Trans. Nonferrous Met. Soc. China 23(2013) 283-288
Preparation of TiCl4 with multistage series combined fluidized bed
Zhang-fu YUAN1, Yuan-qing ZHU2, Liang XI3, Shao-feng XIONG3, Bing-sheng XU1
1. Department of Energy and Resources Engineering, College of Engineering, Peking University, Beijing 100871, China;
2. Department of Environmental Science, College of Environmental Science and Engineering, Peking University, Beijing 100871, China;
3. State Key Laboratory of Multiphase and Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received 14 December 2011; accepted 4 May 2012
Abstract:
In order to solve the agglomeration problem in TiCl4 preparation, a new test in a multistage series combined fluidized bed was studied on a pilot scale. The pilot plant can make full use of titanium slag with a high content of MgO and CaO as the feedstock. Several experimental parameters such as chlorine flow and reaction temperature were discussed and the morphology and components of reaction product were analyzed. According to the results, the conversion rate of TiO2 is up to 90%. It is found that the combined fluidized bed has good anti-agglomeration ability because the accumulation of MgCl2 and CaCl2 on the surface of unreacted slag was carried out of the reactor.
Key words:
combined fluidized bed; titanium tetrachloride; anti-agglomeration; MgO; CaO; MgCl2; CaCl2;
1 Introduction
The matured fluidized chlorination process is mainly used in Chinese industry to prepare titanium tetrachloride (TiCl4) from the titanium-rich material. But the process requires low contents of MgO and CaO in the feedstock to be lower than 1.0% [1]. In this case, the titanium slag produced in Yunnan and Panzhihua in China cannot be applied for further use because the high contents of magnesium and calcium in the slag (the content of the former is above 2.5% while the latter is above 5%) [2]. The operation temperature of chlorination process is normally controlled between 1073 and 1373K [3] in fluidized bed, well above the melting points of CaCl2 and MgCl2 (the former 1055 K while the latter 987 K). CaCl2 and MgCl2 melts then gradually bond and agglomerate, and destroy the boiling steady-state of the bed layer. Finally, the agglomeration of CaCl2 and MgCl2 stops further production in the fluidized bed [4]. To solve this problem, a new process of TiCl4 preparation in a combined fluidized bed has been developed [5]. It is mainly based on the theory that the turbulence of air flow to certain strength can effectively crush the agglomeration [6,7]. The combined fluidized bed was designed with three parts: the primary fast fluidized bed followed by the turbulent fluidized bed and the second fast fluidized bed. The fast fluidized bed promoted the gas-solid up-flow behavior and the turbulent fluidized bed was targeted to treat the semi-dilute flow. Using the turbulent fluidized bed instead of an ordinary circulating tube can prolong the residence time of solid phase for sufficient reaction, while controlling the height of the system is suitable for industrial production. Thus, the productivity of titanium tetrachloride was improved greatly. It was expected that the multistage series combined fluidized bed could reduce and further eliminate the agglomeration influence as mentioned above. Cold-state and hot-state [8] laboratory experiments, as well as a numerical simulation [9,10] of this fluidized bed design have already been carried out. Based on their results, a pilot-scale plant with a production capacity of 4500 t/a was set up for further industrial test. In this work, the operation conditions and key parameters of the pilot-scale process for preparation of TiCl4 in the multistage series combined fluidized bed.
2 Experimental
The starting materials for the test were titanium-rich slag, petro coke and chlorine. Titanium slag, with a particle size of about 130 μm and the composition described in Table 1, was used as starting materials. The composition of petro coke was described in Table 2. Its average particle size was 240 μm. Both the titanium slag and petro coke belong to the particles of the category B, which meet the requirement of the designed pilot system with good fluidizing performances [11]. Chlorine used in the experiment was obtained from volatilization of liquid chlorine. The purity of liquid chlorine was above 99.5% (volume fraction).
Table 1 Composition of high titanium slag (mass fraction, %)

Table 2 Chemical composition of petro coke (mass fraction, %)

The flow chart of tested combined fluidized bed is shown in Fig. 1. A mixture of titanium-rich slag and petro coke was added into the mechanical screw batch charger at the bottom to react with chlorine. After chlorination, the remained gas and dusts flowed from the top of the bed to multistage dedusting system for precipitation and dust removal. Later, the mixture was cooled and absorbed in the multistage spray tower by TiCl4 as coolant. Off gases went through the absorption tower. HCl and Cl2 in it were removed and then discharged to the air.
The combined fluidized bed in the pilot test consisted of a primary fast fluidized bed, followed by the turbulent bed and then the secondary fluidized bed, with sizes of 0.15, 0.30, 0.20 m. The height of the system was 21 m. The material of inner wall was corundum with high-purity that could stand high temperature and corrosion. The bed layer was paved with self-designed distribution plate. A 15 m-vertical pipe was used at the exit to connect with a dust collector. Every part of the combined fluidized bed was equipped with thermocouples, manometers, and observation ports.
According to the result of cold test, the operable ranges of the fluidized bed are: gas flow rate ug=0.9-1.5 m/s;solids flow rate Sv=(4.26-11) t/(m2·h). Within the limitations of chemical equilibrium and heat balance, the operating parameters of real process are ug=(0.9-1.3) m/s;Sv=(4.0-5.5) t/(m2·h), varied with the flux of Cl2. The average temperature is 1073 K.
The components of high titanium slag were analyzed by X-ray fluorescence spectrometry while the particle size was measured by Beckman laser particle size analyzer. All the starting materials, residue of titanium, and chlorination slag were analyzed by JSM-646LV scanning electron microscopy with an energy-dispersive spectrometer (SEM-EDS) to get information concerning the morphology, particle components, and agglomeration observation before and after the reaction. The components of the off-gas were measured with an Orsat gas analyzer [12].

Fig. 1 Flow chart of combined fluidized bed for preparing TiCl4
3 Results and discussion
3.1 Chlorine flow
In the polite test, chlorine was used not only as the reactor but also as the carrier gas. Increase in the flow rate could improve the turbulent effect and mass transfer. But on the other hand, the residence time of the reactor was also reduced. So it is necessary to control the flow in a certain region. Figure 2 shows the variance of chlorine flow with time in the pilot test. The test began with a high flow rate of 580 kg/h, and came to be steady after 6 h. This indicates a similarity between the variance of chlorine flow and the temperature of gasification container as shown in Fig. 2(b), which manifests that the temperature of gasification poses great influences on chlorine flow rate. The rise in temperature caused the acceleration in chlorine flow, and further affected the fluidized state.
The quantitative relation between the chlorine flow rate Qg (kg/h) and the gas velocity ug was calculated according to different diameters and densities of Cl2 in different parts of the combined fluidized bed. The results show that the conversion relations between Qg and ug were 0.00525, 0.00131 and 0.00205 in the primary fast bed, turbulent bed and the secondary fast bed, respectively. When the gasification flow of chlorine is 300 kg/h, the flow rates in the first fast bed, turbulent bed and the second fluidized bed were 1.572, 0.393 and 0.614 m/s, respectively.

Fig. 2 Variations of chloride flow rate (a) and temperature of gasification container (b) with time
3.2 Temperature of fluidized bed
Figure 3 shows the variation of temperature in the six sensing points in the combined fluidized bed after chlorine pumped in. The measuring points of T1 and T2 were closed to the entrance of the flow, where the temperature decreased continuously with run time. This is because the pumped chlorine from the bottom was relatively cold that influenced the temperature of the entrance part of chlorinating furnace. T3, T4 and T5 were the temperatures measured in the turbulent bed. The results showed that the temperatures gradually increased with run time, indicating the normal operation of heat energy in reaction offset the heat loss from the furnace wall and the leaving material. T6 was the temperature of the branch pipe at the top of the chlorinating furnace, which also increased with time. It can be explained that the high temperature gasification products went through the branch pipe to the dedusting system.

Fig. 3 Variations of temperature of six temperature-measuring positions with run time

Fig. 4 SEM images and EDS patterns of products of TiCl4
3.3 SEM-EDS analysis of slag
Figure 4 shows the morphology and component of slag in different parts of the system by SEM and the scanning spectra by EDS, with the reaction interface of Cl2 and slag quite clear. In the scanning graph on surface, the high content of Ti in the slag indicated that Ti did not sufficiently react [13], mainly due to the short residence time in the fluidized bed. The particle size of slag in downtake pipe of furnace top was smaller than slag in the furnace bottom while the contents of Mg and Ca in the former were higher. This suggested that particles with high contents of calcium and magnesium chlorides adhered to the inwall of downtake tube at the top of the furnace during the chlorination process. For the steady operation of the fluidized bed, it is necessary to clean the downtake pipe at a regular interval to keep the flowing section effective.
3.4 Particle size and component of dust slag
Figure 5 shows the analytical results of particle size of dust through different run time in the reaction process at an average temperature of 1073 K. Compared with the raw material of titanium-rich slag and petro coke, the particle size of dust slag decreased remarkably. This suggested that the gas flow rate was relatively high and caused great shear force, which crushed the particle and restrained the agglomeration to a certain degree. The increase in the specific surface at the same time enhanced the reaction extent. With the run time extending, the particle size of dust reduced.

Fig. 5 Analytical results of particle size of dust
In the traditional technology of boiling chlorination, CaCl2 and MgCl2 caused the agglomeration of particles, so the slag after the reaction in the dedusting system contained low contents of CaCl2 and MgCl2. But in this test of multistage series combined fluidized bed, the result of component analysis of dust, shown in Table 3, denoted that the content of Ca was 1.472% (5 h), 16.319% (21 h) and the content of Mg was 8.369% (5 h), 12.371% (21 h). This indicated that a certain amount of chloride which may cause the agglomeration had been brought out of the fluidized bed, thus proved the anti-agglomeration function of the combined fluidized bed. With the run time extending, the components of Ca and Mg in the dust increased.
Table 3 Components of dust collecting residues of pilot test

3.5 Conversion efficiency of TiO2
The real conversion process of TiO2 can be simplified into two scenarios: 1) Cl2 only reacted with TiO2; 2) Cl2 reacted with CaO, MnO, MgO and Fe2O3 before TiO2 chloridization. As the real reaction happened to a extent between the two extreme situations, one way to calculate the conversion rate of TiO2 was derived from the average conversion rate of the two scenarios’ results [14]. Figure 6 shows the conversion data under the experimental and pilot-scale test conditions. The experimental results indicated that the productivity increased as the temperature increased. Considering the actual situation, the pilot test chose 1073 K as the reaction temperature in the chlorination process. Under this condition, the conversion rate of TiO2 was calculated according to the material balance [15] based on the composition of slag and off gases in the pilot-scale test. The final result suggested that the conversion rate in the pilot-scale system could reach 90%. As the pilot devise greatly raised the fluidization efficiency, the final result was higher than the conversion under the same experimental conditions.
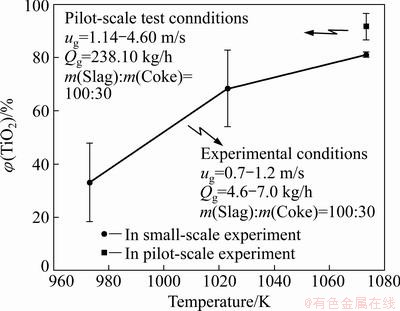
Fig. 6 TiO2 conversion of pilot plant test
4 Conclusions
1) Based on the preparatory work, the TiCl4 preparation in multistage series combined fluidized bed brings good results in the pilot-scale test.
2) According to the result of cold test, the operable parameters of the fluidized bed are: gas flow rate 0.9-1.5 m/s;solids flow rate 4.26-11 t/(m2·h). Within the limitations of chemical equilibrium and heat balance, the operating parameters in real process are 0.9-1.3 m/s;solids flow rate 4.0-5.5 t/(m2·h), varied with the flux of Cl2. The average temperature is 1073 K.
3) The flow rate of chlorine comes to be steady with the run time after 8 h. When it is 300 kg/h, the flow rates in the primary fast bed, turbulent fluidized bed and the second bed are 1.572, 0.393 and 0.614 m/s, respectively.
4) When the reaction temperature is controlled at 1073 K, the conversion rate of TiO2 reaches 90%, higher than the conversation rate under the experimental conditions.
5) CaCl2 and MgCl2 adhere to the surface of the slag, indicating part of the chloride is brought out of the combined fluidized bed in the production. So this system shows its good anti-agglomeration ability.
6) The particle size of dust slag decreases remarkably with prolonging the run time. This suggests that the gas flow rate is relatively high and causes great shear force, which crushes the particle and restrains the agglomeration to a certain degree.
7) Though the pilot-scale test shows good results, it also exposes some problems. The concentration of chlorine in the off gas is too high and the confined nature of the reactor is also challenged.
References
[1] MO W, DENG G Z, LUO F C. Titanium metallurgy [M]. Beijing: Metallurgical Industry Press, 2006: 245. (in Chinese)
[2] WU X, ZHANG J. Geographical distribution and characteristics of titanium resources in China [J]. Titanium Industry Progress, 2006, 23 (6): 8-12.
[3] MORRIS A J. JENSEN R F. Fluidized-bed chlorination rates of Australian rutile [J]. Metall Mater Trans B, 1976, 7: 89-93.
[4] YUAN Z F, XU C, ZHEN S H. Comprehensive utilization of titanium resources in Panzhihua [J]. Modern Chemical Industry, 2003, 23(5): 1-4. (in Chinese)
[5] YUAN Z F, WANG X Q, XU C. A new process for comprehensive utilization of complex titania ore [J]. Minerals Engineering, 2006, 19 (9): 975-978.
[6] TARDOS G, MAZZONE D, PFEFFER R. Destabilization of fluidized beds due to agglomeration (Part I): Theoretical model [J]. Can J Chem Eng, 1985, 63: 377-383.
[7] TARDOS G, MAZZONE D, PFEFFER R. Destabilization of fluidized beds due to agglomeration (Part II): Experimental verification [J]. Can J Chem Eng, 1985, 63(3): 384-389.
[8] XU C, YUAN Z F, WANG X Q. Preparation of TiCl4 with the titanium slag containing magnesia and calcia in a combined fluidized bed [J]. Chinese J Chem Eng, 2006, 14(3): 281-288.
[9] XIONG S F, YUAN Z F, TAN Q Q. Components of off-gas produced by a carbothermal chlorination of titanium slag [J]. The Chinese Journal of Process Engineering, 2009, 9(1): 63-68. (in Chinese)
[10] XU C, YUAN Z F, XIAO W M. One-dimensional modeling of multiple-unit pneumatic transport reactor for producing titanium tetrachloride (I): Mathematical model [J]. The Chinese Journal of Process Engineering, 2004, 4(6): 481-499. (in Chinese)
[11] GELDART D. Types of gas fluidization [J]. Powder Technology, 1973, 7(5): 285-292.
[12] LUO Y Z. Determination of chlorine and oxygen in gas produced by plasma gas-phase oxidation of TiCl4 for preparing TiO2 [J]. Engineering Chemistry & Metallurgy, 1990, 11(1): 63-66. (in Chinese)
[13] XIONG S F, YUAN Z F, XU C, XI L. Composition of off-gas produced by combined fluidized bed chlorination for preparation of TiCl4 [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 128-134.
[14] XU Cong. Study on producing TiCl4 using combined fluidized bed by chloridizing materials of high-level CaO and MgO [R]. PD200404, Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2004. (in Chinese)
[15] XIONG Shao-feng. Applied study on production of TiCl4 by chlorination and its purification [D]. Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2009: 104-106. (in Chinese).
多级复合流化床制备四氯化钛
袁章福1,朱元晴2,席 亮3,熊绍锋3,徐秉声1
1. 北京大学 工学院,能源与资源工程系,北京 100871;
2. 北京大学 环境科学与工程学院,环境科学系,北京 100871;
3. 中国科学院 过程工程研究所,北京100190
摘 要:为了解决四氯化钛制备过程中的粘结问题,采用复合流化床新工艺进行中试试验制备四氯化钛,可以有效利用镁钙含量大于2.5%的高钛渣原料。对氯气流量、流态化温度等参数的影响进行研究,并对反应产物的形貌进行分析,同时计算了反应效率。结果表明:TiO2的转化率达到90%,通过检测发现收尘渣表面富集MgCl2和CaCl2,而流化床中未出现粘结,说明该流化床具有一定的抗粘结能力。
关键词:复合流化床;四氯化钛;抗粘结;MgO;CaO;MgCl2;CaCl2
(Edited by Xiang-qun LI)
Foundation item: Project (2008AA06Z1071) supported by the National High-Tech Research and Development Program of China; Project (20306030) supported by the National Natural Science Foundation of China
Corresponding author: Zhang-fu YUAN; Tel/Fax: +86-10-62758807; E-mail: zfyuan@pku.edu.cn
DOI: 10.1016/S1003-6326(13)62458-9
Abstract: In order to solve the agglomeration problem in TiCl4 preparation, a new test in a multistage series combined fluidized bed was studied on a pilot scale. The pilot plant can make full use of titanium slag with a high content of MgO and CaO as the feedstock. Several experimental parameters such as chlorine flow and reaction temperature were discussed and the morphology and components of reaction product were analyzed. According to the results, the conversion rate of TiO2 is up to 90%. It is found that the combined fluidized bed has good anti-agglomeration ability because the accumulation of MgCl2 and CaCl2 on the surface of unreacted slag was carried out of the reactor.


