via chemical conversion[J]. Hydrometallurgy, 2013, 134/135(3): 47-53.
[16] LIANG Jing, MAO Jian-su. Lead anthropogenic transfer and transformation in China[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1262-1270.
[17] 郝科涛, 吕晓军, 贾 明, 洪 波, 蒋良兴, 方 静, 赖延清, 李 劼, 刘业翔. 铅酸电池负极板栅用Al/Pb复合材料的制备及性能[J]. 中国有色金属学报, 2013, 23(6): 1591-1597.
HAO Ke-tao, LU Xiao-jun, JIA Ming, HONG Bo, JIANG Liang-xing, FANG Jing, LAI Yan-qing, LI Jie, LIU Ye-xiang. Preparation and performance of Al/Pb composite material for lead-acid battery negative grid[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(6): 1591-1597.
[18] 张正洁, 朱忠军. 再生铅反射炉熔炼过程重金属排放规律及影响因素分析[J]. 资源再生, 2014 (8): 66-69.
ZHANG Zheng-jie, ZHU Zhong-jun. Analysis of heavy metal emission rules in the reclaimed lead smelting process with reverberatory furnace and of influence factors[J]. Resource Recycling, 2014 (8): 66-69.
[19] 周洪武. 废铅酸蓄电池铅料特点和冶炼技术选择[J]. 资源再生, 2007(5): 19-22.
ZHOU Hong-wu. Characters of waste lead acid storage and its melting technology options[J]. Resource, 2007(5): 19-22.
[20] 王海荣. 竖炉还原卡尔多炉熔炼渣的试验研究[J]. 中国有色冶金, 2012, 41(4): 63-66.
WANG Hai-rong. Experimental research of reduction of smelting slag from Kaldo furnace by shaft furnace[J]. China Nonferrous Metallurgy, 2012, 41(4): 63-66.
[21] 杜新玲, 王国富. 废旧铅酸蓄电池密闭短窑熔炼的生产实践[J]. 中国有色冶金, 2013, 42(4): 34-38.
DU Xin-ling, WANG Guo-fu. Production practice of smelting waste lead-acid battery with sealing short kiln[J]. China Nonferrous Metallurgy, 2013, 42(4): 34-38.
[22] GENAIDY A M, SEQUEIRA R, TOLAYMAT T, KOHLER J, RINDER M. Evidence-based integrated environmental solutions for secondary lead smelters: pollution prevention and waste minimization technologies and practices[J]. Science of the Total Environment, 2009, 407(10): 3239-3268.
[23] 孙晓娟, 李 卉, 朱新锋, 张 伟, 胡雨辰. 复合脱硫剂对废铅酸蓄电池铅膏脱硫影响的研究[J]. 蓄电池, 2013, 50(4): 147-152.
SUN Xiao-juan, LI Hui, ZHU Xin-feng, ZHANG Wei, HU Yu-chen. Study on effects of complex desulfurizer on the desulfurization ratio of lead pastes from used lead-acid battery[J]. Chinese LABAT Man, 2013, 50(4): 147-152.
[24] 黄 潮, 唐朝波, 唐谟堂, 杨建广, 陈永明, 杨声海, 何 静. 废铅酸蓄电池胶泥的低温熔盐还原固硫熔炼工艺研究[J]. 矿冶工程, 2012, 32(2): 84-87.
HUANG Chao, TANG Chao-bo, YANG Jian-guang, CHEN Yong-ming, YANG Sheng-hai, HE Jing. Sulfur-fixing reduction smelting of spent lead-acid battery colloid sludge in fused salt at low temperature[J]. Mining and Metallurgical Engineering, 2012, 32(2): 84-87.
[25] 陈亚州, 汤 伟, 吴艳新, 李 贵, 赵振波.国内外再生铅技术的现状及发展趋势[J]. 中国有色冶金, 2017, 46(3): 17-22.
CHEN Ya-zhou, TANG Wei, WU Yan-xin, LI Gui, ZHAO Zhen-bo. Present situation and development trend of secondary lead process at home and abroad[J]. China Nonferrous Metallurgy, 2017, 46(3): 17-22.
[26] 杜新玲. 河南豫光金铅富氧底吹处理废铅酸蓄电池生产实践[J]. 有色金属工程, 2013, 3(5): 33-35.
DU Xin-ling. Production practice on treatment of waste lead acid battery with oxygen enriched bottom blowing by Henan Yuguang gold and lead[J]. Nonferrous Metals Engineering, 2013, 3(5): 33-35.
[27] 汪振忠, 柯昌美, 王 茜. 废铅酸蓄电池铅膏脱硫工艺的研究进展[J]. 无机盐工业, 2013, 45(1): 60-62.
WANG Zhen-zhong, KE Chang-mei, WANG Qian. Research progress of desulfurization process for lead paste from waste lead-acid battery[J]. Inorganic Chemicals Industry, 2013, 45(1): 60-62.
[28] LEE H Y. Preparation of basic lead carbonate from lead dust by hydrometallurgical processes[J]. Hydrometallurgy, 2009, 96(1/2): 103-107.
[29] 梁晓蓉, 刘晓荣, 顾怡卿, 史唐明, 樊 鑫, 张中源. 废铅蓄电池湿法再生过程PbO2的还原研究[J]. 上海应用技术学院学报(自然科学版), 2009, 9(2): 126-129.
LIANG Xiao-rong, LIU Xiao-rong, GU Yi-qing, FAN Xin, ZHANG Zhong-yuan. Reduction of PbO2 covered in lead paste of waste lead-acid batteries[J]. Journal of Shanghai Institute of Technology (Natural science), 2009, 9(2): 126-129.
[30] 朱新锋, 杨家宽, 杨丹妮, 孙晓娟, 郭一飞, 陈松涛. 从废铅膏制备超细碳酸铅的表征及热分解性能研究[J]. 功能材料, 2012, 43(17): 2343-2346.
ZHU Xin-feng, YANG Jia-kuan, YANG Dan-ni, SUN Xiao-juan, GUO Yi-fei, CHEN Song-tao. Study on characterization and thermal decomposition of ultra-fine carbonated lead powder from lead paste[J]. Journal of Functional Materials, 2012, 43(17): 2343-2346.
[31] 邱德芬, 柯昌美, 王 茜, 陈 珊, 刘芳芳. 废铅酸蓄电池中二氧化铅还原技术研究进展[J]. 无机盐工业, 2014, 46(3): 15-18.
QIU De-fen, KE Chang-mei, WANG Qian, CHEN Shan, LIU Fang-fang. Research progress of reduction technology for PbO2 from spent lead acid battery[J]. Inorganic Chemical Industry, 2014, 46(3): 15-18.
[32] 刘建斌, 黄志明, 许 民, 刘苏昆. 废铅酸蓄电池渣泥湿法脱硫和还原新工艺研究[J]. 无机盐工业, 2004, 36(1): 47-49.
LIU Jian-bin, HUANG Zhi-ming, XU Min, LIU Su-kun. Study on desulfuration and reduction from paste mud of waste lead storage batteries by hydrometallurgy[J]. Inorganic Chemical Industry, 2004, 36(1): 47-49.
[33] ZHANG Jun-feng, YI Liang, YANG Lin-chun, HUANG Yan, ZHOU Wen-fang, BIAN Wen-jin. A new pre-desulphurization process of damped lead battery paste with sodium carbonate based on a “surface update” concept[J]. Hydrometallurgy, 2016, 160: 123-128.
[34] 陆克源. 固相电解还原提取金属铅[J]. 化工冶金, 1983(3): 67-71.
LU Ke-yuan. Recovery of metallic lead by solid-phase electro-reduction process[J]. Chemical Metallurgy, 1983(3): 67-71.
[35] 韩 召, 李 领, 魏晓磊, 李 杰, 王海川. 硫酸铅在碳酸钠-硝酸钠熔盐中的脱硫转化[J]. 材料热处理学报, 2012, 33(6): 14-19.
HAN Zhao, LI Ling, WEI Xiao-lei, LI Jie, WANG Hai-chuan. Desulfurization and phase transformation of lead sulfate in sodium carbonate-sodium nitrate molten salt[J]. Transactions of Material and Heat Treatment, 2012, 33(6): 14-19.
[36] 吴秀安. 一种处理废旧铅酸蓄电池的工艺[P]. 中国, 201410723350.0, 2015-03-25.
WU Xiu-an. Process for disposing waste lead acid battery[P]. China, 201410723350.0. 2015-03-25.
[37] 高云芳, 董志根. 一种电解还原再生废铅酸蓄电池含铅膏泥中铅资源的方法[P]. 中国, 200710157084.X. 2008-08-27.
GAO Yun-fang, DONG Zhi-gen. Method for recovering lead from waste lead acid battery paste by electroreduction[P]. China, 200710157084.X. 2008-08-27.
[38] VOLPE M, OLIVERI D, FERRARA G, SALVAGGIO M, PIAZZA S, ITALIANO S, SUNSERI C. Metallic lead recovery from lead-acid battery paste by urea acetate dissolution and cementation on iron[J]. Hydrometallurgy, 2009, 96(1/2): 123-131.
[39] 陈维平. 一种湿法回收废铅蓄电池填料的新技术[J]. 湖南大学学报(自然科学版), 1996, 23(6): 112-117.
CHEN Wei-ping. New technology of hydrometallurgical recovering lead from waste battery slurry[J]. Journal of Hunan University(Natural science). 1996, 23(6): 112-117.
[40] ANDREWS D, RAYCHAUDHURI A, FRIAS C. Environmentally sound technologies for recycling secondary lead[J]. Journal of Power Sources, 2000, 88(1): 124-129.
[41] 杨新生. 从废铅蓄电池渣泥中制取铅系列化工产品的试验研究[J]. 江西冶金, 1995(2): 22-23.
YANG Xin-sheng. Experimental research on preparation of lead series chemical products from waste lead acid battery mud[J]. Jiangxi Metallurgy, 1995(2): 22-23.
[42] 齐美富, 郑园芳, 桂双林. 废铅酸蓄电池中铅膏氯盐体系浸取铅的动力学研究[J]. 矿冶工程, 2010, 30(6): 61-64.
QI Mei-fu, ZHENG Yuan-fang, GUI Shuang-lin. Kinetic study on leaching lead from waste lead-acid batteries for lead plaster chloride system[J]. Mining and Metallurgical Engineering, 2010, 30(6): 61-64.
[43] 刘 伟, 杨天足, 周琼华. 碱性多羟基化合物体系在有色冶金中的应用研究进展[J]. 湿法冶金, 2012, 31(1): 1-4.
LIU Wei, YANG Tian-zu, ZHOU Qiong-hua. Research progress on application in hydrometallurgy of nonferrous metals in alkaline solutions containing polyhydric organics[J]. Hydrometallurgy of China, 2012, 31(1): 1-4.
[44] ANDREWS D, RAYCHAUDHURI A, FRIAS C. Environmentally sound technologies for recycling secondary lead[J]. Journal of Power Sources, 2000, 88(1): 124-129.
[45] PRENGAMAN R D, MCDONALD H B. Method of recovering lead values from battery sludge. 1980, U.S. Patent 4229271[P]. 1980-10-21.
[46] 邱德芬, 柯昌美, 王 茜, 张松山. 从废铅膏中回收铅及铅的化合物的方法[J]. 无机盐工业, 2014, 46(7): 16-19.
QIU De-fen, KE Chang-mei, WANG Qian, ZHANG Song-shan. Methods of recovering lead and lead compounds from spend lead paste[J]. Inorganic Chemicals Industry, 2014, 46(7): 16-19.
[47] OLPER M, FRACCHIA P. Hydrometallurgical process for an overall recovery of the components of exhausted lead-acid batteries[P]. Canada. 1333960. 1995-01-17.
[48] OLPER M, MACCAGNI M, BUISMAN C J N, SCHULTZ C E. Electrowinning of lead battery paste with the production of lead and elemental sulphur using bioprocess technologies[C]//DUTRIZAC J E, GONZALEZ J A, HENKE D M, et al. Lead-Zinc 2000. New Orleans: TMS. 2000: 803-813.
[49] ZHU Xin-feng, HE Xiong, YANG Jia-kuang, GAO Lin-xia, LIU Jian-wen, YANG Dan-ni, SUN Xiao-juan, ZHANG Wei, WANG Qin, KUMAR R V. Leaching of spent lead acid battery paste components by sodium citrate and acetic acid[J]. Journal of Hazardous Materials, 2013, 250/251(8): 387-396.
[50] LI Li, HU Yu-chen, ZHU Xin-feng, YANG Dan-ni, WANG Qin, LIU Jian-wen, KUMAR R V, YANG Jia-kuan. Lead citrate precursor route to synthesize nanostructural lead oxide from spent lead acid battery paste[J]. Materials Research Bulletin, 2013, 48(4): 1700-1708.
[51] 朱新锋, 王现丽, 聂鹏茹, 张 伟, 胡雨辰. 湿法回收废铅膏中间产物柠檬酸铅Pb3(C6H5O7)2·3H2O的热分解[J]. 中国有色金属学报, 2016, 26(12): 2686-2693.
ZHU Xin-feng, WANG Xian-li, NIE Peng-ru, ZHANG Wei, HU Yu-chen. Thermal decomposition of lead citrate Pb3(C6H5O7)2·3H2O from recovery spent lead paste by hydrometallurgy process[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(12): 2686-2693.
[52] SONMEZ M S, KUMAR R V. Leaching of waste battery paste components. Part 1: Lead citrate synthesis from PbO and PbO2[J]. Hydrometallurgy, 2009, 95(1/2): 53-60.
[53] SONMEZ M S, KUMAR R V. Leaching of waste battery paste components. Part 2: Leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution[J]. Hydrometallurgy, 2009, 95(1/2): 82-86.
[54] PAN Jun-qing, SUN Yan-zhi, LI Wei, KNIGHT J, MANTHIRAM A. A green lead hydrometallurgical process based on a hydrogen-lead oxide fuel cell[J]. Nature Communications, 2013, 4(4): 2178.
[55] 潘军青, 宋 爽, 马亚强, 孙艳芝, 钮因键. 一种基于原子经济途径回收废旧铅酸电池生产氧化铅的方法[P]. 中国, 201310084392.X. 2013-06-12.
PAN Jun-qing, SONG Shuang, MA Ya-qiang, SUN Yan-zhi, NIU Yin-jian. Method for preparation of lead oxide from waste lead acid battery by atomic economic approach[P]. China, 201310084392.X. 2013-06-12.
[56] LIU Wei-feng, YANG Tian-zu, ZHANG Du-chao, CHEN Lin, LIU You-nian. Pretreatment of copper anode slime with alkaline pressure oxidative leaching[J]. International Journal of Mineral Processing, 2014, 128(5): 48-54.
[57] AHN J G, HAI H T, KIM D J, PARK J S, KIM S B. Direct synthesis of nickel powders from NiO slurry by hydrothermal hydrogen reduction process[J]. Hydrometallurgy, 2010, 102(1): 101-104.
[58] GAO Guo, QIU Pei-yu, QIAN Qi-rong, ZHOU Na, WANG Kan, SONG Hua, FU Hua-lin, CUI Da-xiang. PEG-200-assisted hydrothermal method for the controlled-synthesis of highly dispersed hollow Fe3O4 nanoparticles[J]. Journal of Alloys and Compounds, 2013, 574(27): 340-344.
[59] 吴广龙, 张正洁, 刘俐媛, 丁 琼. 废铅膏冶炼工艺比较分析研究[J]. 蓄电池, 2015, 52(5): 209-211.
WU Guang-hua, ZHANG Zheng-jie, LIU Li-yuan, DING Qiong. A comparative study of smelting technology on lead paste from used lead-acid batteries[J]. Chinese LABAT Man, 2015, 52(5): 209-211.
[60] 朱铁权, 王昌燧, 毛振伟, 李立新, 黄 烘. 我国古代不同时期铅釉陶表面腐蚀物的分析研究[J]. 光谱学与光谱分析, 2010, 30(1): 266-269.
ZHU Tie-quan, WANG Chang-sui, MAO Zhen-wei, LI Li-xin, HUANG Hong. Identification of different corrosion covering the surface of Chinese ancient lead glazed potteries[J]. Spectroscopy and Spectral Analysis, 2010, 30(1): 266-269.
[61] 曾妙先. 火试金法在贵金属元素分析中的应用[J]. 黄金, 2003, 24(5): 48-50.
ZENG Miao-xian. Application of fire assaying to analysis of precious metal element[J]. Gold, 2003, 24(5): 48-50.
Technical idea on lead clean reduction smelting of waste lead paste at low temperature referred to aluminum metallurgy
LIU Wei-feng, ZHANG Kun-kun, DENG Xun-bo, ZHANG Du-chao, CHEN Lin, YANG Tian-zu
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: Waste lead acid battery is the most important renewable lead resource, which is usually dismantled and then recovered separately. Waste lead paste contains lots of lead sulfate, which is a bottleneck for the effective utilization of waste lead acid battery. The conventional pyrometallurgical process and hydrometallurgical process all regard cathode lead as the product, and the former has been widely used. In recent years, material metallurgy aims to directly prepare lead powder for lead acid battery using waste lead paste, which has also been widely studied. Drawing lessons from the idea of aluminum extraction process from bauxite to alumina and aluminum in sequence, a clean lead smelting in low temperature of waste lead paste after hydrothermal reduction conversion was proposed. First, impurities are removed from waste lead paste by leaching in sulphuric acid solution. Then, during the hydrothermal reduction concerting, lead sulfate will be converted into lead oxide with the addition of alkali as conversion agent. At the same time, lead dioxide will be reduced to lead oxide with adding reductant. Final, lead oxide can be reduced to metallic lead at low temperature in molten salt using starch as the reductant. The technology will provide a new idea for the recycle of waste lead paste.
Key words: lead metallurgy; lead acid battery; waste lead paste; hydrothermal conversion; low temperature smelting
Foundation item: Project(51404296) supported by the Young Scientists Fund of National Natural Science Foundation of China; Project(2016M60247) supported by the Postdoctoral Science Foundation of China
Received date: 2018-04-27; Accepted date: 2018-06-25
Corresponding author: LIU Wei-feng; Tel: +86-13548654403; E-mail: liuweifeng@csu.edu.cn
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51404296);中国博士后科学基金资助项目(2016M602427)
收稿日期:2018-04-27;修订日期:2018-06-25
通信作者:刘伟锋,副教授,博士;电话:13548654403;E-mail:liuweifeng@csu.edu.cn
摘 要:废铅酸蓄电池是最重要的再生铅资源,通常先拆解再分别回收利用,由于废铅膏中存在大量硫酸铅,使其成为废铅酸蓄电池资源化利用的瓶颈。废铅膏处理传统火法工艺和湿法工艺均是以阴极铅为目标产物,其中火法工艺获得了广泛应用,近些年材料冶金思路则是绕开废铅膏制备阴极铅的过程,用废铅膏直接制备铅酸蓄电池用的铅粉。借鉴铝冶炼工业由铝土矿到氧化铝再到金属铝的工艺思路,本文提出一种基于水热还原转化的废铅膏低温还原清洁炼铅工艺:首先,废铅膏通过硫酸浸煮脱除杂质;其次,浸煮渣在碱和还原剂同时存在下水热处理,使硫酸铅和二氧化铅均转化为氧化铅;最后,低温熔盐中用淀粉还原氧化铅产出金属铅。该工艺为废铅膏处理的工艺改革提供一种新的思路。
[1] 朱新锋, 刘万超, 杨海玉, 李 磊, 杨家宽. 以废铅酸电池铅膏制备超细氧化铅粉末[J]. 中国有色金属学报, 2010, 20(1): 132-136.
[10] 王升东, 王道藩, 唐忠诚, 唐文彬. 废铅蓄电池回收铅与开发黄丹、红丹以及净化铅蒸汽新工艺研究[J]. 再生资源与循环经济, 2004(2): 24-28.
[11] 潘军青, 边亚茹. 铅酸蓄电池回收铅技术的发展现状[J]. 北京化工大学学报(自然科学版), 2014, 41(3): 1-14.
[12] 李卫锋, 蒋丽华, 湛 晶, 张传福. 废铅酸蓄电池铅再生技术现状及进展[J]. 中国有色冶金, 2011, 40(6): 53-56.
[13] 宋剑飞, 李丹, 陈昭宜. 废铅蓄电池的处理及资源化——黄丹红丹生产新工艺[J]. 环境工程, 2003, 21(5): 48-50.
[18] 张正洁, 朱忠军. 再生铅反射炉熔炼过程重金属排放规律及影响因素分析[J]. 资源再生, 2014 (8): 66-69.
[19] 周洪武. 废铅酸蓄电池铅料特点和冶炼技术选择[J]. 资源再生, 2007(5): 19-22.
[20] 王海荣. 竖炉还原卡尔多炉熔炼渣的试验研究[J]. 中国有色冶金, 2012, 41(4): 63-66.
[21] 杜新玲, 王国富. 废旧铅酸蓄电池密闭短窑熔炼的生产实践[J]. 中国有色冶金, 2013, 42(4): 34-38.
[23] 孙晓娟, 李 卉, 朱新锋, 张 伟, 胡雨辰. 复合脱硫剂对废铅酸蓄电池铅膏脱硫影响的研究[J]. 蓄电池, 2013, 50(4): 147-152.
[24] 黄 潮, 唐朝波, 唐谟堂, 杨建广, 陈永明, 杨声海, 何 静. 废铅酸蓄电池胶泥的低温熔盐还原固硫熔炼工艺研究[J]. 矿冶工程, 2012, 32(2): 84-87.
[25] 陈亚州, 汤 伟, 吴艳新, 李 贵, 赵振波.国内外再生铅技术的现状及发展趋势[J]. 中国有色冶金, 2017, 46(3): 17-22.
[26] 杜新玲. 河南豫光金铅富氧底吹处理废铅酸蓄电池生产实践[J]. 有色金属工程, 2013, 3(5): 33-35.
[27] 汪振忠, 柯昌美, 王 茜. 废铅酸蓄电池铅膏脱硫工艺的研究进展[J]. 无机盐工业, 2013, 45(1): 60-62.
[29] 梁晓蓉, 刘晓荣, 顾怡卿, 史唐明, 樊 鑫, 张中源. 废铅蓄电池湿法再生过程PbO2的还原研究[J]. 上海应用技术学院学报(自然科学版), 2009, 9(2): 126-129.
[30] 朱新锋, 杨家宽, 杨丹妮, 孙晓娟, 郭一飞, 陈松涛. 从废铅膏制备超细碳酸铅的表征及热分解性能研究[J]. 功能材料, 2012, 43(17): 2343-2346.
[31] 邱德芬, 柯昌美, 王 茜, 陈 珊, 刘芳芳. 废铅酸蓄电池中二氧化铅还原技术研究进展[J]. 无机盐工业, 2014, 46(3): 15-18.
[32] 刘建斌, 黄志明, 许 民, 刘苏昆. 废铅酸蓄电池渣泥湿法脱硫和还原新工艺研究[J]. 无机盐工业, 2004, 36(1): 47-49.
[34] 陆克源. 固相电解还原提取金属铅[J]. 化工冶金, 1983(3): 67-71.
[35] 韩 召, 李 领, 魏晓磊, 李 杰, 王海川. 硫酸铅在碳酸钠-硝酸钠熔盐中的脱硫转化[J]. 材料热处理学报, 2012, 33(6): 14-19.
[36] 吴秀安. 一种处理废旧铅酸蓄电池的工艺[P]. 中国, 201410723350.0, 2015-03-25.
WU Xiu-an. Process for disposing waste lead acid battery[P]. China, 201410723350.0. 2015-03-25.
[37] 高云芳, 董志根. 一种电解还原再生废铅酸蓄电池含铅膏泥中铅资源的方法[P]. 中国, 200710157084.X. 2008-08-27.
[39] 陈维平. 一种湿法回收废铅蓄电池填料的新技术[J]. 湖南大学学报(自然科学版), 1996, 23(6): 112-117.
[41] 杨新生. 从废铅蓄电池渣泥中制取铅系列化工产品的试验研究[J]. 江西冶金, 1995(2): 22-23.
[42] 齐美富, 郑园芳, 桂双林. 废铅酸蓄电池中铅膏氯盐体系浸取铅的动力学研究[J]. 矿冶工程, 2010, 30(6): 61-64.
[43] 刘 伟, 杨天足, 周琼华. 碱性多羟基化合物体系在有色冶金中的应用研究进展[J]. 湿法冶金, 2012, 31(1): 1-4.
[46] 邱德芬, 柯昌美, 王 茜, 张松山. 从废铅膏中回收铅及铅的化合物的方法[J]. 无机盐工业, 2014, 46(7): 16-19.
[55] 潘军青, 宋 爽, 马亚强, 孙艳芝, 钮因键. 一种基于原子经济途径回收废旧铅酸电池生产氧化铅的方法[P]. 中国, 201310084392.X. 2013-06-12.
[59] 吴广龙, 张正洁, 刘俐媛, 丁 琼. 废铅膏冶炼工艺比较分析研究[J]. 蓄电池, 2015, 52(5): 209-211.
[60] 朱铁权, 王昌燧, 毛振伟, 李立新, 黄 烘. 我国古代不同时期铅釉陶表面腐蚀物的分析研究[J]. 光谱学与光谱分析, 2010, 30(1): 266-269.


