Trans. Nonferrous Met. Soc. China 26(2016) 2925-2929
Effect of sintering atmosphere on corrosion resistance of Ni/(NiFe2O4-10NiO) cermet inert anode for aluminum electrolysis
Zhong-liang TIAN, Wei-chang GUO, Yan-qing LAI, Kai ZHANG, Jie LI
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 30 December 2015; accepted 12 April 2016
Abstract:
A comparative study on the corrosion resistance of 17Ni/(NiFe2O4-10NiO) cermet inert anode prepared in different sintering atmospheres was conducted in Na3AlF6-Al2O3 melt. The results indicate that the corrosion rates of NiFe2O4-based cermet anodes prepared in the vacuum and the atmosphere with oxygen content of 2×10-3 (volume fraction) are 6.46 and 2.71 cm/a, respectively. Though there is a transition layer with lots of holes or pores, a densified layer is formed on the surface of anode due to some reactions producing aluminates. For the anode prepared in the atmosphere with oxygen content of 2×10-3, the thickness of the densification layer (about 50 μm) is thicker than that (about 30 μm) formed on the surface of anode prepared in the vacuum. The contents of NiO and Fe(II) in NiFe2xO4-y-z increase with the decrease of oxygen content in sintering atmosphere, which reduces the corrosion resistance of the material.
Key words:
sintering atmosphere; corrosion resistance; NiFe2O4-based cermet; inert anode; aluminum electrolysis;
1 Introduction
For almost 120 years, aluminum has been produced electrochemically by the  . This technology uses carbon anode and liquid aluminum cathode to decompose alumina dissolved in the molten cryolite [1]. The use of inert anode instead of carbon eliminates the generation of the greenhouse gas CO2 when the oxygen liberated from the dissociation of alumina reacts with anode carbon. It also reduces perfluorocarbon(PFC) byproducts and other polluting emissions such as PAHs. So, the development of inert anode is a long standing dream of researchers [2,3].
. This technology uses carbon anode and liquid aluminum cathode to decompose alumina dissolved in the molten cryolite [1]. The use of inert anode instead of carbon eliminates the generation of the greenhouse gas CO2 when the oxygen liberated from the dissociation of alumina reacts with anode carbon. It also reduces perfluorocarbon(PFC) byproducts and other polluting emissions such as PAHs. So, the development of inert anode is a long standing dream of researchers [2,3].
However, the development of viable non-carbon anodes is very challenging due to numerous restrictive requirements for inert anode material in the highly aggressive environment of Al electrolysis cell. After plenty of research efforts from Al producers and academic laboratories, though some materials such as alloy, cermet and oxide ceramic were regarded as promising candidates, no acceptable inert anode material has yet been found for long-term use in industrial aluminum electrolysis [1,4-8]. In recent years, the oxide ceramic is abandoned gradually because of its low electrical conductivity, high brittleness and other problems such as being difficult to connect the metal rod [2]. The cermet anodes are ranked as one of the most appropriate candidates because of their combination of high corrosion resistance of ceramic and high electrical conductivity of metal [9-11]. NiFe2O4 is often utilized as ceramic matrix for cermet inert anode because of its high melting point, excellent corrosion resistance, stable thermal and chemical properties [2,5,7,12].
To improve the mechanical properties of ceramic material, modifying agent or sintering assistant was used during the preparation process, and some progress was made [13]. The effects of sintering atmosphere on the mechanical properties of NiFe2O4-based cermets were also studied in our previous studies, and the results showed that the alteration of oxygen content in the sintering atmosphere could affect the microstructure and mechanical properties of NiFe2O4-based cermets [14]. However, it is known to all that the corrosion resistance of material is the most important performance for inert anodes, it is not only related to the life of inert anode, but also affects the quality of metal Al.
In this work, 17Ni/(NiFe2O4-10NiO) cermet inert anodes were prepared in different sintering atmospheres by cold pressing-sintering process. And the influence of oxygen content in the sintering atmosphere on its corrosion resistance to Na3AlF6-Al2O3 melt was studied. The purpose is to explore the impact of the sintering atmosphere on the corrosion resistance of Ni/(NiFe2O4-10NiO) cermet inert anode.
2 Experimental
2.1 Fabrication of anodes
The raw materials, metal Ni powder, NiO and Fe2O3 were all of reagent grade. The samples of 17Ni/ (NiFe2O4-10NiO) cermet were prepared by the same process as Ref.[14]. A proper amount of Fe2O3 and 10% (mass fraction) excess NiO, compared with that of the stoichiometric NiFe2O4, were mixed by using a ball mill, and then calcined in a muffle furnace at 1200 °C for 6 h to obtain NiFe2O4-10NiO ceramic powder. The calcined ceramic powder was mixed again with metal Ni powder by ball milling in the media containing dispersant and adhesive that were organic to avoid the metal oxidation. Finally, the mixed ceramic-metal powder was dried and pressed into cylindrical blocks (d20 mm × 40 mm), which were sintered at 1350 °C for 4 h in the vacuum or an atmosphere with the oxygen content of 2×10-3 (volume fraction) respectively to get the desired Ni/(NiFe2O4-10NiO) cermet inert anode samples.
2.2 Chemical and bath preparation
The bath was made up of raw materials Na3AlF6, AlF3, CaF2 and Al2O3. Among them, Na3AlF6, CaF2 and Al2O3 were reagent grade. AlF3 was purified by sublimation three times and its purity was above 99.5%. All the components were dried at 150 °C for at least 48 h in the vacuum oven to remove the water before being used. The mole ratio of NaF to AlF3 (n(NaF)/n(AlF3)) in Na3AlF6-AlF3-CaF2-Al2O3 melt was 2.3:1, and the contents of CaF2 and Al2O3 were both 5.0% (mass fraction). The powder components were mixed and the initial mass of electrolyte was 800 g.
2.3 Cell design and electrolysis procedure
A sketch of the experimental cell is presented in Fig. 1. A hole was drilled at the bottom of graphite crucible and some metal aluminum (about 90 g, the purity was 99.5% and obtained from Yunnan Aluminum Co., Ltd.) was added. Thus, a steady cathode surface could be obtained during electrolysis. Alumina sleeve was set in the graphite crucible and about 800 g electrolyte was contained. Under the operating conditions of the laboratory test, the cell could not be thermally self-sustaining. It was necessary to provide extra heat by placing the experimental cell with the anode together in a vertical laboratory furnace and the furnace was heated to the desired temperature.
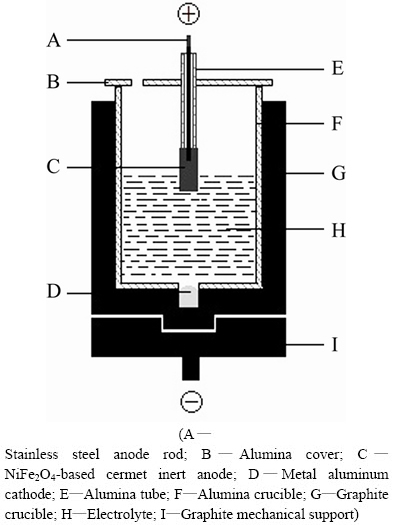
Fig. 1 Sketch of experimental cell for electrolysis
Metal aluminum was added prior to electrolysis. The cell with NiFe2O4-based cermet inert anode was heated to the required temperature of 960 °C and kept for 2 h before immersing the anode and electrifying 20 min afterwards. The electrolysis temperature was maintained at 960 °C during testing. The temperature of Na3AlF6- AlF3-CaF2-Al2O3 melt was measured with Pt/Pt- 10%Rh thermocouple once 1 h and maintained below a range of ±3 °C during testing. The temperature accuracy of the furnace was controlled to ±1 °C by TCE-II programmable temperature control unit.
The anode-bath contacting area was controlled by the immersion depth of anode, which was 1.0 cm. The current and the cell voltage were supplied and monitored by a multi-purpose potentiostat/galvanostat (model 273A/10, Perkin-Elmer Instruments). The current density of anode bottom was 1 A/cm2 and the current was kept constant throughout the experiment. Al2O3 was supplied once every 15 min and its content could be kept constant during the experiment.
After electrolysis, the anode was raised out of the bath while maintaining polarization so as to prevent reduction of the anode material by dissolved metal aluminum. The cell was left to cool with the anode resting above the electrolyte. The metal aluminum recovered was also analyzed by X-ray fluorescence spectrum (Philips 8424 TW2424) (analytic error lower than 5%) for getting the contents of Ni and Fe. The anodes tested were sectioned, mounted, polished, and analyzed by XRMA (JSM-5600LV) using a quantitative energy dispersive spectrometer (EDS) connected to the SEM. The phase composition of NiFe2O4-based cermet inert anode before electrolysis was also analyzed by Rigaku3014 X-ray diffractometer.
3 Results and discussion
3.1 Material performance
The results from our previous studies indicated that it took approximately 4-6 h for stoichiometric NiFe2O4 in Na3AlF6-AlF3-Al2O3 melt to reach a steady-state content, which was regarded as the solubility [15]. The study carried out by OLSEN and THONSTAD [16] also confirmed this. Therefore, the electrolysis experiments lasted for 10 h here.
After testing, the contents of Ni and Fe in the metal aluminum recovered at the cathode were analyzed by XRF. The increments of impurities Ni and Fe are listed in Table 1. As can be seen, for test No. 1, the increment of impurity Ni in aluminum is 0.222 g, while for test No. 2, the value is only 0.094 g. It can be calculated that the increment of Ni is decreased significantly (by 57.7%) due to the change of sintering atmosphere during preparation. The same is true for the impurity Fe, although its increment is decreased by only 36.8%. It can be concluded that the alteration of oxygen content in the sintering atmosphere is helpful for improving the quality of the metal aluminum.
Table 1 Increment of impurities Ni and Fe and corrosion rate of anode

Generally speaking, the corrosion rate of anode is nearly the same as the deposition rate of impurity into the aluminum cathode after the corrosion reaches a steady-state. Therefore, the corrosion rate of inert anode can be calculated according to the deposition rate of some kinds of impurities at the cathode [17]. As for 17Ni/(NiFe2O4-10NiO) cermet inert anode, its corrosion rate can be obtained by the change of Ni content in the metal aluminum recovered at the cathode based on the following equation [17]:
 (1)
(1)
where vc (cm/a) is the corrosion rate of anode, △mNi(Al)(g) is the increment of Ni in the metal aluminum at the cathode, wNi(cer) (%) is content (mass fraction) of Ni in the anode, ρ (g/cm3) is the density of anode, S(cm2) is the area of the anode surface immersed in the bath, and τ (h) is the time of electrolysis.
The corrosion rate based on Eq. (1) is calculated to be 6.46 cm/a for 17Ni/(NiFe2O4-10NiO) cermet inert anode prepared in the vacuum. However, for the inert anode prepared in the atmosphere with the oxygen content of 2×10-3, its corrosion rate is 2.71 cm/a. This indicates that the corrosion resistance of Ni/(NiFe2O4- 10NiO) cermet to Na3AlF6-AlF3-Al2O3 melt can be improved by changing the oxygen content of sintering atmosphere.
3.2 Microstructure analysis
The XRD analysis results of 17Ni/(NiFe2O4-10NiO) cermet prepared in different atmospheres are shown in Fig. 2. It is found that both the samples obtained in the vacuum and the atmosphere with oxygen content of 2×10-3 contain the phases Ni, NiFe2O4 and NiO, and no other impurity appears. But the characteristic peak of NiFe2O4 is more obvious for the anode prepared in the atmosphere with oxygen content of 2×10-3 than that for the anode prepared in the vacuum.
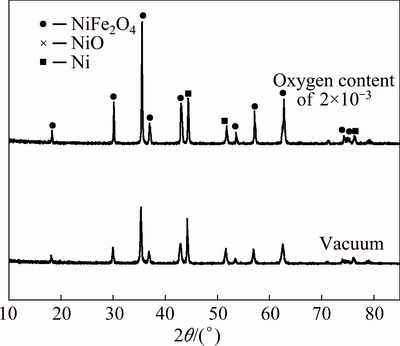
Fig. 2 XRD patterns of 17Ni/(NiFe2O4-10NiO)
With the change of oxygen content in the sintering atmosphere, the following reaction may occur:
NiFe2O4 NiFe2xO4-y-z+NiO+O2↑ (2)
NiFe2xO4-y-z+NiO+O2↑ (2)
Thus, the ceramic NiFe2O4, which is one of the components of anodes tested, will be decomposed into NiFe2xO4-y-z and NiO if the oxygen content in the atmosphere decreases. Although the stoichiometric compound NiFe2O4 and the nonstoichiometric compound NiFe2xO4-y-z cannot be distinguished by XRD, there are two forms for the element Fe in NiFe2xO4-y-z, which are Fe(Ⅱ) and Fe(Ⅲ). When the oxygen content in the sintering atmosphere decreases, Fe(Ⅲ) in NiFe2xO4-y-z is reduced to Fe(Ⅱ) and the content of Fe(Ⅱ) increases. In Na3AlF6-AlF3-Al2O3 melt, lower valence state Fe(Ⅱ) in NiFe2xO4-y-z may dissolve more easily than higher valence state Fe(Ⅲ) [11]. Therefore, the corrosion resistance of 17Ni/(NiFe2O4-10NiO) cermet prepared in the vacuum decreases.
At the same time, Reaction (2) also indicates that the content of NiO decreases and the content of NiFe2O4 increases if the anode is sintered at high oxygen content. The results from the XRD also show that the mass fractions of phases NiFe2O4 and NiO are 71.5% and 17.8% respectively for the anode prepared in the atmosphere with the oxygen content of 2×10-3. If the anode is prepared in the vacuum, the mass fractions of phases NiFe2O4 and NiO are 64.4% and 20.4%, respectively. The results of Ref. [18] show that the corrosion resistance of NiFe2O4 is better than that of NiO. So, if the cermet inert anode is prepared in the atmosphere with the oxygen content of 2×10-3, its corrosion resistance is better than that prepared in the vacuum.
To obtain more information, SEM images of cross-sections of the anode surfaces after electrolysis are obtained and shown in Fig. 3. There are some visible structural changes for the anodes sintered in different atmospheres when going from the unchanged interior of the electrode to the surface. A distinct densification layer is formed on the surface of anode. The results from EDS analysis of the densification layer show that there is element Al, which is not added to the anode during the preparation process, existing after electrolysis besides elements Ni, Fe and O. This phenomenon may appear via some reactions forming aluminates such as NiFe2O4, NiAl2O4 and FeAl2O4 [16]. Further studies should be conducted to reveal the reason that the densification layer forms.
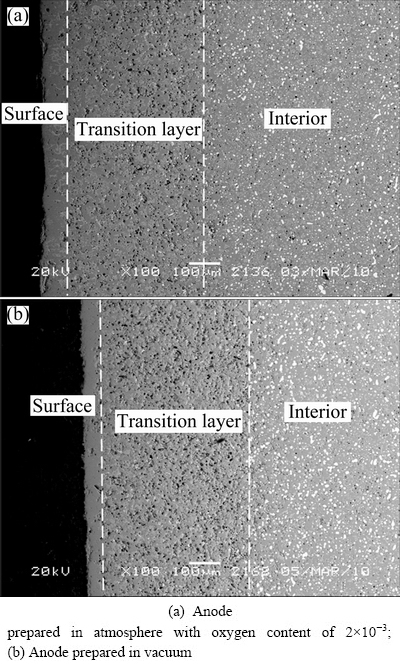
Fig. 3 SEM images of anodes after electrolysis
For the anodes prepared in different atmospheres, another point which should be noted is that there is a porous layer between the surface and the interior of anode after electrolysis. And in this layer, which is called “transition layer” here, the metal phase Ni disappears and there are some holes or pores left. The amounts of holes or pores in the transition layer are more than those in the anode material itself. The elements of Ni, Fe, Al, Na, O and F were detected. And the thickness of the transition layer for both the anodes prepared in different atmospheres is almost the same, which is about 500 μm. This may be due to the electrochemical reaction (Reaction (3)) between the metal Ni and the electrolyte infiltrated into the anode, which causes the preferential dissolution of the metal Ni during electrolysis [19].
3Ni+2AlF3=3NiF2+2Al (3)
It is very obvious for the anodes prepared in different atmospheres that the thickness of the densification layer on the surface is different. The densification layer with thickness of about 50 μm is formed for the anode prepared in the atmosphere with the oxygen content of 2×10-3 after electrolysis for 10 h. However, the thickness is only about 30 μm for the anode prepared in the vacuum. Since the surface layer of this anode is denser than the anode material itself which contains some pores due to its poor sintering characteristics, it is helpful for NiFe2O4-based cermet inert anode to improve its corrosion resistance against Na3AlF6-AlF3-Al2O3 melt. Therefore, the corrosion rate of the anode prepared in the atmosphere with the oxygen content of 2×10-3 is lower than that prepared in the vacuum.
4 Conclusions
1) The corrosion rate of 17Ni/(NiFe2O4-10NiO) cermet during electrolysis can be reduced by properly improving the oxygen content of sintering atmosphere. Based on the change of Ni in the metal aluminum recovered at the cathode, the corrosion rate of anode prepared in the atmosphere with the oxygen content of 2×10-3 is 2.71 cm/a. However, for the anode prepared in the vacuum, its corrosion rate is 6.46 cm/a.
2) The decrease of the oxygen content in the sintering atmosphere will cause the increase of the content of Fe(II) in NiFe2xO4-y-z, which may dissolve more easily than that of Fe(III). Meanwhile, the content of NiO also increases. It is adverse to improve the corrosion resistance of the anode during electrolysis.
3) A distinct densification layer is formed on the surface, and a transition layer with lots of holes or pores also comes into being between the surface and the interior. The thicknesses of the densification layer for the anodes prepared in the atmosphere with oxygen content of 2×10-3 and in the vacuum are about 50 and 30 μm, respectively. Further studies should be conducted to reveal the reason that the distinct layer forms.
References
[1] THONSTAD J, FELLNER P, HAARBERG G M,  J, KVANDE H, STERTEN
J, KVANDE H, STERTEN  . Aluminium—Fundamentals of the
. Aluminium—Fundamentals of the  process [M]. 3rd ed.
process [M]. 3rd ed.  Verlag, 2001: 3-8.
Verlag, 2001: 3-8.
[2] PAWLEK R P. Inert anodes: An update [C]//GRANDFIELD J. Light Metals. Hoboken, NJ: TMS, 2014: 1309-1313.
[3] XIAO S J, MOKKELBOST T, PAULSEN O, RATVIK ARNE P, HAARBERG GEIR M. SnO2-based gas (hydrogen) anodes for aluminum electrolysis [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3917-3921.
[4] GOUPIL G, HELLE S, DAVIS B, GUAY D,  L. Anodic behavior of mechanically alloyed Cu-Ni-Fe and Cu-Ni-Fe-O electrodes for aluminum electrolysis in low-temperature KF-AlF3 electrolyte [J]. Electrochimica Acta, 2013, 112: 176-182.
L. Anodic behavior of mechanically alloyed Cu-Ni-Fe and Cu-Ni-Fe-O electrodes for aluminum electrolysis in low-temperature KF-AlF3 electrolyte [J]. Electrochimica Acta, 2013, 112: 176-182.
[5] CONSTANTIN V. Influence of the operating parameters over the current efficiency and corrosion rate in the Hall-Heroult aluminum cell with tin oxide anode substrate material [J]. Chinese Journal of Chemical Engineering, 2015, 23: 722-726.
[6] TIAN Zhong-liang, LAI Yan-qing, LI Zhi-you, CHAI Deng-peng, LI Jie, LIU Ye-xiang. Further development on NiFe2O4-based cermet inert anodes for aluminum electrolysis [J]. JOM, 2014, 66(11): 2229-2234.
[7] WELCH B. J. Inert anodes-the status of the materials science, the opportunities they present and the challenges that need resolving before commercial implementation [C]//BEARNE G. Light Metals. Warreudale, PA: TMS, 2009: 971-978.
[8] LIU Jian-yuan, LI Zhi-you, TAO Yu-qiang, ZHANG Dou,ZHOU Ke-chao. Phase evolution of 17(Cu-10Ni)-(NiFe2O4-10NiO) cermet inert anode during aluminum electrolysis [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 566-572.
[9] HELLE S, PEDRON M, ASSOULI B, DAVIS B, GUAY D,  L. Structure and high-temperature oxidation behaviour of Cu-Ni-Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis [J]. Corrosion Science, 2010, 52: 3348-3355.
L. Structure and high-temperature oxidation behaviour of Cu-Ni-Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis [J]. Corrosion Science, 2010, 52: 3348-3355.
[10] GALLINO I, KASSNER M E, BUSCH R. Oxidation and corrosion of highly alloyed Cu-Fe-Ni as inert anode material for aluminum electrowinning in as-cast and homogenized conditions [J]. Corrosion Science, 2012, 63: 293-303.
[11] HE Han-bing, WANG Yuan, LONG Jia-ju, CHEN Zhao-hui. Corrosion of NiFe2O4-10NiO-based cermet inert anodes for aluminium electrolysis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3816-3821.
[12] WANG Gui-hua, SUN Xiao-fei. Electrochemical behavior of cermet anodes in Na3AlF6-K3AlF6-based low-melting electrolytes for aluminium electrolysis [C]//SADLER B. Light Metals. Kingwood, TX: TMS, 2013: 1295-1298.
[13] DU Jin-jing, LIU Yi-han, YAO Guang-chun, LONG Xiu-li, ZHANG Xiao. Effect of MnO2 addition on early-stage sintering behavior and properties of NiFe2O4 ceramics [C]//SUAREZ C E. Light Metals. Washington, PA: TMS, 2012: 1385-1388.
[14] CHEN Duan, ZOU Zhong, TIAN Zhong-liang, XIN Peng-fei, LIU Kai, LAI Yan-qing, LI Jie. Effect of sintering atmosphere on phase composition and mechanical properties of 5Ni/(10NiO-NiFe2O4) [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 124-128.
[15] LAI Yan-qing, TIAN Zhong-liang, QIN Qing-wei, ZHANG Gang, LI Jie. Solubility of composite oxide ceramics in Na3AlF6-Al2O3 melts [J]. Journal of Central South University of Technology, 2003, 34(3): 245-248. (in Chinese)
[16] OLSEN E, THONSTAD J. Nickel ferrite as inert anodes in aluminium electrolysis. Part I: Material fabrication and preliminary testing [J]. Journal of Applied Electrochemistry, 1999, 29: 293-299.
[17] BLINOV V, POLYAKOV P, THONSTAD J, IVANOV V, PANKOV E. Behaviour of inert anodes for aluminium electrolysis in a low temperature electrolyte: Part I [J]. Aluminium, 1997, 73(12): 906-912.
[18] LI Jie, DUAN Hua-nan, LAI Yan-qing, TIAN Zhong-liang, LIU Ye-xiang. Effect of NiO content on corrosion behaviour of Ni-xNiO-NiFe2O4 cermets in Na3AlF6-Al2O3 melts [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(6): 1180-1186.
[19] TARCY G P. Corrosion and passivation of cermet inert anodes in cryolite-type electrolytes [C]// MILLER R E. Light Metals. New Orleans, LA: TMS, 1986: 309-320.
烧结气氛对铝电解Ni/(NiFe2O4-10NiO)金属陶瓷惰性阳极耐腐蚀性能的影响
田忠良,郭伟昌,赖延清,张 凯,李 劼
中南大学 冶金与环境学院,长沙 410083
摘 要:对比研究了不同烧结气氛条件下制备的17Ni/(NiFe2O4-10NiO)金属陶瓷惰性阳极在Na3AlF6-Al2O3熔体中的耐腐蚀性能。研究结果表明,在真空和氧含量为2×10-3(体积分数)气氛下制备的NiFe2O4基金属陶瓷阳极电解腐蚀率分别为6.46和2.71 cm/a。尽管电解后阳极过渡层中出现了许多孔洞,但在反应新生成的铝酸盐作用下,阳极表面形成了一层致密层。对于氧含量为2×10-3气氛下制备的阳极,其表面电解后生成的致密层厚度(约为50 μm)大于真空条件下阳极表面所生成的致密层厚度(约为30 μm)。随着烧结气氛中氧含量的降低,所获材料中NiO和NiFe2xO4-y-z中Fe(II)的含量均增加,材料的抗腐蚀能力降低。
关键词:烧结气氛;耐腐蚀性能;NiFe2O4基金属陶瓷;惰性阳极;铝电解
(Edited by Wei-ping CHEN)
Foundation item: Project (51474238) supported by the National Natural Science Foundation of China
Corresponding author: Yan-qing LAI; Tel:+86-731-88830649; E-mail: csulyqmelt@126.com
DOI: 10.1016/S1003-6326(16)64422-9
Abstract: A comparative study on the corrosion resistance of 17Ni/(NiFe2O4-10NiO) cermet inert anode prepared in different sintering atmospheres was conducted in Na3AlF6-Al2O3 melt. The results indicate that the corrosion rates of NiFe2O4-based cermet anodes prepared in the vacuum and the atmosphere with oxygen content of 2×10-3 (volume fraction) are 6.46 and 2.71 cm/a, respectively. Though there is a transition layer with lots of holes or pores, a densified layer is formed on the surface of anode due to some reactions producing aluminates. For the anode prepared in the atmosphere with oxygen content of 2×10-3, the thickness of the densification layer (about 50 μm) is thicker than that (about 30 μm) formed on the surface of anode prepared in the vacuum. The contents of NiO and Fe(II) in NiFe2xO4-y-z increase with the decrease of oxygen content in sintering atmosphere, which reduces the corrosion resistance of the material.


