Trans. Nonferrous Met. Soc. China 22(2012) 2972-2978
Preparation of ultra-fine fibrous Fe-Ni alloy powder by coordinated co-precipitation-direct reduction process
ZHANG Chuan-fu, YAO Yong-lin, ZHANG Yin-liang, ZHAN Jing
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 9 October 2011; accepted 17 February 2012
Abstract:
The precursor prepared by coordinated co-precipitation was direct reduced by hydrogen to ultra-fine fibrous Fe-Ni alloy powder. The effects of concentrations of reactants, pH value, reaction temperature and additive on the preparation of precursor were systematically investigated. The structures, thermal decomposition processes and morphologies of the precursors were characterized by X-ray diffraction (XRD), thermal gravity-differential thermal analysis (TG-DTA) and scanning electron microscoy (SEM). The results show that using 2% polyvinylpyrrolidone (PVP) (in mass fraction) as additive, a well-dispersed precursor with a uniform morphology can be obtained in a solution with Fe2+ and Ni2+ total concentration (1:1) of 0.8 mol/L, pH value of 6.2 at 60 °C, and a pure and well dispersed fibrous iron-nickel powder can be prepared by direct reduction of this precursor in a mixed atmosphere of nitrogen and hydrogen at the temperature of 420 °C.
Key words:
Fe-Ni alloy powder; Fe2+; Ni2+; precursor; fiber; shape-controlled synthesis;
1 Introduction
Ultra-fine Fe-Ni alloy powder, because of the characteristics of small and uniform size, large specific surface area, high chemical activity, special surface magnetism, and excellent magnetoelectric properties, is widely applied in magnetic materials, hard alloy, alloy plating and so on [1]. Fibrous Fe-Ni alloy powder is an ideal polycrystalline iron fiber for absorbing materials with high permeability and electrical conductivity [2,3]. Ultra-fine fibrous Fe-Ni alloy powder has unique shape anisotropy that its axial permeability and conductivity are greater than radial ones. Compared with traditional metal powder absorbing materials, ultra-fine fibrous Fe-Ni alloy powder has a very strong dielectric loss, eddy current loss and hysteresis loss in a wide frequency band with high absorption rate because of its layered direction arrangement, and its mass can be reduced by 40%-60%, with the coating mass density of only 1.5-2.5 kg/m2. Therefore, ultra-fine fibrous Fe-Ni alloy powder is a good light radar absorbing material [4,5].
To the best of our knowledge, ultra-fine Fe-Ni alloy powder has been studied by many researchers and some preparation methods have been used, such as machine-alloying [6], mechanochemical process [7], electrodeposition method [8], chemical carbonyl method [9], liquid reduction method [10], chemical reduction [11], sol-gel method [12], micro-emulsion method [13,14], template method [15,16] and combustion method [17]. In these methods, some impurities will be introduced by machine-alloying and liquid reduction method, and other methods have a higher cost. At the same time, most of the methods can be only used to prepare particles with spherical shape, tetragonal shape and amorphism, and the one-dimensional fiber particles can be prepared by template method but difficult to be industrialized.
Based on the past studies of our research group [18-20], the authors used ethylenediamine as regulator to coordinate the iron-nickel ions in the solution to control the morphology of the precursor powder. The precursor powder was directly reduced to Fe-Ni alloy powder in the mixed atmosphere of nitrogen and hydrogen. The effects of experiment conditions on the size and morphology of product during the process of precursor-preparation and thermal decomposition reduction were studied in detail. The obtained Fe-Ni alloy powder has an average size of 300 nm, with even dispersion and well-fibrous morphology.
2 Experimental
2.1 Synthesis of fibrous Fe-Ni alloy powder
FeSO4·7H2O, NiSO4·6H2O, oxalic acid, ethylene- diamine, and alcohol were used as raw materials. All of the reagents were of analytical grade, the reaction solutions were all prepared on the same day and insoluble impurities were filtered. A certain concentration of oxalic acid solution was prepared in reactor and PVP used as surfactant was added. The oxalic acid was stirred in a constant temperature water bath which was heated to the reaction temperature and maintained at the constant temperature. The stoichiometric mixed solution of FeSO4 and NiSO4 was prepared and added into the reactor. Ethylenediamine was used as a coordination agent and pH value regulator. After filtration, the precipitation was repeatedly washed with deionized water and soaked in ethanol for 30 min, then it was put into a vacuum drying oven at 60 °C and dried for 24 h, and the final product obtained is oxalate iron-nickel composite powder (for ease of description, hereinafter known as precursor). The dried precursor powder was put into a porcelain boat and placed into a tubular heater, controlling the heating temperature, heating rate, holding time and reduction atmosphere to process the hydrogen direct reduction. After reaction, the powder cooled in the nitrogen atmosphere is the desired Fe-Ni alloy powder.
2.2 Characterization
The structures and compositions of precursor and Fe-Ni alloy powders were characterized by D/max-2400 XRD, using Cu Kα radiation, with λ=0.154056 nm, tube voltage of 50 kV, current of 10 mA, pace width of 0.01°, scan speed of 4 (°)/min, and scan range (2θ) of 10°-90°. The morphologies, particle sizes and dispersions of the precursor and Fe-Ni alloy powders were scanned with JSM-5600LV SEM. The temperature range of the direct reduction of precursor was determined by TG-DTA analysis. The specific surface area was measured by Autosorb-1 gas adsorption apparatus produced by Quantachrome Company with adsorbed gas nitrogen at 77.35 K.
3 Results and discussion
3.1 XRD analysis of precursor
Figure 1 shows the XRD patterns of the precursor powder, and FeC2O4·2H2O and NiC2O4·2H2O prepared with the same method. There are obvious characteristics peaks, which indicates that the material has a high crystallinity. Compared with Figs. 1(b) and (c), no FeC2O4·2H2O and NiC2O4·2H2O can be found in the precursor, which shows that the iron and nickel oxalate precipitated together and formed a complex salt. No JCPDS card can be found to accord with the precursor, so we suppose that during the sediment process, the ethylenediamine in solution entered into the sediment and formed a precipitation of oxalic acid iron-nickel complex salt which may be structured as [NixFe1-x(C2N2H8)y]C2O4·nH2O. Some similar results were reported in other studies about oxalate precursors of transition metals [21,22].
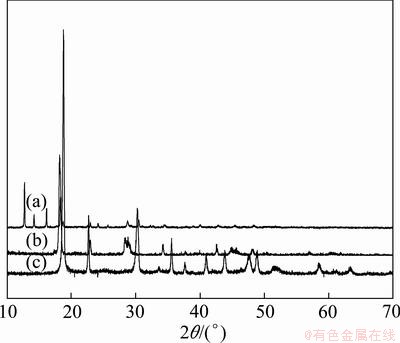
Fig. 1 XRD patters of iron-nickel alloy precursor (a), FeC2O4·2H2O (b) and NiC2O4·2H2O (c)
3.2 Impact of parameters of precipitation
3.2.1 Impact of metal ion concentration
The concentration of metal ions has a great impact on the morphology of the precursor. The SEM photographs of precursor powders produced with different metal ion concentrations are shown in Fig. 2. When the total metal ion concentration (n(Fe)/n(Ni)=1:1) was 0.4 mol/L, the precursor was rod-like, but the uniformity of the particle was poor, with different lengths, serious damages among the particles, as shown in Fig. 2(a). With the concentration increasing to 0.6 mol/L, there is not obvious change on the morphologies of the precursors (Fig.2 (b)). When the total metal ion concentration was 0.8 mol/L, a good dispersion, increased aspect ratio, significant particle interface were observed. According to the classical crystallographic theory, particle formation includes nucleation and crystal growth. At a low metal ion concentration, the supersaturation is relatively low, inducing that the crystal growth will predominate and a bigger particle will be obtained. Continuing to improve the concentration of metal ions, the solution saturation and nucleation rate would increase, leading to the formation of a large number of crystal nuclei. Thus the frequency of collisions between the crystal nuclei increased, resulting in more serious reunion. The size of precursor obtained at high concentration (greater than 1 mol/L) is uneven as indicated in Fig.2 (d). The results show that the total concentration of metal ions should be controlled at about 0.8 mol/L.
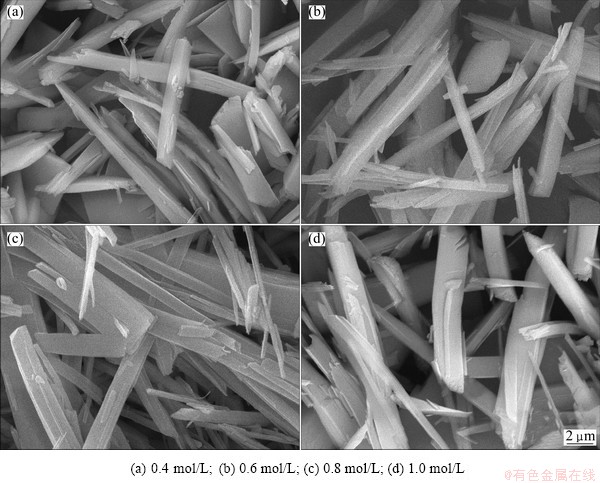
Fig. 2 SEM morphologies of precursors prepared under different concentrations of metal ions at 50 °C and pH=5.8
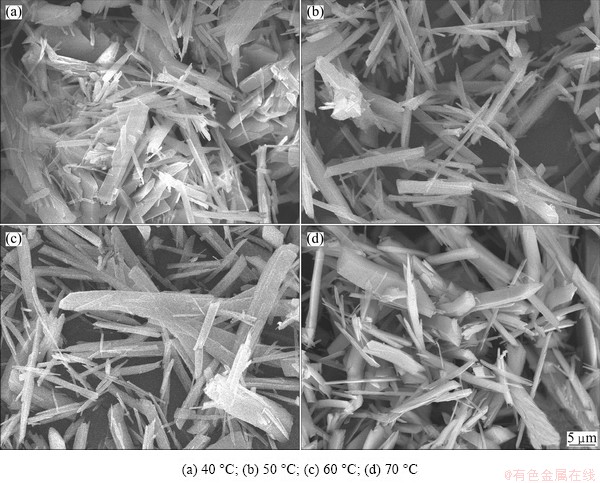
Fig. 3 SEM morphologies of precursors prepared at different temperatures when the total concentration was 0.8 mol/L at pH=5.8
3.2.2 Impact of temperature
The size and shape of the precursor are greatly affected by the reaction temperature. The SEM photographs of precursor produced at different temperatures are shown in Fig. 3. It can be seen that, at a lower temperature, because of the higher viscosity of solution and lower diffusion rate of metal ions, the particles had serious reunion, and non-uniform size, as shown in Fig. 3(a). With the increase in temperature, there will be a better condition for nucleation and crystal growth, so the aspect ratio of particles became large, the reunion was lightened, and the border became obvious. At 70 °C, the size of particles is big because of the quick growth speed at high temperature, as indicated in Fig. 3(d). Therefore, to prepare precursor powder with small size and good dispersion, the best reaction temperature is 50-60 °C.
3.2.3 Impact of pH value
The SEM photographs of precursors produced at different pH values are shown in Fig. 4, which shows that the reaction pH value has a great influence on precursor’s shape. At a low pH value, the precursor shape is irregular, but presents a better dispersion, as shown in Fig. 4(a). With the increase of pH value, the precursor becomes cubic-like with a uniform size, as shown in Fig. 4(b). At the pH values of 6.0 and 6.2, the appearance of the precursor is rod-like, with a smaller size, a larger aspect ratio, and a better dispersion, as shown in Figs. 4(c) and (d). Ethylenediamine was used to adjust the pH value and at different pH values, the amount of ethylenediamine was also different. It can be used to control the speed of precipitation that ethylenediamine can complex with iron and nickel ions, which is conducive to prepare precursor powder with good dispersion. At a lower concentration of ethylenediamine, there will be more free metal ions which will combine the oxalic ion quickly and form cubic FexNi1-xC2O4·2H2O particles. Improving the concentration of ethylenediamine, more metal ions will combine with ethylenediamine to form complex first which will decrease the concentration of free metal ions and be benefit for the oriented growth of crystal, and then the complex will combine with oxalic ion to form fibrous precursor. The results show that the reaction pH value should be controlled at 6.0-6.2.
3.2.4 Impact of surfactant
The surfactant can greatly improve the dispersion of precursor powder. In the growing up process of precipitation particles, the surfactant adsorbed on the solid-liquid interface can reduce the interface free energy, thus the thermodynamics of the spontaneous cohesion process was weakened. In addition, the surfactant adsorbed on the surface of particles played as a steric, making the powder not easy to agglomerate. The SEM photographs of precursors prepared with different amounts of additive are shown in Fig. 5. It can be seen from Fig. 5(a) that there is a very serious reunion of the precursor, and some cubic particles appear. A certain amount of additive is helpful to decrease the reunion of particles and obtain a good dispersion. In the experiment, adding 2% PVP as additives could improve the morphology of powder to a greater degree to obtain precursor with good dispersion and large aspect ratio, as shown in Fig. 5(b).
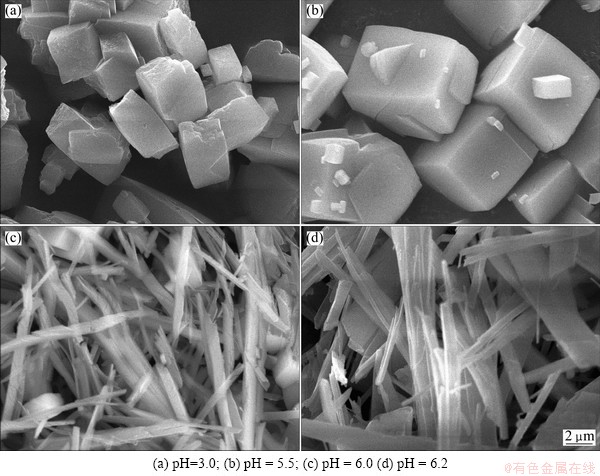
Fig. 4 SEM morphologies of precursors prepared at different pH values when the total concentration was 0.8 mol/L at 60 °C
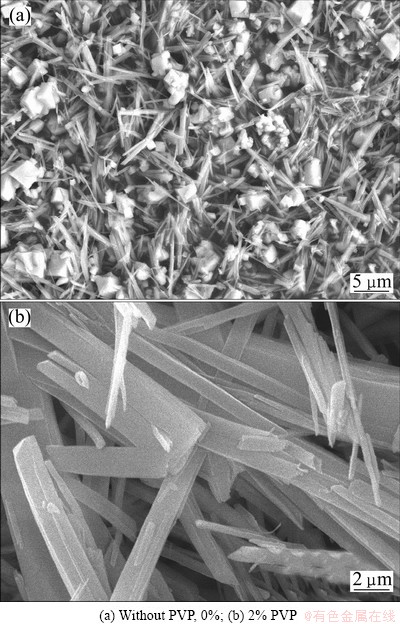
Fig. 5 SEM photographs of precursors prepared with different amounts of additive when the total concentration was 0.8 mol/L at 50 °C and pH=5.8
3.3 TG-DTA analysis of precursor powder
The TG-DTA analysis of precursor powder was done to roughly determine the temperature range of the following direct reduction of precursor. Figure 6 shows the TG-DTA curves in the nitrogen atmosphere, with a heating rate of 10 °C/min. We can see from Fig. 6 that the thermal decomposition of precursor in nitrogen can be divided into three steps: before 100 °C, there is a slow mass loss of 8.591%, and the corresponding DTA curve shows a clear endothermic peak, which is the removal of the ethylenediamine in precursor; there is an endothermic peak at 200.75 °C, corresponding to a mass loss of 5.575%, the removal process of the crystallization water in precursor; the endothermic peak at 386.11 °C corresponds to the mass-loss platform of 52.36%, which is the thermal decomposition process of oxalic acid iron-nickel complex salt. Above all, the total decomposition process can be described by the following reactions:
[NixFe1-x(C2N2H8)y]C2O4·nH2O=[NixFe1-x]C2O4·nH2O+yC2N2H8↑ (1)
[NixFe1-x]C2O4·nH2O=[NixFe1-x]C2O4+nH2O↑ (2)
[NixFe1-x]C2O4=NixFe1-xO+CO↑+CO2↑ (3)
3.4 Characterization of Fe-Ni alloy powder
Direct reduction experiment of the precursor
powder was carried out under nitrogen and hydrogen atmosphere at 420 °C. The XRD pattern and SEM image of the obtained product are shown in Fig. 7(a) and Fig. 7(b), respectively. It can be seen from Fig. 7(a) that the sample shows a good crystallinity. The peaks match well with those of the JCPDS (No. 47—1417) data of the γ-(Fe,Ni) with face-centered cubic phase. So the obtained thermal decomposition product is Fe-Ni alloy powder. The product prepared under this temperature shows no impurity peak on the XRD pattern, indicating that the product has a high purity. Using (111) crystal plane diffraction peak as a benchmark, and Scherrer formula d = 0.90λ/(βcosθ) for calculation, the particle size is about 300-400 nm. The specific surface area is about 25.0 m2/g. It can be seen from Fig. 7(b) that the iron-nickel alloy powder prepared at 420 °C presents uniform granularity, fibrous structure, and good dispersion, and its morphology has a good inheritance of the precursor.
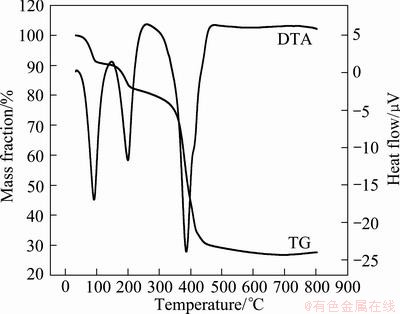
Fig. 6 TG-DTA curve of iron-nickel alloy precursor powder
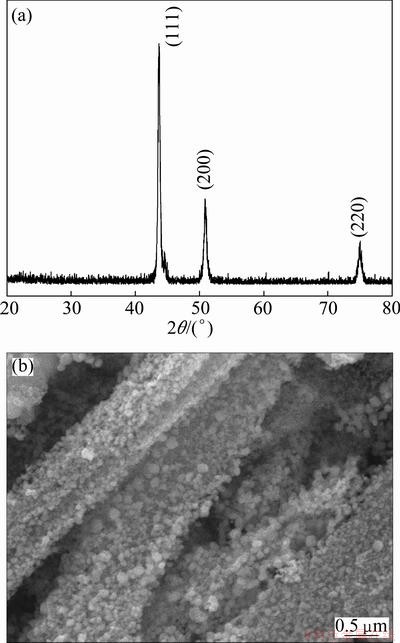
Fig. 7 XRD pattern (a) and SEM morphology (b) of ultra-fine iron-nickel alloy powder obtained at 420 °C
4 Conclusions
1) The precursor of fibrous iron-nickel powder can be prepared by coordinated co-precipitation process with FeSO4·7H2O and NiSO4·6H2O as raw materials, oxalic acid as precipitator, and ethylenediamine as regulator. The experimental study shows that using 2%PVP in mass fraction as additive, well-dispersed fibrous precursor with a uniform morphology can be obtained in a solution with Fe2+ and Ni2+ total concentration of 0.8 mol/L, pH value of 6.2 at 60 °C.
2) Well dispersed fiber iron-nickel powder with a high purity, uniform granularity can be prepared by direct reduction of this precursor in a mixed atmosphere of nitrogen and hydrogen at the temperature of 420 °C. The Fe-Ni alloy has a face-centered cubic phase and the particle size is 300-400 nm, the specific surface area is about 25.0 m2/g.
References
[1] FRASE H N, SHULL R D, HONG L B, STEPHENS T A, GAO Z Q, FULTZ B. Soft magnetic properties of nanocrystalline Ni3Fe and Fe75Al12.5Ge12.5 [J]. Nanostructured Materials, 1999, 11(8): 987-993.
[2] LIU Yong-sheng, ZHANG Jin-cang, YU Li-ming, JIA Guang-qiang, JING Chao, CAO Shi-xun. Magnetic and frequency properties for nanocrystalline Fe-Ni alloys prepared by high-energy milling method [J]. Journal of Magnetism and Magnetic Materials, 2005, 285: 138-144.
[3] DIVINSKI S V, HISKER F, KANG Y S, LEE J S, HERZIG C. Ag diffusion and interface segregation in nanocrystalline γ-FeNi alloy with a two-scale microstructure [J]. Acta Materialia, 2004, 52: 631-645.
[4] TANAKA A, YOON S H, MOCHIDA I. Formation of fine Fe-Ni particles for the non-supported catalytic synthesis of uniform carbon nanofibers [J]. Carbon, 2004, 42: 1291-1298.
[5] SUH Y J, JANG H D, CHANG H, KIM W B, KIM H C. Size-controlled synthesis of Fe-Ni alloy nanoparticles by hydrogen reduction of metal chlorides [J]. Powder Technology, 2006, 161: 196-201.
[6] HAMZAOUI R, ELKEDIM O, GRENECHE J M, GAFFET E. X-ray diffraction and Mossbauer studies of mechanically alloyed Fe-Ni nanostructured powders [J]. Journal of Magnetism and Magnetic Materials, 2005, 294: 145-149.
[7] QIN X Y, LEE J S, NAM J G, KIM B S. Synthesis and microstructural characterization of nanostructured γ-Ni-Fe powder [J]. Nanostructured Materials, 1999, 11(3): 383-397.
[8] TRUDEAU M L. Nanocrystalline Fe and Fe-riched Fe-Ni through electrodeposition [J]. Nanostructured Materials, 1999, 12(1-4): 55-60.
[9] CHEN Li-min, QI Jia-zhong, ZHU Xue-qin, GE Fu-ding. Microstructure and microwave absorptivity of nanometer γ-(Fe, Ni) alloy particles [J]. Ordnance Material Science and Engineering, 1999, 22(4): 3-6. (in Chinese)
[10] DONG Guo-jun, ZHANG Jie, HAN Huan-bo, ZHANG Mi-lin. Preparation and catalytic performance for hydrogen production of Fe-Ni nanosized alloy [J]. Journal of Harbin Engineering University, 2006, 27(5): 786-790. (in Chinese)
[11] MOUSTAFA S F, DAOUSH W M. Synthesis of nano-sized Fe-Ni powder by chemical process for magnetic applications [J]. Journal of Materials Processing Technology, 2007, 181: 59-63.
[12] SHEN Hong-fang, CHEN Wen-ge, GU Chen-qing. Preparation and characterization of Fe-Ni nano-composite powders [J]. Materials for Mechanical Engineering, 2005, 29(4): 31-33. (in Chinese)
[13] ZHANG Chao-ping, DENG Wei, HU Lin, LUO Yu-ping, HU Zong-chao, GAO Xiang, SHEN De-jun. Preparation of ultrafine Ni-Fe composite particles by microemulsion [J]. Journal of Inorganic Materials, 2001, 16(3): 481-485. (in Chinese)
[14] HU Lin, ZHANG Chao-ping. Preparation and characterization of Ni/Fe composite nanoparticles [J]. Journal of Fudan University, 2003, 42(1): 35-38. (in Chinese)
[15] DUHAMEL C, CHAMPION Y, TENCE M, WALLS M. Synthesis of controlled-chemistry ultrafine FexNi1-x ferromagnetic powders [J]. Journal of Alloys and Compounds, 2005, 393: 204-210.
[16] WU Hua-qiang, CAO Yun-jie, YUAN Pin-shi, XU Hong-yan, WEI Xian-wen. Controlled synthesis, structure and magnetic properties of Fe1-xNix alloy nanoparticles attached on carbon nanotubes [J]. Chemical Physics Letters, 2005, 406: 148-153.
[17] LI An-lun, FANG Zhao-xun, DAI Ming-feng. Combustion synthesis of nanometer Fe-Ni alloy powder and the preparation and character of coating gold [J]. Chinese Journal of Process Engineering, 2004, 4(S): 212-217. (in Chinese)
[18] ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui, LI Chang-jun. Preparation and characterization of fibrous NiO particles by thermal decomposition of nickelous complex precursors [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(4): 713-717.
[19] ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui, GUO Xue-yi, OKIDO M. Preparation of fibrous nickel oxide particles [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(6): 1440-1443.
[20] ZHANG Chuan-fu, WU Lin-lin, LI Chang-jun, WU Jian-hui. Preparation of fiber precursor of Ni-Co alloy powder [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(1): 182-186. (in Chinese)
[21] ZHAN Jing, ZHOU Di-fei, ZHANG Chuan-fu. Shape-controlled synthesis of novel precursor for fibrous Ni-Co alloy powders [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 544-551.
[22] ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui, LI Chang-jun. Preparation and characterization of fibrous NiO particles by thermal decomposition of nickelous complex precursors[J]. Transactions of Nonferrous Metals Society of China, 2004, 14(4): 713-717.
配位共沉淀-直接还原法制备超细纤维状铁镍合金粉
张传福,姚永林,张银亮,湛 菁
中南大学 冶金科学与工程学院,长沙 410083
摘 要:采用配位共沉淀制备了铁镍合金前驱体,用氢气直接还原前驱体得到了超细纤维状铁镍合金粉。系统研究了反应物浓度、pH值、反应温度及添加剂等参数对前驱体制备过程的影响。分别采用XRD、TG-DTA和SEM对前驱体的结构、热分解过程和形貌进行了表征。 结果表明,采用质量分数2%的PVP作为添加剂,当Fe2+和Ni2+ (1:1)总浓度为0.8 mol/L,pH值为6.2,反应温度为60 °C时,可以制得分散良好、形貌均匀的纤维状前驱体。前驱体在420 °C的氮气和氢气混合气氛下直接还原可以得到纯度高、分散性好的纤维状铁镍合金粉。
关键词:铁镍合金粉;Fe2+;Ni2+;前驱体;纤维;形貌控制合成
(Edited by YANG Hua)
Foundation item: Project (20090162120080) supported by the Research Fund for Doctoral Program of Higher Education of China; Project (2010FJ3011) supported by the Program of Science and Technology of Hunan Province, China; Project supported by the Open-End Fund for the Valuable and Precision Instruments of Central South University, China
Corresponding author: ZHAN Jing; Tel: +86-731-88836048; E-mail: zhanjing2001@hotmail.com
DOI: 10.1016/S1003-6326(11)61558-6
Abstract: The precursor prepared by coordinated co-precipitation was direct reduced by hydrogen to ultra-fine fibrous Fe-Ni alloy powder. The effects of concentrations of reactants, pH value, reaction temperature and additive on the preparation of precursor were systematically investigated. The structures, thermal decomposition processes and morphologies of the precursors were characterized by X-ray diffraction (XRD), thermal gravity-differential thermal analysis (TG-DTA) and scanning electron microscoy (SEM). The results show that using 2% polyvinylpyrrolidone (PVP) (in mass fraction) as additive, a well-dispersed precursor with a uniform morphology can be obtained in a solution with Fe2+ and Ni2+ total concentration (1:1) of 0.8 mol/L, pH value of 6.2 at 60 °C, and a pure and well dispersed fibrous iron-nickel powder can be prepared by direct reduction of this precursor in a mixed atmosphere of nitrogen and hydrogen at the temperature of 420 °C.


