
Low-temperature sintering process for UO2 pellets in partially-oxidative atmosphere
YANG Xiao-dong(杨晓东)1, 2, GAO Jia-cheng(高家诚)1, WANG Yong(王 勇)1, CHANG Xin(畅 欣)2
1. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. Yibin Nuclear Fuel Element Plant, Yibin 644000, China
Received 17 January 2007; accepted 22 June 2007
Abstract:
Low-temperature sintering(LTS) experiments of UO2 pellets and their results were reported. Moreover, a routine process of LTS for UO2 pellets was primarily established. Being sintered at 1 400 ℃ for 3 h in a partially-oxidative atmosphere, the relative density of the pellet can be up to around 94%. Pellets with such a high density are of benefit for following-up reduction-sintering processes. Orthogonal test indicates that the importance of factors affecting the density decreases in the sequence of partial-oxidative sintering temperature and time, reduction-sintering time and temperature, and sintering atmosphere. It is found that it is helpful to introducing a small amount of water vapor into the sintering atmosphere during the latter stage. It is believed that it is the key factor to raise the O/U ratio of original powder in order to improve the properties of the low-temperature sintered pellets.
Key words:
UO2 pellet; low-temperature sintering; sintering temperature; sintering time;
1 Introduction
In the process of UO2 pellet manufacturing, the sintering is an extremely important and indispensable procedure. The high-temperature sintering technology, that is to say, sintering at 1 750 ℃ or higher for 6-9 h, is widely adopted by the nuclear fuel manufacturers in the world[1]. The low-temperature sintering(LTS) is also called the two-stage sintering[2-3], i.e., sintering firstly in the partially-oxidative atmosphere at 1 200 ℃ and then in the reductive atmosphere at about 1 400 ℃[4-5]. The research for the UO2 low-temperature sintering technology was very popular in the world and the definite achievements were accomplished[6-7].
With the uptight situation of energy sources and the strengthened consciousness of environment protection in recent years, the low-temperature sintering technology is actively developed in the world so that the production cost of nuclear fuel will be reduced and the UO2 fuel with higher performance will be explored and manufactured. The research in this field forms a new climax and the considerable achievement is acquired. A large amount of technological researches have been done by CEA Laboratory (French Atomic Committee) and FRAMATOME, France. The pellets sintered at low temperature have been tested in reactors. It is a tendency to put the LTS into the industrial production[8]. In this work, the research of the LTS technology was conducted.
2 Experimental
As emphasis was put on the establishment of sintering parameters in two sintering steps, the production method for green pellets was the same as that used in the normal production. Meanwhile, the physical and chemical measurements used for the normal products were also adopted for these sintered pellets.
2.1 Original powder
The primary properties of original UO2 powders are indicated in Table 1.
Table 1 Primary properties of original UO2 powders

Two types of green pellets are used in this work. One is the so called “additive-type pellets”, the other is called “matrix-type pellets” added with a very small amount of additives. Additive-type pellets were produced in the same way as usual: mixing, compacting, crashing and pressing to form green pellets. Matrix-type pellets were made by a testing process, in which about 0.20% of ammonium oxalate (as pore-former) and 0.20% of zinc stearate were added.
2.2 Furnace and sintering process
The furnace used in the test was manufactured in College of Material Science and Engineering, Chongqing University, China, which was a vertical batch furnace. The sintering temperature was (1 500±2) ℃. The mixture of N2 and CO2 was used as partially-oxidative atmospheres.
2.3 Test methods
The density of sintered pellets was measured by the immersion technique (in the deionized water) according to Archimedes principal. O/U ratio, grain size and thermal stability of the pellets were measured by the standard test methods.
3 Results and discussion
3.1 Density of tested pellets
The low-temperature sintering test was carried out in a partially-oxidative atmosphere of N2+CO2. Relative densities of pellets sintered at different temperatures are listed in Table 2. From Table 2, it can be deduced that the density is mainly affected by temperature; the higher the temperature is, the higher the density is. The relationship can be expressed by the following equation:
(ρs-ρ0)/(ρth-ρ0)=Aexp(BT) (1)
where ρs, ρ0 and ρth represent sintering density, density of green pellets and theoretical density of UO2, respectively; T is absolute temperature; A and B are coefficients, which are (1.12-1.26)×10-3 and (3.46- 3.91)×10-3, respectively.
Table 2 Relative densities of pellets sintered in partially- oxidative atmosphere at different temperatures (%)
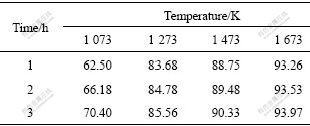
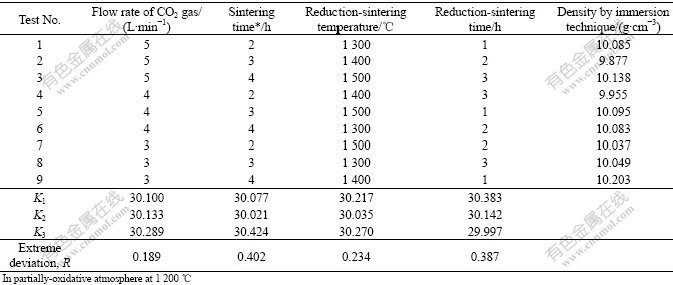
The results of further orthogonal tests (the sintering temperature fixed at 1 473 K, in a partially-oxidative atmosphere) are listed in Table 3. The data in Table 3 indicate that the importance of factor affecting density decrease in the sequence of sintering time, reduction- sintering time, reduction-sintering temperature, and flow rate of CO2 gas. After studying the density difference of sintered pellets, the following optimized parameters can be obtained: CO2 flow rate 3 L/min (flux to mass ratio less than 1.5%), sintering time in a partially-oxidative atmosphere 4 h, reduction-sintering temperature 1 773 K, and reduction-sintering time 1 h. The sintering time is the most significant one among the above-mentioned parameters in partially-oxidative atmosphere. The shorter the time of the pellets sintered in the reduction atmosphere, the higher the density of the sintered pellets obtain. Temperature during the reduction-sintering stage only slightly affects the density of the sintered pellets.
Table 3 Result of orthogonal test on factors affecting pellet characteristics
In order to further improve the density of low-temperature sintered pellets, sintering tests were carried out at 1 673 K in partially-oxidative atmosphere. Both the additive-type pellets and the matrix-type pellets were used in the test. The result is indicated in Table 4. It can be seen that it is feasible to choose 1 473-1 673 K as the sintering temperature. Several batches of validation tests indicate that the densities of the additive-type pellets and the matrix-type pellets are 10.286 g/cm3 (or 93.85%) and 10.286 g/cm3 (or 95.22%), respectively.
Table 4 Densities of pellets sintered in partially-oxidative atmosphere at 1 673 K (g/cm3)
3.2 Other physico-chemical properties of pellets
When being sintered in the partially-oxidative atmosphere, density, dimension, porosity, O/U ratio and other properties of the pellets are surely altered, and so do their microstructures. The dependences of some characteristics on sintering parameters are shown in Fig.1. Fig.1(b) shows that the open pore percentage of sintered pellets drops sharply at 1 200 ℃, so this proves again that it is feasible to choose 1 200-1 400 ℃ as the sintering temperatures. Fig.2 shows the appearance and the microstructure of the sintered pellets.
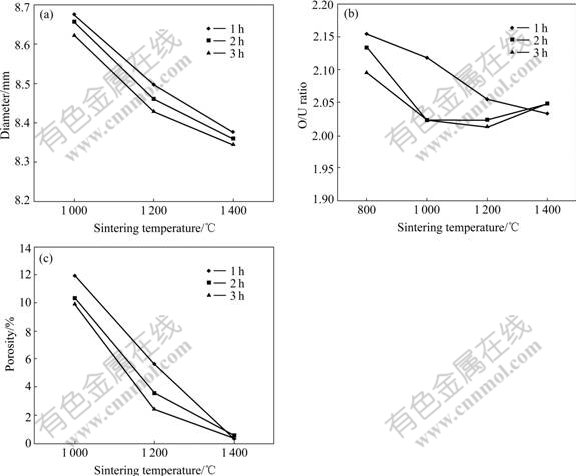
Fig.1 Properties of sintered pellets vs sintering- temperature and time: (a) Diameter; (b) O/U ratio; (c) Porosity
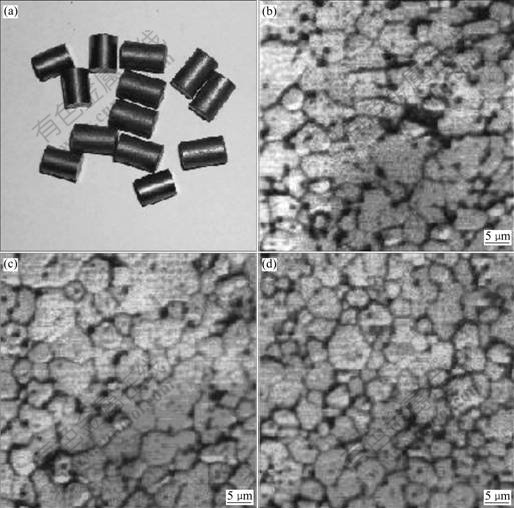
Fig.2 Appearance (a) and microstructures (b, c, d) of sintered pellets (Average grain size: 4.5 mm): (b) Edge; (c) Interspace; (d) Center
Sintering temperature has a great effect on the density of sintered pellets. So orthogonal sintering tests are performed at the fixed temperature of 1 200 ℃ (Table 3), and the results are listed in Table 5. It can be deduced that, as for grain size of sintered pellets, the importance of factors decreases in the sequence of sintering time, reduction-sintering time, flow rate of CO2 gas, and reduction-sintering time. The optimum parameters of the sintering process are CO2 flow rate of 3 L/min, sintering time of 4 h in a partially-oxidative atmosphere, reduction-sintering temperature of 1 400 ℃, and 1 h reduction-sintering. For the ratio of open pores, the importance of factors decreases in the sequence of
Table 5 Data gathered from orthogonal test
sintering time, reduction-sintering time, reduction- sintering temperature, and flow rate of CO2 gas. The optimum parameters of the sintering process are CO2 flow rate of 3 L/min, sintering time of 4 h in a partially- oxidative atmosphere, reduction-sintering temperature of 1 500 ℃, and reduction-sintering time of 1 h. In general, when the temperature of LTS in partially-oxidative atmosphere is selected, the optimum parameters for the LTS process should be 3 L/min CO2, 4 h of sintering in partially-oxidative atmosphere, reduction-sintering temperature of 1 500 ℃, and reduction-sintering time of 1 h.
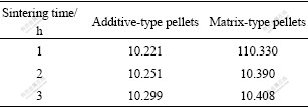
The characteristics of sintered pellets at a fixed temperature of 1 400 ℃ in partially-oxidative atmosphere are listed in Table 6. For both types of sintered pellets, the densities increase with prolonging the sintering time, but the increment is very small. The ratio of pores tends to be stable after sintering for 1 h, and just slightly changes with prolonging sintering time. All these results indicate that the densification process of pellets is completed almost in 1 h when being sintered at 1 400 ℃ in partially-oxidative atmosphere. After that the grains begin to grow up. It should be noted that the thermal stability of the sintered pellets is negative, which means that the low-temperature sintered pellets expand after re-sintering.
Table 6 Properties of additive-type and matrix-type pellets sintered at 1 673 K
3.3 Other results
Sintering temperature in partially-oxidative atmos- phere is 1 673 K, sintering time is 6 h, reduction-sintering temperature is 1 773 K, and reduction-sintering time is 1 h. The results of validation test are listed in Table 7. Under the same sintering conditions, the density of matrix-type pellets is about 0.14 g/cm3 higher than that of additive-type pellets, and the thermal stability of additive-type pellets is better than that of matrix-type pellets. It is also found that the density of pellets sintered at 1 400 ℃ for 3 h in partially- oxidative atmosphere is almost the same as that of pellets sintered at the same temperature and atmosphere for 6 h and then sintered at 1 500 ℃ in hydrogen for 1 h. So are the grain size, the ratio of total pore and the ratio of small pore.
Table 7 Data of validation tests for comparison
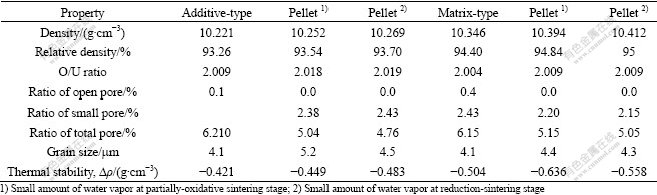
Several experiments were also carried out with a small amount of water vapor in the atmosphere at different sintering stages. Table 8 lists the results. It can be seen that the presence of water vapor in the atmosphere actually influences the density of pellets in terms of growing up of grains and reducing of small pores and total pore as well. In general, the effect of a small amount of water vapor at the partially-oxidative sintering stage is slighter than that at the reduction-sintering stage.
Table 8 Data of tests with water vapor in atmosphere
4 Discussion
4.1 Mechanism of LTS
Low-temperature sintering is essentially an activation sintering technique. The variations of UO2 pellet properties during LTS processes can be accelerated and improved by some additional factors. So, compared with traditional process, UO2 pellets with higher sintering density can be obtained at much lower sintering temperature. The sintering of uranium dioxide is controlled by diffusing processes. The diffusing coefficient of oxygen is larger than that of uranium by several magnitudes, so it is the uranium atom that controls the sintering process of UO2. In the later stage of sintering, uranium atoms diffuse along the grain boundaries. In hyper-stoichiometric uranium dioxide, the activation energy for diffusion of uranium atom drops exponentially as the surplus oxygen increases. Therefore, the presence of an amount of surplus oxygen in uranium dioxide can decrease the activation energy for diffusion and get a higher diffusing coefficient of uranium atom, and the LTS can thus be performed. When being sintered in a reductive atmosphere, the surplus oxygen in UO2+x is reduced and removed very fast. But when being sintered in partially-oxidative atmosphere, the surplus oxygen in UO2+x is remained in equilibrium with the oxygen in the atmosphere. The O/U ratio remains constant during the whole sintering process. Therefore, partially-oxidative atmosphere is essential for LTS. It has been proved that nitrogen-based atmosphere with a small amount of CO2 can be used as the partially-oxidative atmosphere for LTS of UO2 pellets. It is obvious that the CO2 in the sintering atmosphere promotes the O/U ratio in UO2, decreases the activation energy for diffusion of uranium atom, and enhances the diffusion rate of uranium atom. In hyper-stoichiometric uranium dioxide, as mentioned above, the activation energy for diffusion of uranium atom drops exponentially as the surplus oxygen increases, and the growing rate of grain at the anaphase of the sintering process is controlled by diffusion. This principal is utilized in the LTS technique to accelerate the sintering process. Therefore, the sintering process and growing up of grain are promoted by enhancing the x value of UO2+x.
1) Small amount of water vapor at partially-oxidative sintering stage; 2) Small amount of water vapor at reduction-sintering stage
Theoretically, the densification rate of crystalline solid during sintering is controlled by the diffusion of atoms, which means that the diffusion coefficient is the most important factor. For diffusion sintering, the typical densification equation is
x5/a2=40σΩDt/(KT) (2)
D=D0exp[-ΔG/(RT)] (3)
For a certain type of green pellets, a, σ, Ω, K and R are constants. So the densification rate of sintering depends on the value of Dt/T. With the diffusion coefficient increasing, sintering time can be reduced or sintering temperature T can be lowered. That is low- temperature activation sintering. For example, at 1 100 ℃, for UO2+x with x equal to 0.000 01, D is 3.9×10-25 cm/s; and with x equal to 0.03, D is 3.3×10-18 cm/s. As oxygen content increases, the diffusion coefficient increases exponentially because the activation energy of diffusion (ΔG) decreases exponentially.
The promoting effect of water vapor on sintering can also be explained by the LTS mechanism. As water vapor is introduced into the sintering atmosphere, H2O is disassociated, and then OH- and H+ are released. Uranium dioxide reacts with water vapor during the sintering process. Thus, the O/U ratio is enhanced, which makes the sintered pellets be made of UO2 and U4O9. The activation energy of diffusion for uranium atom is then lowered, with the diffusion rate of uranium atom enhanced. In hyper-stoichiometric uranium dioxide, as mentioned above, the activation energy for diffusion of uranium atom drops exponentially as the surplus oxygen increases, and the growing rate of grains at the anaphase of the sintering process is controlled by diffusion. This principle is utilized in the LTS technique to accelerate the sintering process. Therefore, the sintering process and the growing up of grains are promoted by enhancing the x value of UO2+x.
On the other hand, water vapor in atmosphere can take away impurities in the pellets. For example, reacting with H+ of H2O and being decomposed under high-temperature, boron, fluorine and chlorine can form boric acid, HF and HCl respectively, which are expelled from the furnace with the flowing sintering atmosphere. Zinc oxide can be reduced to Zn, which also volatilize very fast due to its low melting point.
4.2 Pore distribution of low-temperature sintered pellets
The data in Tables 2 and 6 are plotted in Fig.3. The closed porosity is calculated by the following equation:
ρc=1-ρr-ρo (4)

Fig.3 Change of open and close porosity vs relative density of LTS pellets
Fig.3 shows that the open porosity drops as the density of sintered pellets increases. When the density rises to 10.192 g/cm3 (relative density 92%), the open porosity drops to less than 1%.
All these show that the pore distribution of pellets by LTS has no other special feature, and the regularity is similar as that of high-temperature sintered (HTS) pellets. Further studies on the difference of pore configuration between LTS pellets and HTS pellets shall be carried out.
4.3 Effect of additives on thermal stability of sintered pallets in LTS
Thermal stability is used to verify the dimension stability of sintered pellets. In general, microstructure of pellets is the most important factor that affects the thermal stability of pellet dimensions. Pellets with low density, small pores and small grain size usually have a poor thermal stability. On the contrary, pellets with high density, big pores and big grain size usually have a good thermal stability. Pore configuration includes the shape, size and distribution of pores, which are the most important characteristics for the thermal stability of pellets in the reactor.
Uranium dioxide powder is piled up by particles or agglomerated particles. The green pellets made of this powder contain a large number of pores connecting with each other. The distribution of pores in green pellets shows an “S” shape. The “S” shape indicates that there are two pore sizes in the green pellets, which are big pores and tiny pores. The tiny pores exist in the original powder, and the thick/big pores form during the pressing process of green pellets. During sintering of UO2 green pellets, the porosity is lowered but the density is increased. The densification of pellets is the result of element diffusion toward pores. Tiny pores at the interface of granules disappear firstly, and then do big ones. Therefore, all the changes of pellets happened during sintering are related directly or indirectly to the changes of contact between particles.
Additives are usually used to improve microstructure, especially for better pore shape and distribution. U3O8 and some organic pore-formers are used to adjust the density of pellets most frequently. As a high valence oxide, U3O8 changes its crystal structure during sintering to leave some pores in pellets. It acts as a pore optimizer and regulator. Compared with U3O8, organic pore-formers such as ammonium oxalate completely volatilize during sintering processes, and leave bigger pores in the pellets. So, organic pore-former has much stronger effect on lowering density than U3O8 does.
From Tables 6 and 7, it can be found that the density of matrix-type pellets is higher than that of additive-type pellets by about 1.2%-1.4% under the same sintering condition. And additives show no obvious effect on ratio of open pores and of small pores. Detailed study on effect of additives on microstructure (especially pore configuration) of low temperature sintered pellets shall be carried out. The adding of U3O8 and organic pore-former is beneficial to the thermal stability of sintered pellets.
5 Conclusions
1) Green pellets taken from normal production process are used in the experiments of low temperature sintering. The low temperature sintering routine and the parameters are established. Pellets are sintered in partially-oxidative atmosphere at 1 400 ℃ for 6 h and then sintered in reductive atmosphere at 1 500 ℃ for 1 h. The relative density of resulted additive-type pellets is about 94%.
2) Experimental results indicate that the importance of sintering factors, which affect characteristics of sintered pellets, decreases in the following sequence of partially-oxidative sintering time, reduction-sintering time, reduction-sintering temperature and flow rate of CO2 gas.
3) Experimental results show that the pore distribution vs density curve of the low temperature sintered pellets is in agreement with that of high temperature sintered pellets.
References
[1] YANG X D, GAO J C, WU Z M, WANG Y. Low-temperature sintering of UO2 pellets in low-partial-pressure oxidizing atmosphere [J]. Atomic Energy Science and Technology, 2006, 40: 682-687.
[2] AYAZ B, BILGE A N. The possible usage of ex-ADU uranium dioxide fuel pellets with low-temperature sintering [J]. Journal of Nuclear Materials, 2000, 280: 45-50.
[3] YANG X D, GAO J C, WU Z M. Low-temperature sintering of UO2 pellets at partial-pressure oxidization atmosphere [J]. Atomic Energy Science and Technology, 2005, 39(Suppl): 122-124.
[4] HARADA Y. UO2 sintering in controlled oxygen atmospheres of three-stage process [J]. Journal of Nuclear Materials, 1997, 245: 217 -223.
[5] GAO J C, LI R, ZHONG F W, YANG. X D, WANG Y. Progress in processes of uranium dioxides pellets [J]. Journal of Functional Materials, 2006, 37: 849-852.
[6] CHEVREL H, DEHAUDT P, FRANCOIS B, BAUMARD J F. Influence of surface phenomena during sintering of overstoichiometric uranium dioxide UO2+x [J]. Journal of Nuclear Materials, 1992, 189: 175-182.
[7] SONG K W, KIM S H, NA S H, LEE Y W. Effects of Nb2O5 addition on grain growth and densification in UO2 pellets under reducing and/or oxidizing atmospheres [J]. Journal of Nuclear Materials, 1994, 209: 280-285.
[8] YANG X D. Technological research on low-temperature sintering of UO2 pellets [D]. Chongqing: Chongqing University, 2004: 4-8.
Corresponding author: GAO Jia-cheng; Tel: +86-23-65111520; Fax: +86-23-65102821; E-mail: gaojch@cqu.edu.cn
(Edited by YANG Bing)


