Trans. Nonferrous Met. Soc. China 24(2014) 3944-3948
Electrical conductivity and viscosity of cryolite electrolytes for solar grade silicon (Si-SoG) electrowinning
Michal KORENKO1, Zuzana  1,
1,  1, Michal
1, Michal  1, Marta
1, Marta  2, Zhong-ning SHI3
2, Zhong-ning SHI3
1. Institute of Inorganic Chemistry, Slovak Academy of Sciences, Dúbravská cesta 9, SK–84536 Bratislava, Slovakia;
2. Institute of Inorganic Chemistry, Technology and Materials, Slovak University of Technology, Radlinskeho 9, SK–81237, Bratislava, Slovakia;
3. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 17 October 2013; accepted 24 November 2014
Abstract:
Electrical conductivity of molten binary and ternary mixtures based on the system NaF-AlF3-SiO2 was investigated by means of a tube–cell (made of pyrolytic boron nitride) with stationary electrodes. Viscosity of the binary system Na3AlF6-SiO2 was measured by computerized torsion pendulum method. It was found that conductivity and viscosity varied linearly with temperature in all investigated mixtures. Obtained content dependence of electrical conductivity (isotherms) was divided into two parts. First, one represented the content region up to 10% (mole fraction) of SiO2; second, the region was with a higher content of SiO2 (from 10% up to 40%). While the conductivity considerably decreased with content of SiO2 in the second part; it surprisingly rose in the low content range. A small addition of SiO2 to the molten cryolite (up to 10%) could slightly increase viscosity, but had no influence on the slope of this dependence since it is responsible for a glassy-networks formation in the melt. Further addition of SiO2 to the molten cryolite had a huge effect on the viscosity.
Key words:
electrical conductivity; viscosity; solar grade silicon; molten salts; molten cryolite–silica melts;
1 Introduction
Solar power is the most abundant form of renewable energy. It is not yet clear as to what portion of our energy will ultimately be solar-based, although it has the potential to far exceed the total energy demand of the globe. One of the current hurdles against solar energy becoming a major contributor to the energy basket is its high cost. Silicon, being the chief photovoltaic material, accounts for 25%-50% of the cost of solar arrays [1], thus an increasing attention has been paid to the generation of a so-called solar grade silicon (Si-SoG) at low cost. Even though prices of solar-cell modules keep on decreasing, it is still not cheaper in comparison with conventional ways of generating electricity, e.g. fossil fuel or nuclear generation.
High price of Si-SoG caused by limited supply has thus become the bottlenecks of the photovoltaic industry to achieve its much anticipated growth. It is therefore essential to develop a method for the synthesis of Si-SoG which is efficient energy and will deliver inexpensive feedstock material. As a promising approach, the electrodeposition of silicon from fluoride-based electrolytes at a relatively low temperature (1000 °C), similar method to Hall–Héroult process for Al production has been investigated by different researchers [2-13].
Previous examinations of several molten salt electrolytes have revealed that cryolite-based salts could be suitable for the electrolysis of silicon with respect to the product purity and current efficiency [7,8]. Nevertheless, the process has not been commercialized successfully to date due to one major problem: the high melting point of Si (1412 °C), which prevents formation of liquid silicon at the typical electrolysis temperature of 1000 °C. Moreover, a detailed knowledge of the physico- chemical properties of the different cryolite-based electrolytes is also very limited [6,10,14-16].
The present work is a part of larger project undertaken to obtain the primary physico-chemical properties like density, viscosity, electrical conductivity, vapour pressure and surface properties of different fluoride melts (contenting silicon species), mainly based on cryolite (Na3AlF6)–silica(SiO2) systems. The final object is thus to obtain the base for a physico-chemical description of molten electrolytes needed for the future electrowinning of Si-SoG.
Viscosity and particular electrical conductivity of the electrolytes have a primary importance for any electrochemical applications. Besides the mentioned above, some consideration on structure and transport theories of electrolytes may be also tested by means of these data. Although the physico-chemical properties of different molten cryolite systems are a subject of a long-term research due to the Hall–Héroult technology, data concerning the electrical conductivity and viscosity of the specific system Na3AlF6–SiO2 are rather rare [6,14,15].
2 Experimental
Synthetic cryolite (NaF–AlF3 of eutectic composition) was used for the preparing of the samples (AlF3 sublimated under low pressure (ca. 100 Pa) at 1100 °C). Analytical grade compounds like NaF (FlukaTM) and SiO2 (Johnson Mathey ChemicalsTM, U.K.) were used. For determination of the cell constant specpure NaCl and KCl (Johnson Mathey ChemicalsTM, U.K.) were used. All chemicals (except a sublimated AlF3) were dried under vacuum at 400 °C for several hours before the melting.
The conductivity cell consisted of a pyrolytic boron nitride (pBN) capillary (Boralloy, MomentiveTM, U.S.A.) of 4 mm in iner diameter and 100 mm in length. The electrode consisted of a platinum-rhodium alloy (10 % of rhodium) rod (1 mm outer diameter) in a fixed position inside the pBN capillary. A melt container (crucible made of the same alloy) was served as a counter electrode. Crucible containing ca. 35 g of salt mixture was placed in a closed vertical laboratory furnace under the inert argon atmosphere with some overpressure. The pBN capillary, with the platinum– rhodium electrode inside, was moved up and down to the melt several times in order to stir an electrolyte. A Pt-Pt10Rh thermocouple was used for the temperature measuring. An impedance/gain-phase analyzer (National InstrumentsTM a high-performance modular chassis controlled by LabviewTM software) was used to measure the cell impedance. Further experimental details of the procedure could be found elsewhere [17].
The torsion pendulum method based on the measurement of the logarithmic decrement of damping, caused by the friction in the melt, was used for the viscosity measurement. The measuring device was described in detail elsewhere [18]. The platinum cylinder with the diameter of 15 mm and the height of 20 mm was used as the measuring body. The oscillations of the pendulum system were registered by means of two phototransistors, placed in the path of a light beam reflected from a mirror attached to the pendulum. The measurements were carried out in the temperature interval of approximately 100-150 K. The measurement in the cooling direction was performed until the temperature of approximately 20 K above the temperature of primary crystallization.
The necessary amount of the mixture (50 g) was placed in the Pt crucible and then quickly transferred into the furnace that was preheated at 573 K, positioned just below the measuring body, where the sample was held under an atmosphere of dried nitrogen. After melting of the sample, the pendulum was immersed in the melt, and the surface of the melt was kept always 2 mm over the top of the cylinder. The depth of immersion was controlled using the electrical contact. The whole measuring device, including the furnace temperature was controlled by computer. After all the input data and the required temperature profile were inserted, the measurement of the viscosity at the desired temperatures was performed automatically. The experimental error in the viscosity measurement did not exceed 2.5%.
3 Results and discussion
A possibility of the chemical reactions in the melt containing Na3AlF6-SiO2 is of crucial importance in regard to reproducibility of the measurements performed in this system. According to ABRAMOV et al [19] and BELYAEV et al [20], silica reacts with AlF3 and Na3AlF6 under the formation of SiF4:
4AlF3(diss)+3SiO2(diss)  3SiF4(g)+2Al2O3(diss) (1)
3SiF4(g)+2Al2O3(diss) (1)
4Na3AlF6(l)+3SiO2(diss)  3SiF4(g)+12NaF(diss)+2Al2O3(diss) (2)
3SiF4(g)+12NaF(diss)+2Al2O3(diss) (2)
2Na3AlF6(l)+2SiO2(diss)  2SiF4(g)+4NaF(diss)+2NaAlO2(diss) (3)
2SiF4(g)+4NaF(diss)+2NaAlO2(diss) (3)
2NaAlO2(diss)+2SiO2(diss)  Na2O·Al2O3·2SiO2(diss) (4)
Na2O·Al2O3·2SiO2(diss) (4)
Changes in the Gibbs free energy corresponding to these reactions are pretty small [6] (-ΔG1026 °C=77.82 kJ; -ΔG1026 °C= 4.35 kJ). Regarding to the thermodynamic probability of reactions (1) and (2), MONNIER and BARAKAT [21], as well as WEILL and FYFE [16] assumed that rates of the above mentioned reactions are fairly small and a change in the composition of the melt is negligible.
On the other hand, mechanism proposed by MONNIER and BARAKAT [21] resulted in the dissociation into Si4+ and O2- ions. However, it seems to be fairly reasonable to assume that SiO2 dissolved in cryolite will form some types of complex anions that could substantially reduce activity of silicon in the melt [6]. Moreover, FELLNER and  [22] later reported chemical investigations in the molten system Na3AlF6-SiO2-Al2O3-AlF3. It was found that the components of this system reacted under the formation of SiF4 that resulted in the mass loss during experiments.
[22] later reported chemical investigations in the molten system Na3AlF6-SiO2-Al2O3-AlF3. It was found that the components of this system reacted under the formation of SiF4 that resulted in the mass loss during experiments.
As can be seen in Fig. 1 the conductivity in all of the investigated mixtures varied linearly with the temperature within the limits of error. Slope of the temperature dependence was the same for all mixtures, except for the last mixture (46.67% Na3AlF6+50%AlF3+ 3.33% SiO2, mole fraction). An addition of silica to cryolite had in general negative effect on the electrical conductivity of cryolite melts, except for the lower content of silica (5% and 10%, mole fraction). In Fig. 1, an influence of AlF3 addition on conductivity of the cryolite–silica systems is shown as well. Cryolite systems containing AlF3 are considered a main constituent for a new so-called acidic electrolyte in the Hall-Héroult process [23-25]. A main reason to measure an influence of SiO2 on the conductivity of acidic cryolite melts is to verify if a small addition of SiO2 will have a similar (positive) effect on the conductivity like in the case of non-acidic cryolite melts. As can be seen from Fig. 1, the small addition of SiO2 (3.33%, mole fraction) decreases conductivity of the acidic cryolite melt (cryolite with higher concentration of AlF3).
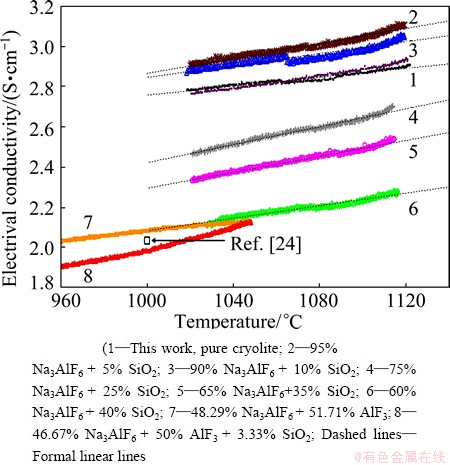
Fig. 1 Electrical conductivity of mixture Na3AlF6-AlF3-SiO as function of temperature
Transport properties of mixtures are usually presented in the diagrams showing isotherms. The conductivity of the investigated systems as a function of the content of SiO2 is shown in Fig. 2 (the binary systems based on Na3AlF6–SiO2). As can be seen from Fig. 2, the isothermal dependency can be divided into two parts. First one represents the region up to 10% of SiO2, and the second one is the region with composition from 10% to 40% (and probably more) of SiO2. While the conductivity considerably decreases with the concentration of SiO2 in the second part according to BELYAEV [14], GRJOTHEIM et al [7] and SOKHANVARAN et al [15], it surprisingly slightly rises with concentration of SiO2 in the low concentration range. This trend of conductivity isotherms (at small concentration of SiO2) is not in agreement with the previous works.
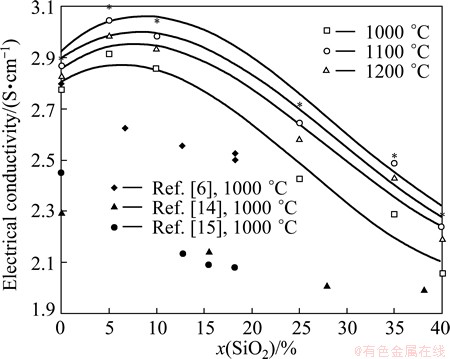
Fig. 2 Electrical conductivity isotherms of mixture Na3AlF6–SiO2 and literature data
In general, the deviation in certain values of electrical conductivity is in the case of GRJOTHEIM et al [6] up to ca. 6%, in the case of BELYAEV [14] up to ca. 20%, in the case of  et al [24] up to ca. 5%, and in the case of SOKHANVARAN et al [15] up to ca. 20 %.
et al [24] up to ca. 5%, and in the case of SOKHANVARAN et al [15] up to ca. 20 %.
Electrical conductivity of pure cryolite as well as cryolite–silica mixtures mostly differs from that measured by BELYAEV [14] and SOKHANVARAN et al [15]. The difference between this work and literature data can be generally explained by the difference between experimental procedures. Needless to say, sufficiently insulated and inert materials (such as pyrolytic boron nitride) were not available [14,7]. Moreover, an experimental value of the electrical conductivity of pure cryolite (at 1000 °C) reported by SOKHANVARAN et al [15] is very low (ca. 2.42 S/cm) compared with the value of other authors, e.g., for pure cryolite, it is (2.80±0.02) S/cm [23-26]. Likewise, the melting point of pure cryolite (998.3±2) °C, mentioned in Ref. [15], shows considerable deviation from the most cited value of 1011.6 °C [23].
The most interesting point of the present study is that the electrical conductivity of molten cryolite slightly increases with the small addition of silica (up to 10% of SiO2). This surprising behaviour is likely caused by the reaction between molten cryolite and silica (reactions 2, 3 and 4) under the formation of NaF [19,20,22]. This means that the small addition of silica to the molten cryolite a little bit changes so-called cryolite ratio (CR) which is the mole ratio of NaF to AlF3, thus a pure cryolite occurs at the ratio of 3. It is generally accepted that the shift in CR extremely affects the electrical conductivity of the molten cryolite (for CR=1, ca. 1.2 S/cm; for CR=3, ca. 2.8 S/cm; and for CR=9, ca. 4.2 S/cm) [26]. Thus, a small addition of silica to the molten cryolite could (in this way) slightly increase the electrical conductivity. Further addition of silica to the molten cryolite has, however, a negative effect on the conductivity, since it is responsible for a glassy-networks formation in the melt and is accompanied by a substantial increase of viscosity and thus degradation of the transport properties (see Fig. 3).
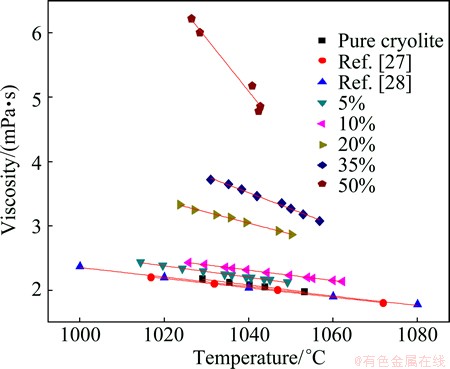
Fig. 3 Viscosity of mixture Na3AlF6–SiO2
This assumption is also indirectly supported by the results obtained in acidic cryolite melts (Na3AlF6-SiO2- AlF3 system, see Fig. 1). As can be seen from Fig. 1, a small addition of SiO2 to the acidic cryolite has no positive influence on the conductivity like it is in the case of pure cryolite. Silica could react with AlF3 without the formation of NaF (reaction (1)). A possible formation of NaF (by reactions (2), (3) or (4)) is moreover buffered by the presence of a huge amount of AlF3 in the melt that results in the retention of CR in the acidic melt.
Figure 3 shows the experimental results of the viscosity measurement in the Na3AlF6-SiO2 system. These results are shown in the form of relationship between viscosity and temperature at different contents of silica in cryolite. It also shows the values of the viscosity of pure cryolite from previous works [27,28]. The results are in good agreement.
As can be seen from Fig. 3, the viscosity in all investigated mixtures varies linearly with the temperature within the limit of error. Slope of the temperature dependence is the same only in a small content of silica (ca. up to 10% SiO2). At higher contents, the slope linearly varies with the content of silica in molten cryolite. A small addition of silica to the molten cryolite (up to 10%) could slightly increase viscosity, but has no influence on the slope of this dependence. Further addition of silica to the molten cryolite has, however, great effects on the viscosity, since it is responsible for a glassy-network formation in the melt. Higher addition of silica has even the influence on the slope of the temperature dependence of viscosity.
4 Conclusions
1) The electrical conductivity and viscosity vary linearly with temperature in all investigated mixtures of the Na3AlF6-SiO2 system. Slope of the temperature dependence is in the case of electrical conductivity, the same for all of the investigated mixtures.
2) Addition of AlF3 to this system decreases the electrical conductivity. While the conductivity considerably decreases with content of SiO2 in the second part, and it surprisingly slightly rises with the content of SiO2 in the low content range. This behavior is likely caused by the reaction between molten cryolite and silica under the formation of NaF, which changes the cryolite ratio in the melt and thus affects the conductivity.
3) Further addition of silica has a negative effect on the conductivity, since it is responsible for a glassy-network formation in the melt and is accompanied by a substantial increase of viscosity and thus degradation of the transport properties.
Acknowledgments
This work was supported by the Science and Technology Assistance Agency (APVV-0460-10 and SK-CN-0029-12), Slovak Grant Agency (VEGA 2/0116/14 and VEGA 2/0095/12) and the National Natural Science Foundation of China (51322406) and the Program for New Century Excellent Talents (NCET-13-0107), Ministry of Education of China. The authors want to also acknowledge the China-Slovakia Inter-governmental S&T Cooperation Program.
References
[1] ISTRATOV A A, BUONASSISI T, PICKETT M D, HEUER M, WEBER E R. Control of metal impurities in “dirty” multicrystalline silicon for solar cells [J]. Mater Sci Eng B, 2006, 134: 282-286.
[2] ELWELL D, RAO G M. Electrolytic production of silicon [J]. J Appl Electrochem, 1988, 18(1): 15-22.
[3] ELWELL D, FEIGELSON R S. Electrodeposition ofsolarsilicon [J]. Sol Energ Mat Sol C, 1982, 6: 123-145.
[4] MONNIER R, GIACOMETTI J C. Recherches sur le raffinage  du silicium [J]. Helv Chim Acta, 1964, 47(6): 345-353. (in French)
du silicium [J]. Helv Chim Acta, 1964, 47(6): 345-353. (in French)
[5] OLSON J M, CARLETON K L. A semipermeable anode for silicon electrorefinning [J]. J Electrochem Soc, 1981, 128(12): 2698-2699.
[6] GRJOTHEIM K,  FELLNER P,
FELLNER P,  A. Electrolytic deposition of silicon and silicon alloys [J]. Canadian Metallurgical Quarterly, 1971, 10: 79-82.
A. Electrolytic deposition of silicon and silicon alloys [J]. Canadian Metallurgical Quarterly, 1971, 10: 79-82.
[7] MONNIER R, BARAKAT D, GIACOMETTI J C. Refining of silicon and germanium: US, 3254010 [P]. Gen. Trustee Company Inc, 1966.
[8] MONNIER R, BARAKAT D. Dual cell refining of silicon and germanium: US, 3219561 [P]. Gen. Tustee Company Inc., 1965.
[9] BOEN R, BOUTEILLON J. The electrodeposition of silicon in fluoride melts [J]. J Appl Electrochem, 1983, 13: 277-288.
[10] GRJOTHEIM K,  FELLNER P. The electrodeposition of silicon from cryolite melts [J]. Light Metals, 1982: 333-341.
FELLNER P. The electrodeposition of silicon from cryolite melts [J]. Light Metals, 1982: 333-341.
[11] OLSON K S, MARTINEZ A M, ROLSETH S, GUNDBRANSEN H, JUEL M, HAARBERG G M. Electrodeposition of crystalline silicon films from alkali fluoride mixtures [J]. Electrochem Soc Trans, 2010, 33: 429-438.
[12] OISHI T, WATANABE M, KOYAMA K, TANAKA M, SAEGUSA K. Process for solar silicon production by molten salt electrolysis using aluminium-silicon liquid alloy [J]. J Electrochem Soc, 2011, 158(9): E93-E99.
[13] BIEBER A L, MASSOT L, GIBILARO M, CASSAYRE L, TAXIL P, CHAMELOT P. Silicon electrodeposition in molten fluorides [J]. Electrochim Acta, 2012, 62: 282-289.
[14] BELYAEV A I. Fiziko-khimicheskie protsessy pri elektrolize alyuminiya [M]. Moscow: Metallurgizdat, 1947. (in Russian)
[15] SOKHANVARAN S, THOMAS S, BARATI M. Charge transport properties of cryolite-silica melts [J]. Electrochim. Acta, 2012, 66: 239-244.
[16] WEIL D F, FYFE W S. The 1010 °C and 800 °C isothermal section in the system Na3AlF6-Al2O3-SiO2 [J]. J Electrochem Soc, 1964, 111: 582-585.
[17] KORENKO M,  Electrical conductivity of systems based on Na3AlF6-SiO2melt [J]. Chem Pap, 2013, 67: 1350-1354.
Electrical conductivity of systems based on Na3AlF6-SiO2melt [J]. Chem Pap, 2013, 67: 1350-1354.
[18]  V. Physicochemical analysis of molten electrolytes [M]. Amsterdam, the Netherlands: Elevier, 2006: 370.
V. Physicochemical analysis of molten electrolytes [M]. Amsterdam, the Netherlands: Elevier, 2006: 370.
[19] ABRAMOV G A, VETYUKOV M M, GUPALO I P, KOSTYUKOV A A. Teoreticheskie osnovy elektrometallurgii alyuminia. Metallurgizdat [M]. Moscow: 1953. (in Russian)
[20] BELYAEV A I, ZHEMCHUZINA Y A, FIRSANOVA L A. Fyzicheskaya khimia rasplavlennykh solei [M]. Metallurgizdat, Moscow, 1957. (in Russian)
[21] MONNIER R, BARAKAT D. Contribution a l`étude du comportement de la silice danes les bains de cryolithe fondue [J]. Helv Chim Acta, 1957, 47: 2041-2045. (in French)
[22] FELLNER P,  Chemical reactions in molten Na3AlF6-SiO2-Al2O3-AlF3 [J]. Chem Pap, 1973, 27: 737-741.
Chemical reactions in molten Na3AlF6-SiO2-Al2O3-AlF3 [J]. Chem Pap, 1973, 27: 737-741.
[23] THONSTAD J, FELLNER P, HAARBERG G M,  J, KVANDE H, STERTEN
J, KVANDE H, STERTEN  . Aluminium electrolysis [M]. 3rd ed. Düsseldorf: Aluminium – Verlag, 2001.
. Aluminium electrolysis [M]. 3rd ed. Düsseldorf: Aluminium – Verlag, 2001.
[24]  J, THONSTAD J, STERTEN
J, THONSTAD J, STERTEN  , FELLNER P. Electrical conductivity of molten cryolite-based mixtures obtained with a tube-type cell made of pyrolytic boron nitride [J]. Met Mater Trans B, 1996, 27: 255-261.
, FELLNER P. Electrical conductivity of molten cryolite-based mixtures obtained with a tube-type cell made of pyrolytic boron nitride [J]. Met Mater Trans B, 1996, 27: 255-261.
[25] FELLNER P, KOBBELTVELD O, STERTEN  , THONSTAD J. Electrical conductivity of molten cryolite-based binary mixtures obtained with a tube-type cell made of pyrolytic boron nitride [J]. Electrochim Acta, 1993, 38: 589-592.
, THONSTAD J. Electrical conductivity of molten cryolite-based binary mixtures obtained with a tube-type cell made of pyrolytic boron nitride [J]. Electrochim Acta, 1993, 38: 589-592.
[26] GRJOTHEIM K, KROHN C,  K, THONSTAD J. Aluminium electrolysis: Fundamentals of the Hall –Héroult process [M]. Düsseldorf: Aluminium–Verlag, 1982.
K, THONSTAD J. Aluminium electrolysis: Fundamentals of the Hall –Héroult process [M]. Düsseldorf: Aluminium–Verlag, 1982.
[27] ROBELIN C H, ChARTRAND P. A viscosity model for the (NaF+AlF3+CaF2+Al2O3) electrolyte [J]. J Chem Thermodynamics, 2011, 13: 761-771.
[28] BROCKNER W, TORKLEP K,  H A. Viscosity of sodium fluoride-aluminium fluoride melt mixtures [J]. Ber Bunsenges Phys Chem, 1979, 83(1): 12-19.
H A. Viscosity of sodium fluoride-aluminium fluoride melt mixtures [J]. Ber Bunsenges Phys Chem, 1979, 83(1): 12-19.
太阳能级硅电沉积用冰晶石熔盐的电导率和黏度
Michal KORENKO1, Zuzana  1,
1,  1,Michal
1,Michal  1 Marta
1 Marta  2, 石忠宁3
2, 石忠宁3
1. Institute of Inorganic Chemistry, Slovak Academy of Sciences, Dúbravská cesta 9, SK–84536 Bratislava, Slovakia;
2. Institute of Inorganic Chemistry, Technology and Materials, Slovak University of Technology, Radlinskeho 9, SK–81237, Bratislava, Slovakia;
3. 东北大学 材料与冶金学院,沈阳 110819
摘 要:以热解碳化硼管作电导池,用固定电导池常数法研究由NaF-AlF3-SiO2构成的二元系和三元系熔盐的电导率;用扭摆法测定Na3AlF6-SiO2二元系的黏度。研究发现,所研究熔盐体系的电导率和黏度均与温度呈直线关系。Na3AlF6-SiO2二元系的电导率与SiO2含量关系曲线可分为0~10%和10%~40%(摩尔分数)两段,当SiO2含量超过10%之后,电导率随着SiO2含量的增加而快速下降,而当SiO2含量小于10%时,电导率随着SiO2含量的增加而缓慢增加。在SiO2含量大于10%的冰晶石熔盐中添加少量的SiO2,熔盐的黏度增加,但增加的趋势基本相同,这与熔盐中形成玻璃网状结构的离子团有关。当在冰晶石熔盐中继续增大SiO2含量到50%时,黏度发生急剧变化。
关键词:电导率;黏度;太阳能级硅;熔盐;冰晶石-氧化硅熔盐
(Edited by Xiang-qun LI)
Corresponding author: Michal KORENKO; Tel: +421-02-59410463; E-mail: Michal.Korenko@savba.sk;
Zhong-ning SHI; Tel: +86-24-83686464; E-mail: znshi@mail.neu.edu.cn
DOI: 10.1016/S1003-6326(14)63554-8
Abstract: Electrical conductivity of molten binary and ternary mixtures based on the system NaF-AlF3-SiO2 was investigated by means of a tube–cell (made of pyrolytic boron nitride) with stationary electrodes. Viscosity of the binary system Na3AlF6-SiO2 was measured by computerized torsion pendulum method. It was found that conductivity and viscosity varied linearly with temperature in all investigated mixtures. Obtained content dependence of electrical conductivity (isotherms) was divided into two parts. First, one represented the content region up to 10% (mole fraction) of SiO2; second, the region was with a higher content of SiO2 (from 10% up to 40%). While the conductivity considerably decreased with content of SiO2 in the second part; it surprisingly rose in the low content range. A small addition of SiO2 to the molten cryolite (up to 10%) could slightly increase viscosity, but had no influence on the slope of this dependence since it is responsible for a glassy-networks formation in the melt. Further addition of SiO2 to the molten cryolite had a huge effect on the viscosity.


