Trans. Nonferrous Met. Soc. China 29(2019) 1082-1089
Selective flotation of smithsonite from dolomite by using novel mixed collector system
Li WANG1,2, Guang-yan HU1,2, Wei SUN1,2, Sultan Ahmed KHOSO1,2, Run-qing LIU1,2, Xiang-feng ZHANG1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing
Mineral Resources, Central South University, Changsha 410083, China
Received 2 June 2018; accepted 14 January 2019
Abstract:
A novel mixed collector (BHOA) was prepared by mixing benzohydroxamic acid (BHA) and sodium oleate (NaOL) and applied to the flotation separation of smithsonite from dolomite. Flotation results showed that NaOL alone had good collecting performance on smithsonite and common gangue mineral dolomite but had poor selectivity. By using a BHA/NaOL mixed system with a molar ratio of 2:1, the recoveries of smithsonite and dolomite reached approximately 90% and 5%, respectively. Surface tension analysis showed that the surface activity of BHOA was a little higher than that of a single NaOL because of synergistic effects. Zeta potential and X-ray photoelectron spectroscopy measurements indicated that surfactants BHA and NaOL co-absorbed on the smithsonite surface and only NaOL was present on the dolomite surface in the presence of BHOA.
Key words:
flotation; smithsonite; dolomite; sodium oleate; benzohydroxamic acid; sulfuration;
1 Introduction
Flotation is the most effective pretreatment technique for zinc oxide ores, and the flotation of zinc oxide ores has been extensively researched. The sulfide–xanthate method is one of the main flotation methods for zinc oxide recovery. However, the recovery efficiency is not high because of the short carbon chains and weak collection performance of xanthate [1], and the sulfide–xanthate method has several notable drawbacks: (1) it is sensitive to slime, thus necessitating removal of slime beforehand and resulting in a low total recovery of zinc; (2) it is unsuitable for zinc oxide ores with high iron contents; (3) zinc oxide minerals are difficult to recover from the silicate phase; (4) the control of sodium sulfide dosage as a sulfide agent is difficult; and (5) sulfuration consumes a considerable amount of energy during heating and thus increases costs. Therefore, the sulfide–xanthate method is only suitable for the treatment of smithsonite with low iron oxide and slime contents [2-4].
The feasibility of fatty acids as collectors for zinc oxides has been extensively investigated because of their low costs [5]. Fatty acid collectors can be used in the reverse flotation process owing to their good flotation performance for quartz or clay gangue minerals in zinc oxide ores [6]. Fatty acids exhibit excellent flotation performance but have relatively poor selectivity for smithsonite [7-9]. The strong collection of fatty acids on iron oxide ores prevents the separation of zinc oxide from iron oxide gangues. Benzohydroxamic acid is commonly used as an anionic chelating collector and widely used in the flotation of oxidized ores, such as scheelite and cassiterite [10]. However, the use of a single anion collector often results in poor selectivity, although selective separation can be enhanced by using activators and depressants [11].
The synergy between individual components considerably enhances the performance of resulting recovery system when the surfactants are mixed at certain proportions [12-15]. Mixed surfactant systems often show synergistic behaviors that decrease critical micelle concentration, improve the efficiency of wetting, decrease surface tension of water, and promote solubilization and foaming [16]. The combination of different flotation reagents can enhance the treatment effect and flotation index and reduce the total dosage of treatment, thereby improving the economic benefit of production [17]. The mixed anionic and cationic (catanionic system) collectors for smithsonite were firstly investigated in solutions containing DDA/KAX collectors [18,19]. Although anionic collectors are generally used in mineral flotation industries, their adsorption and flotation mechanisms remain unknown.
The purpose of this study is to prepare a novel mixed collector system by mixing benzohydroxamic acid (BHA) and sodium oleate (NaOL) for the selective flotation separation of smithsonite from dolomite. The difference between the flotation effects of NaOL and BHA on smithsonite and dolomite was evaluated before and after sulfuration. The surface activity and adsorption mechanism of a mixed collector on the surface of smithsonite and dolomite were investigated.
2 Experimental
2.1 Materials and reagents
Smithsonite and dolomite samples were purchased from Yunnan Province and Hunan Province, China, respectively. After the impurities were discarded, the samples were ground with a ceramic ball mill. Single mineral flotation experiments were performed on 0.074-0.037 mm size fraction. The fraction with size less than 0.037 mm was used for further analysis of surface activity and adsorption mechanisms. The X-ray diffraction (XRD) spectra of pure smithsonite and dolomite minerals are shown in Fig. 1. The purities of smithsonite and dolomite were 98.78% and 98.93%, respectively, which satisfied the purity requirement of the mineral tests. Benzohydroxamic acid, sodium oleate, and sodium sulphide were purchased from Sinopharm Chemical Reagent Co., Ltd. All the reagents had an analytical purity of 99.9%. The pH value of the solution was adjusted with reagent-grade hydrochloric acid and sodium hydroxide, and deionized water was used in all the processes.
2.2 Flotation tests
Single-mineral flotation tests were conducted by using a 40 mL XFG flotation machine operated at a fixed rotational speed of 1800 r/min. Pulp was prepared by mixing 2 g of mineral in 40 mL of water and agitated for 1 min. Pulp pH was controlled by the addition of an acid or alkali stack solutions. The collector was then added to the pulp and conditioned for 3 min. Then, pine oil (120 g/t) was added. The conditioning time was 2 min. Finally, froth collection was performed for 3 min. The concentrates and tailings received were filtered, dried, weighed, and sent for further analysis, and the yield rate and recovery were calculated. Flotation results were represented by the average value of three different measurements.
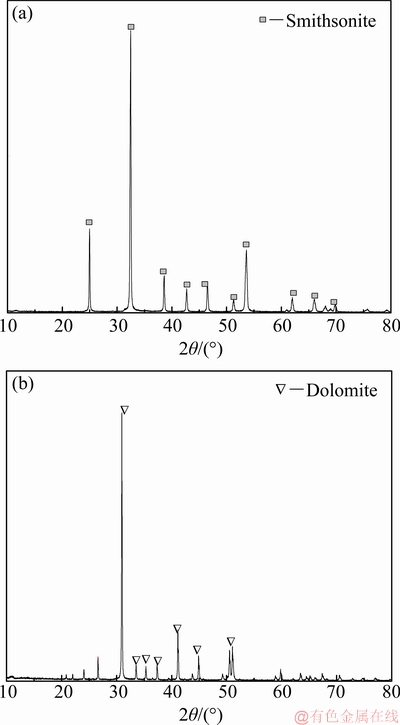
Fig. 1 XRD spectra of smithsonite (a) and dolomite (b)
2.3 Surface tension measurements
Surface tension values of NaOL, BHA, and BHOA solutions were determined through the Wihlmy–Cooper gold plate hanging piece method. An ILMS surface tester (GBX Company, France) was used. Firstly, 15 mL of the sample solution was placed in a glass dish, which was in turn placed onto the sample stage of the surface tension instrument. The surface tension value of the solution was obtained. Then, anhydrous ethanol and ultra-pure water were used to clean the glass dish and the hanging piece, and an alcohol lamp was used to dry the hanging piece. All tests were conducted through the same procedure. Each test was repeated three times for each sample, and the average was taken as the measured value.
2.4 Zeta potential measurements
Zeta potentials of minerals before and after the treatment with reagents were measured by a JS94H microelectrophoresis instrument (Shanghai Zhongchen Digital Technic Apparatus Co., China) [20]. Exactly 0.2 g of the sample and 20 mL of ultra-pure water were mixed to a 50 mL beaker. The pH of the slurry was adjusted with hydrochloric acid or sodium hydroxide. A fixed concentration of the collector was added to the slurry, and the mixture was placed on a magnetic stirrer, which was set to a fixed speed for 5 min. Finally, the zeta potential of the slurry was measured. The results presented are the average of three independent measurements with a typical variation of ±2 mV.
2.5 X-ray photoelectron spectroscopy measurements
X-ray photoelectron spectroscopy (XPS) analysis was performed on an ESCALAB 250Xi model spectro- meter (ThermoFisher-VG Scientific, Massachusetts, USA). In accordance with the single-mineral flotation test, 2.0 g of the mineral sample and 40 mL of water were added to a 40 mL flotation cell. The mixture was stirred for 1 min, and the collector solution was added to the sample. The sample was thoroughly stirred, then filtered, and finally rinsed with ultra-pure water. The sample was repeatedly rinsed five times and then filtered and dried in a vacuum chamber at 25 °C prior to further testing.
3 Results and discussion
3.1 Flotation performance of single mineral
The flotation efficiency of smithsonite with different proportions of BHA and NaOL was studied extensively. The total concentration of the collector was set to be 6×10-4 mol/L. Smithsonite flotation recovery at pH 9.0 is shown in Fig. 2(a). Smithsonite was not recovered by flotation when only BHA was used. By contrast, the flotation recovery for smithsonite was as high as 94% when only NaOL was used. The mixed collector system (BHA/NaOL) exhibited a good smithsonite recovery (>80%). When the molar ratio of the BHA/NaOL system was set to be 1:2, the flotation recovery of smithsonite reached approximately 92%, which is nearly equal to that obtained when only NaOL was used. Therefore, a mixed collector system with BHA/NaOL molar ratio of 1:2 was proposed and designated as BHOA. Figures 2(b) and (c) show the effects of pH and Na2S dosage on the flotation recovery of smithsonite, respectively. The optimum flotation pH was 9.0, and the optimum Na2S dosage was 2500 g/t.
Figure 3 shows the effect of collector concentration on the flotation recoveries of smithsonite and dolomite. As shown in Figs. 3(a) and (b), the flotation recovery of smithsonite was considerably high in the presence of sodium sulfide, and sodium sulfide slightly influenced the recovery of dolomite.
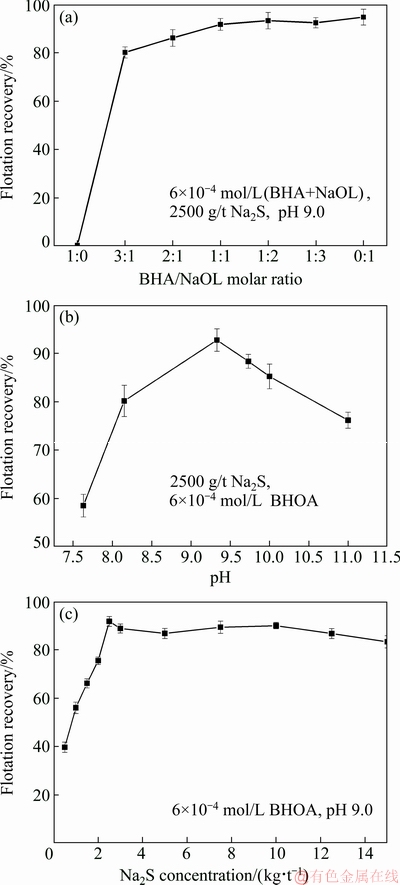
Fig. 2 Effects of BHA/NaOL molar ratio (a), pH (b) and Na2S dosage (c) on smithsonite flotation recovery
Furthermore, the recovery of smithsonite increased rapidly with increasing the collector concentration in the presence of BHOA and then reached a maximum recovery of 95% when the collector concentration was 6×10-4 mol/L. The recovery of smithsonite decreased slightly, and the recovery of dolomite remained below 10% when the collector concentration was less 6×10-4 mol/L. When only NaOL was used, the flotation recovery of smithsonite showed a quit equivalent trend with BHOA, and the amount of recovered dolomite was slightly higher than that in the case of BHOA. Thus, the selective separation of these two minerals is difficult when NaOL is used alone. Therefore, smithsonite can be selectively separated from dolomite by using BHOA and controlling BHOA concentration.
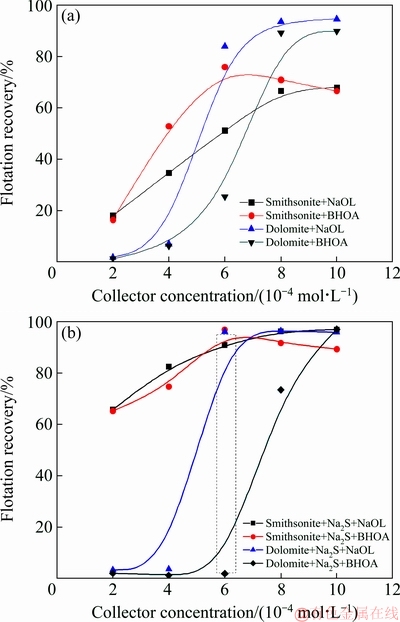
Fig. 3 Recoveries of smithsonite and dolomite at different collector concentrations without (a) and with (b) sulfuration at pH 9.0
3.2 Surface properties of BHOA, NaOL and BHA
The equilibrium surface tension values of NaOL, BHA, and BHOA as function of their concentrations in ultrapure water at pH 9 are presented in Fig. 4.

Fig. 4 Equilibrium surface tension values of NaOL, BHA, and BHOA as function of their concentrations in ultrapure water at pH 9.0
The critical micelle concentration (CMC) values for surfactant mixtures were determined from Fig. 4 by separately fitting straight lines to rapidly decreasing, stable, and increasing portions of the curves and then calculating the surfactant concentrations where the lines intersected [21]. As shown in Fig. 4, BHA showed a relative low surface activity because of its high surface tension. The surface tension of the NaOL solution decreased rapidly when its concentration changed from 1×10-5 to 1×10-4 mol/L. Moreover, the surface tension remained unchanged when the concentration of the NaOL solution exceeded 1×10-4 mol/L. The surface tension of the BHOA solution rapidly decreased at concentrations ranging from 1×10-5 to 1×10-4 mol/L (see Fig. 4).
By comparing the surface tension curves of BHOA, NaOL, and BHA solutions, BHOA surfactants were found to be more efficient than single collector in decreasing air-water interfacial tension, particularly in low surfactant concentration conditions. This finding indicated that the mixture of NaOL and BHA has a synergistic effect on surface tension.
3.3 Zeta potential measurements
The adsorption mechanisms of the collectors on smithsonite and dolomite surfaces were investigated. Zeta potential measurements were carried out before and after the addition of collectors. As shown in Fig. 5(a), the isoelectric point of smithsonite was pH 8.2, which was similar to that reported in Ref. [22]. Figure 5(b) shows the surface potential of smithsonite as a function of concentration of collectors. After the addition of sodium sulfide, the zeta potential of smithsonite remained unchanged. This response indicated that BHA was not adsorbed on the smithsonite surface. Before the addition of sodium sulfide, NaOL or BHOA caused the increase in the negative surface zeta potential of smithsonite. The higher the concentration of NaOL or BHOA solution is, the greater the negative value of the surface zeta potential of smithsonite is. This relationship indicates the adsorption of large amount of these collectors onto the smithsonite surface. Furthermore, under the same concentration, BHOA caused a higher increase in the negative surface zeta potential of smithsonite than NaOL in the presence or absence of sodium sulfide. This result indicated that BHA also played an important role in the adsorption behavior of mixed collectors on smithsonite surface.
Figure 6(a) shows that the surface zeta potential of dolomite varies with changes in pH. The isoelectric point of dolomite was at pH 6.5. As shown in Fig. 6(b), the zeta potential of dolomite remained unchanged after the addition of sodium sulfide and BHA, and thus no adsorption or limited adsorption occurred on the surface of dolomite. After sulfuration, the negative zeta potential of dolomite was high in the NaOL or BHOA system.

Fig. 5 Surface potentials of smithsonite at different pH values (a) and collector concentrations at pH 9.0 (b)
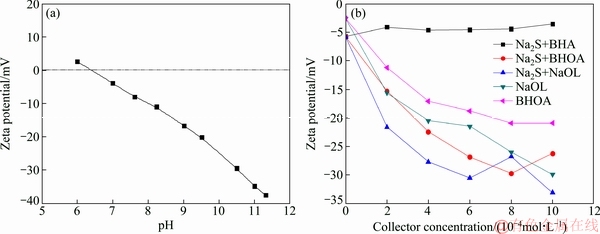
Fig. 6 Surface potentials of dolomite at different pH values (a) and collector concentrations at pH 9.0 (b)
These results may be attributed to the negative effect of sodium sulfide and to the increased adsorption of the reagent. The effect of a fixed concentration of BHOA on the surface zeta potential of dolomite was equivalent to the effect of NaOL. Therefore, in the presence of mixed collectors, BHA had no effect on the surface and surface zeta potential of dolomite.
3.4 XPS analysis
XPS uses X-ray photons to excite the inner electrons of atoms on the material surface. An energy spectrum is obtained by analyzing the excitation of inner electrons. Therefore, XPS can be used for the qualitative, quantitative, or semi-quantitative analysis of the composition and chemical state of the surface of a solid sample [23]. In this work, we conducted XPS analysis to characterize the differences between treated and untreated smithsonite and dolomite samples with different collectors, and the results are presented in Figs. 7-9.
Figure 7 shows XPS spectra of smithsonite, Zn 2p, S 2p, and C 1s of smithsonite before and after treatment with different collectors. Figure 7(a) shows the full spectra of the smithsonite surface before and after treatment in the binding energy range of 1020-1060 eV. Figure 7(b) shows that new absorption peaks at 1020.25 eV were observed after the treatment with sodium sulfide, indicating that a new Zn-containing compound was produced on the smithsonite surface. Figure 7(c) shows that the S 2p peak in the spectra of smithsonite and sodium sulfide appeared at 161.9 eV. The spectra of Zn 2p and S 2p before and after the action of smithsonite and sodium sulfide indicated that S2- and Zn2+ reacted together to produce ZnS after smithsonite and sodium sulfide treatment.
In Fig. 7(d), the C 1s absorption peaks at 285 eV corresponded to the organic carbon in alkyl, whereas those at 290 eV corresponded to CO2-3 .In the presence of Na2S and NaOL, the intensities of the absorption peaks at 285 eV significantly increased. The increase indicated that NaOL was adsorbed on the smithsonite surface. The XPS spectra of smithsonite after Na2S and BHOA treatment showed that the intensity of C 1s absorption peak at 285 eV was significantly enhanced relative to that in the XPS spectra of pure smithsonite. Thus, NaOL or BHA was adsorbed on the smithsonite surface after vulcanization. In N 1s spectra, the absorption peaks at 405 eV corresponded to N atoms in BHA (see Fig. 8), indicating that low amounts of BHA were weakly adsorbed on smithsonite.
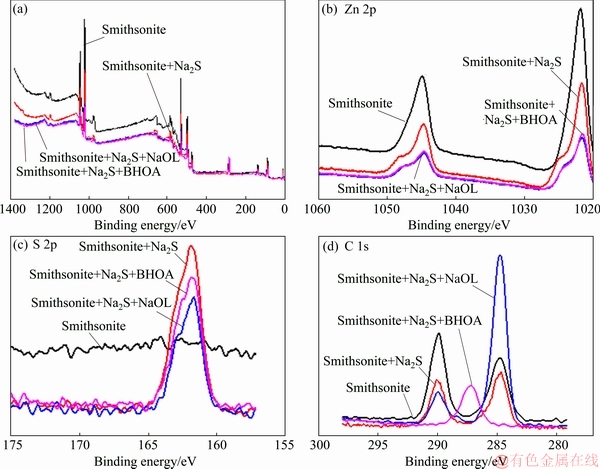
Fig. 7 XPS spectra of smithsonite (a), Zn 2p (b), S 2p (c), and C 1s (d) of pure smithsonite before and after treatment with different collectors
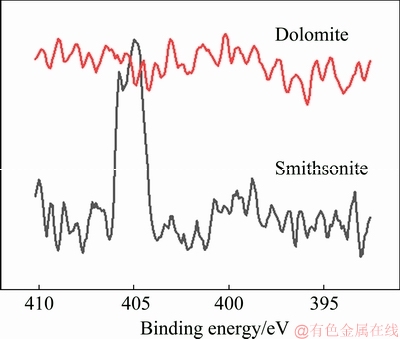
Fig. 8 XPS spectra for N atoms of BHOA after reaction with sodium sulfide
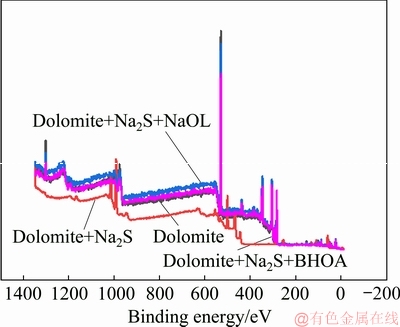
Fig. 9 XPS spectra of dolomite before and after treatment with different collectors
Figure 8 shows that the XPS spectra of dolomite remained unchanged after Na2S treatment, and Na2S and dolomite did not react to form sulfides. Figure 9 shows the XPS spectra of the dolomite surface before and after treatment with different collectors. The increased intensity of the C 2p absorption peak at 285 eV after Na2S and NaOL treatment indicated that NaOL was adsorbed on the dolomite surface. As shown in Figs. 8 and 9, no significant absorption peaks were observed in the N 1s spectra in the presence of Na2S and BHOA. The absence of these peaks indicated that only NaOL was adsorbed onto the dolomite surfaces in the mixed collectors.
In the XPS results, the reaction of sodium sulfide with smithsonite produced ZnS on the surface of smithsonite. In the presence of Na2S and BHOA, NaOL was adsorbed on the surfaces of smithsonite and dolomite. Meanwhile, BHOA only affected smithsonite, thus increasing the selectivity of the mixed collector system.
4 Conclusions
(1) A novel mixed collector (BHOA) was prepared by mixing BHA and NaOL and used for the selective flotation separation of smithsonite from dolomite. The separation mechanism of BHOA was evaluated by flotation tests, surface tension analysis, zeta potential, and XPS.
(2) BHOA has a better collecting efficiency for smithsonite than NaOL. The flotation recovery of smithsonite reached 92% when the NaOL/BHA molar ratio was set to be 2:1. Meanwhile, a poor dolomite recovery of less than 20% was obtained.
(3) The BHOA shows better surface activity than pure collectors because of synergistic effects and thus it is much more suitable for the flotation separation of smithsonite.
(4) Negative surface potentials of smithsonite and dolomite increased considerably when NaOL or BHOA was used alone. However, the BHOA system only increased the negative surface potential of smithsonite. Meanwhile, NaOL affected the negative surface potential of dolomite, and the effect was independent of BHA.
(5) NaOL chemically reacted with smithsonite and dolomite and the BHA in BHOA collector only reacted with smithsonite, thus increasing the selectivity of the collector for smithsonite.
References
[1] ESPIRITU E R L, SILVA G R D, AZIZI D, LARACHI F, WATERS K E. The effect of dissolved mineral species on bastnasite, monazite and dolomite flotation using benzohydroxamate collector [J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2018, 539: 319-334.
[2] YANG Jin-lin, ZHANG Hong-mei, MO Wei, MA Shao-jian, SU Xiu-juan. Flotation tests of zinc oxide ore with iron [J]. Advanced Materials Research, 2013, 826: 57-60.
[3] CHEN Jin-quan, ZHOU De-yan, WEI Zong-wu, CHEN Jian-hua. Experimental study on flotation of a mudded lead-zinc oxide ore with high iron content [J]. Mining Research & Development, 2007, 17: 475-484.
[4] CHEN Zhi-wei. Experimental study on flotation technology of iron-bearing and sliming zinc oxide ore [J]. Mining & Metallurgical Engineering, 2008, 28: 51-53.
[5] ZHANG Ye, HU Yue-hua, SUN Ning, LIU Run-qing, WANG Zhen, WANG Li, SUN Wei. Systematic review of feldspar beneficiation and its comprehensive application [J]. Minerals Engineering, 2018, 128: 141-152.
[6] WAN R Y, LEVIER M K, CLAYTON R B. Hydrometallurgical process for the recovery of precious metal values from precious metal ores with thiosulfate lixiviant [J]. Minerals Engineering, 1995, 8: 939.
[7] HOSSEINI S H, FORSSBERG E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid [J]. Minerals & Metallurgical Processing, 2006, 23: 87-96.
[8] SHI Qing, FENG Qi-ming, ZHANG Guo-fan, DENG Hong. Electrokinetic properties of smithsonite and its floatability with anionic collector [J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2012, 410: 178-183.
[9] SHI Qing, ZHANG Guo-fan, FENG Qi-ming, DENG Hong. Effect of solution chemistry on the flotation system of smithsonite and calcite [J]. International Journal of Mineral Processing, 2013, 119: 34-39.
[10] TIAN Meng-jie, LIU Run-qing, GAO Zhi-yong, CHEN Pan, HAN Hai-sheng, WANG Li, ZHANG Chen-yang, SUN Wei, HU Yue-hua. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector [J]. Minerals Engineering, 2018, 119: 31-37.
[11] LYU Fei, GAO Jian-de, SUN Ning, LIU Run-qing, SUN Xiao-dong, CAO Xue-feng, WANG Li, SUN Wei. Utilisation of propyl gallate as a novel selective collector for diaspore flotation [J]. Minerals Engineering, 2019, 131: 66-72.
[12] VIDYADHAR A, KUMARI N, BHAGAT R P. Adsorption mechanism of mixed cationic/anionic collectors in quartz–hematite flotation system [J]. Journal of Colloid and Interface Science, 2007, 306(2): 195-204.
[13] MEHDILO A, ZAREI H, IRANNAJAD M, ARJMANDFAR H. Flotation of zinc oxide ores by cationic and mixed collectors [J]. Minerals Engineering, 2012, 36-38: 331-334.
[14] XU Long-hua, HU Yue-hua, TIAN Jia, WU Hou-qin, YANG Yao-hui, ZENG Xiao-bo, WANG Zhen, WANG Jin-ming. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors [J]. Minerals Engineering, 2016, 89: 84-92.
[15] WANG Li, SUN Wei, HU Yue-hua, XU Long-hua. Adsorption mechanism of mixed anionic/cationic collectors in muscovite-quartz flotation system [J]. Minerals Engineering, 2014, 64: 44-50.
[16] WANG Li, SUN Ning, WANG Zhen, HAN Hai-sheng, YANG Yue, LIU Run-qing, HU Yue-hua, TANG Hong-hu, SUN Wei. Self-assembly of mixed dodecylamine-dodecanol molecules at the air/water interface based on large-scale molecular dynamics [J]. Journal of Molecular Liquids, 2019, 276: 867-874.
[17] HOSSEINI S H, FORSSBERG E. Smithsonite flotation using mixed anionic/cationic collector [J]. Mineral Processing and Extractive Metallurgy, 2007, 118: 186-190.
[18] HOSSEINI S H, FORSSBERG E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector [J]. Minerals Engineering, 2007, 20: 621-624.
[19] WANG Zhen, XU Long-hua, WANG Jin-ming, WANG Li, XIAO Jun-hui. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector [J]. Applied Surface Science, 2017, 426: 1141-1146.
[20] WANG Zhen, WANG Li, ZHENG Yong-xing, XIAO Jun-hui. Role of calcium dioleate in the flotation of powellite particles using oleate [J]. Minerals Engineering, 2019, 138: 95-100.
[21] AHN C K, WOO S H, PARK J M. Selective adsorption of phenanthrene in nonionic–anionic surfactant mixtures using activated carbon [J]. Chemical Engineering Journal, 2010, 158: 115-119.
[22] SHI Q, FENG Q, ZHANG G, DENG H. Electrokinetic properties of smithsonite and its floatability with anionic collector [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 410: 178-183.
[23] NOWAK P, LAAJALEHTO K. Oxidation of galena surface: An XPS study of the formation of sulfoxy species [J]. Applied Surface Science, 2000, 157: 101-111.
复配捕收剂从白云石中选择性浮选菱锌矿
王 丽1,2,胡广艳1,2,孙 伟1,2,Sultan Ahmed KHOSO1,2,刘润清1,2,张祥锋1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 战略含钙矿产资源清洁高效利用湖南省重点实验室,长沙410083
摘 要:将苯甲羟肟酸(BHA)与油酸钠(NaOL)混合,制备一种新的复配捕收剂(BHOA),并将其应用于从白云石中浮选分离菱锌矿。浮选结果表明,单独使用油酸钠对菱锌矿和白云石均有较好的捕收性能,但选择性较差。在BHA/NaOL摩尔比为2:1的复配体系中,菱锌矿与白云石的回收率分别为90%和5%左右。表面张力分析表明,由于协同效应,复配捕收剂BHOA的表面活性高于单一油酸钠。Zeta电位和XPS测量结果显示,在复配捕收剂BHOA存在的情况下,BHA与NaOL在菱锌矿表面发生共吸附,白云石表面仅观察到油酸钠。
关键词:浮选;菱锌矿;白云石;油酸钠;苯甲羟肟酸;硫化作用
(Edited by Wei-ping CHEN)
Foundation item: Project (51704329) supported by the National Natural Science Foundation of China; Project (2018YFC1901901) supported by the National Key Scientific Research Project of China
Corresponding author: Xiang-feng ZHANG; Tel: +86-731-88830482; Fax: +86-731-88660477; E-mail: 095611077@csu.edu.cn
DOI: 10.1016/S1003-6326(19)65016-8
Abstract: A novel mixed collector (BHOA) was prepared by mixing benzohydroxamic acid (BHA) and sodium oleate (NaOL) and applied to the flotation separation of smithsonite from dolomite. Flotation results showed that NaOL alone had good collecting performance on smithsonite and common gangue mineral dolomite but had poor selectivity. By using a BHA/NaOL mixed system with a molar ratio of 2:1, the recoveries of smithsonite and dolomite reached approximately 90% and 5%, respectively. Surface tension analysis showed that the surface activity of BHOA was a little higher than that of a single NaOL because of synergistic effects. Zeta potential and X-ray photoelectron spectroscopy measurements indicated that surfactants BHA and NaOL co-absorbed on the smithsonite surface and only NaOL was present on the dolomite surface in the presence of BHOA.


