
J. Cent. South Univ. (2019) 26: 88-97
DOI: https://doi.org/10.1007/s11771-019-3984-z
Tribological and flame retardant modification of polyamide-6 composite
YOU Yi-lan(游一兰)1, 2, LIU Chen-ming(刘晨明)2, LI Du-xin(李笃信)3,LIU Shi-jun(刘士军)1, HE Guo-wen(贺国文)2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. College of Materials and Chemical Engineering, Hunan City University, Yiyang 413000, China;
3. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
A series of wear and flame resistant polyamide 6 (PA6) composites were prepared using glass fiber (GF) and talc (T) as reinforcer, polytetrafluoroethylene (PTFE) and graphite (Gr) as solid lubricants, red phosphorus (RP) and zinc borate (ZB) as flame retardant. The tribological property, mechanical property, flame retardant property and the flame retardant mechanism were investigated. The tests show that the formula of the wear resistant PA6 composite (WRPA 6) is PA6/GF/T/PTFE/Gr in the ratio of 100/15/5/10/5 by mass. Because this composite exhibits the lowest friction coefficient (0.1429) and no wear mass loss, the introduction of RP and ZB can increase the flame resistance of WRPA6, and the synergistic effect of RP and ZB is obtained. Detailedly, the composite with 4 parts of ZB and 12 parts of RP shows the best flame retardant property, achieving the highest limiting oxygen index (LOI) (30.2 vol%) and a UL94 V-0 rating, and the flame retardant mechanisms may be gas phase along with condense phase mechanism.
Key words:
wear-resistant; flame retardant; polyamide-6 composite;
Cite this article as:
YOU Yi-lan, LIU Chen-ming, LI Du-xin, LIU Shi-jun, HE Guo-wen. Tribological and flame retardant modification of polyamide-6 composite [J]. Journal of Central South University, 2019, 26(1): 88–97.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-3984-z1 Introduction
Polyamide 6 (PA6) is widely used in friction mechanical component like gear and conveyor roller due to its excellent performance such as high specific strength, easy processing and forming, good corrosion resistance and self- lubrication.But PA6 used alone cannot meet the need of wear resistant and noninflammability in certain areas, so the tribological modification and flame retardant modification is necessary.
It is well known that adding solid lubricant, such as graphite (Gr) [1], polytetrafluoroethylene (PTFE) [2], ultrahigh molecular weight polyethylene (UHMWPE) [3] and disulfide (MoS2) [4], is an effective way to reduce the friction coefficient of polymer. There is a common point that solid lubricants are more effective in lowering friction coefficient than wear rate, and some even are harmful for reducing wear rate [5]. But the reinforcement can improve the antiwear performance of polymer composite [6]. Especially when the solid lubricant and reinforcement are used together as modifications, the polymer composite will become even more wearable. In Ref. [7], the tribological properties of polyformaldehyde (POM) was not changed obviously by adding PTFE/MoS2, but the wear volume of POM reduced more than 30% when nano-Al2O3 is added, due to the synergistic effect of reinforcement (nano-Al2O3) and solid lubricants (PTFE/MoS2). The same result can be found in Ref. [8], where the friction coefficient and wear rate of polyetheretherketone (PEEK) decreased sharply when both carbon fiber (CF) and solid lubricant are filled. These synergetic effect are also found in polyester [9], epoxy [10], polyether ketone [11] composites. The wear resistance can be improved by exploring different strengthening agent and solid lubricant combinations.
Nevertheless, these wear resistant materials show poor fire resistance because of its petroleum origin, so flame retardant modification is necessary. With the enhancement of environment protection awareness, halogen-free flame retardant has become an important trend.The common halogen-free flame retardant are mostly inorganic, such as phosphorus-containing compounds [12], borates [13], nitrogen compound [14], metal hydroxides [15], etc. Among them, phosphorous-containing compounds producing non-optical-dense and non-corrosive smoke have been widely used as halogen substitutes, because they work mainly in the condensed phase, by forming a charred layer through dehydration and further crosslinking reactions [16]. Although elemental phosphorus is highly flammable, its amorphous form, red phosphorus (RP), has been found to be an effective flame retardant additive in rubber [17], fabric [18] and plastics [19]. For polyamide, the flame retardant effect of RP is more obvious, because RP could induce char formation much more easily in polymer chains containing nitrogen or oxygen. As reported by SCHARTEL et al [20], the amount of residue of glass fiber reinforced PA66 increased by adding RP. Zinc borate (ZB) is wildly used as a typical inorganic flame retardant due to its good smoke-suppressing properties and low cost. For example, ZB could promote the char generation of polyvinyl chloride and reduce the amount of harmful gases during combustion [21].
In recent years, much effort has been expended to look for the flame retardants which can keep a balance between cost, performance and environment. This effort promoted many researchers to study the synergistic effects between flame retardants. For flame retardant modification of PA6 and its blend, synergistic effects between hyperbranched polyphosphate bisphenol-S ester (HPPES) and melamine pyrophosphate (MPP) [22], organophilic montmorillonite (OMT) and red phosphorus-magnesium hydroxide (RP-MH) [23, 24], MH, MH and methyl-blocked novolac (MBN) [25], aluminum diethylphosphinate (AlPi) and organic bentonite (OBT), zinc oxide (ZnO), zeolite 4A (4A) [26] were presented. It was reported that these combinations could promote the formation of compact char layers, so the flame resistance increased.
In a word, the tribological and flame retardant properties can be improved if the formulation was optimized, but the study about both the wear resistance and flame retardant properties is quite scarce. In this study, a wear and flame resistant material is hoped to be prepared. Firstly, it aims to improve the tribological properties of PA6 by reinforcement and solid lubricant; secondly, endow the wear resistance materials with fire resistance by RP and ZB; thirdly, study the wear and flame retardant mechanism.
2 Experimental
2.1 Specimen preparation
Firstly, PA6 and the continuous glass fiber reinforced PA6 (PA6/GF) (100/15, in weight) were dried under a vacuum at 90 °C for 8 h to remove moisture, then the components (PA6/GF, talc(T), PTFE, Gr, RP and ZB) were mixed equally according to their proportion. The diameter of GF was 13 to 24 μm, and the particles size of Gr, PTFE, T and ZB were 25 μm, 60 μm, 100–1800 mesh and 325 mesh, respectively. RP was in nanoscale and encapsulated in PA6 resin. Secondly, the mixed components were extruded in a double-screw extruder with working temperature between 210 and 240 °C and cut into granules. Thirdly, the granules were dried again as described above and injected into the tested samples under the injection temperature of 230–250 °C.
2.2 Characterization
Friction and wear studies were carried out on a UMT-3 friction and wear tester, the assembly diagram of the frictional pairs was shown in Figure 1. The chromium steel ball was used as counterface. During testing, the ball was fixed and the test sample moved forth and back in line direction in a trace of 10 mm and duration of 120 min under 100 N and 200 r/min (a reciprocating moving of the test sample was defined as one round).
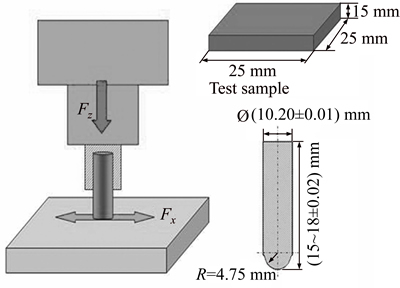
Figure 1 Schematic diagram and sample size of friction and wear test (Fz–Load direction; Fx–Friction direction)
Tensile tests were carried out in accordance with ISO 527-2:1993.
Vertical firing test was performed according the UL94 burning testing standard using the specimen with dimensions of 130 mm×13 mm×3.2 mm. According to the ASTM standard D2863, limiting oxygen index (LOI) measurements were carried out on the limiting oxygen index analyzer with a sample size of 100 mm×6.5mm× 3.0 mm.
Thermogravimetric analysis technique (TGA) was used to study the thermal stability using a NETZSCHST449C apparatus at a heating rate of 10 °C/min under nitrogen flow.
The morphologies of the burning residue in the LOI test and the worn surface were observed by a scanning electron microscope (SEM, QUANTA- FEG250) after being coated with a gold layer.
3 Results and discussion
The glass fiber (GF) and talc (T) were used as reinforcers to increase strength and wear resistance; polytetrafluoroethylene (PTFE), graphite (Gr) and its combination were used as solid lubricants to decrease the friction coefficient; red phosphorus (RP), zinc borate (ZB) and its combinations were used as flame retardants to improve the flame resistance. The tribological properties, flame retardant properties and tensile strength of these composites were studied.
3.1 Tribological properties
Glass fiber (GF), talc (T), polytetrafluoroethylene (PTFE) and graphite (Gr) were used to reduce the wear mass loss and friction coefficient of PA 6, indeed the goal was achieved. By adding these fillers, the wear mass loss of the composites was too small to detect, and the effect of them on the friction coefficient of PA 6 is shown in Figure 2. It is clearly seen that the friction coefficient of PA6 decreases by adding the fillers in appropriate proportion. For the formula with PTFE, as shown in Figure 2(b) , the friction coefficient of PA6 decreases first with the PTFE content up to 10 parts and then increases thereafter, and the lowest friction coefficient in this case is 0.1552, which is 54% lower than that of PA6. But for the formula with Gr, as Figure 2(a) shows, the friction coefficient is lowered only when the composite contains 5 parts of Gr, and it becomes even higher than PA6 when the Gr content exceeds the 5 parts level. Then the synergistic effects of PTFE and graphite were studied. The results reveal that the combination of 10 parts PTFE and 5 parts Gr contributes to the lowest friction coefficient (the value is 0.1429) which is even lower than the composite with 10 parts PTFE, as illustrated in Figure 2(c). It is attributed to the high thermal conductivity of Gr which can reduce the contact temperature during sliding, so the contact area is reduced and the reduced friction coefficient is obtained [5]. In conclusion, the best blending ratio of PA6/GF/T/PTFE/Gr is 100/15/5/10/5 in mass, and this formula is defined as wear-resistant PA6 composite (WRPA6) in this study.
The effects of flame retardants on the tribological of WRPA 6 are shown in Figure 3. The friction coefficient and wear mass loss are on the rise with the increase of RP content. Maybe the continuity of the matrix is wrecked and the optimized WRPA6 formulation is destroyed, so the tribological properties deteriorate. While 4 parts RP is superseded by equal amount of ZB, that is to say, when the ratio of RP/ZB is 12/4 by mass, both the friction coefficient and wear mass loss are reduced. This is due to the strengthening function of ZB which mass can improve the plough resistance of the composite. In this case, the friction coefficient and wear loss is 0.2387 and 3.5 mg, respectively, which is lower than 0.34 and 3.8 mg of pure PA6. But the friction coefficient and wear mass loss reversely increase when more RP is replaced by ZB, this maybe because the excessive ZB increases the abrasive wear loss of the composites.
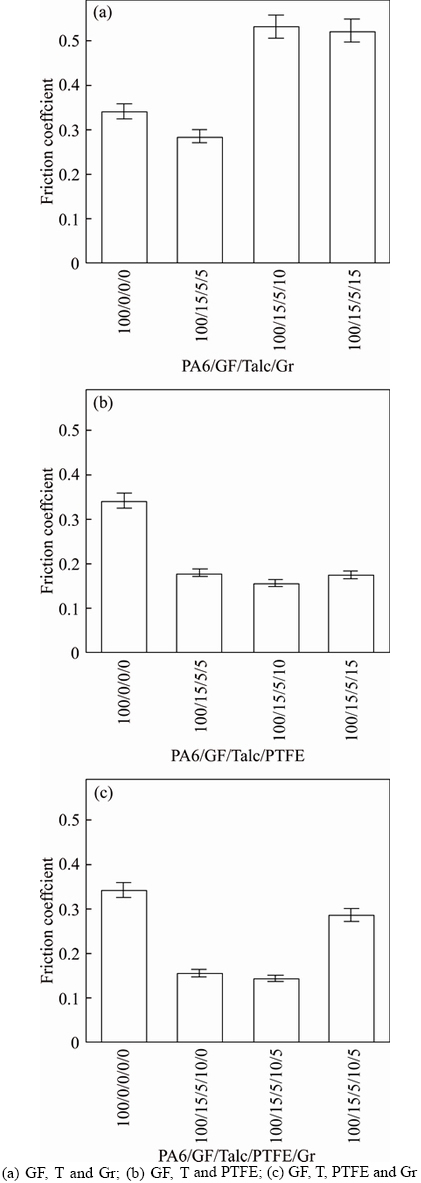
Figure 2 Friction coefficient of PA6 composites filled with GF, T, PTFE and Gr:
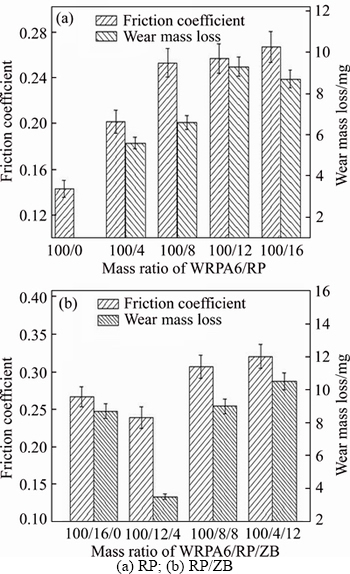
Figure 3 Effect of flame retardants on tribological properties of WRPA 6:
3.2 SEM analysis of worn surface
To further understand the wear mechanism, the worn surfaces were examined by scanning electron microscopy. Figures 4(a)–(c) show the worn surfaces of PA6, WRPA 6 and WRPA6 with 12 parts of RP and 4 parts of ZB. Some cracks perpendicular to the sliding direction and some grooves parallel to the sliding direction are observed in Figure 4(a), which illustrate that the fatigue wear is dominating, and grain wear occurs synchronously during the wear of PA6. When micro cracks extend to the wear surface, the surface will be easily peeled off which is the main cause of PA6 wear. Then in Figure 4(b), there are no cracks, and only a small amount of the removal of the surface material is observed. It may be that the GF and T can enhance the material’s anti-cutting ability and reduce the plastic deformation, so the wear mass loss is markedly decreased. In this time, the wear mechanism is mainly adhesion wear. In Figure 4(c), a lot of little hollows appear; this may be due to the addition of RP and ZB, resulting in the increase of the fillers and the interface combination becoming worse, so the glass fiber falls off more easily and serves as the abrasive, causing a slight increase in the wear.
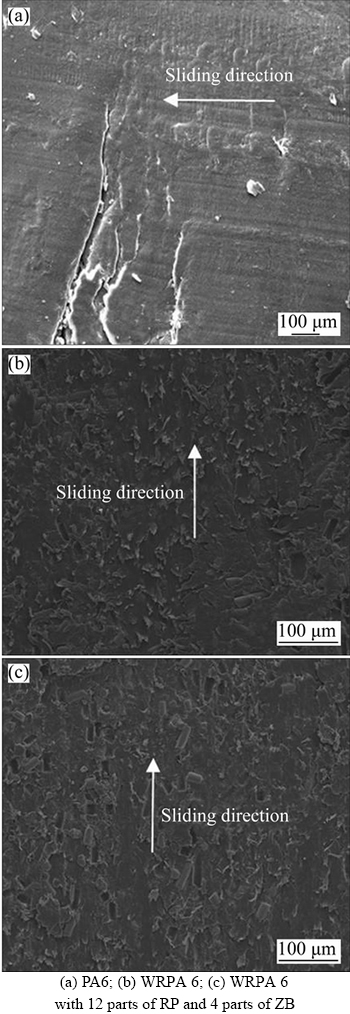
Figure 4 SEM morphologies of worn surfaces of different composites:
3.3 Mechanical property
Figure 5 illustrates the influence of GF, T, PTFE and Gr on the tensile strength of PA6. As we can see, by adding these filler combinations, the tensile strength increases. This is mainly due to the enhancement of GF and T. Also, for the formula containing Gr (Figure 5(a)), the tensile strength increases with the increase of Gr content. It indicates that Gr also shows enhanced effect.

Figure 5 Tensile strength of PA6 composites filled with GF, T, PTFE and Gr:
Therefore, the non-inorganic rigid components which can transmit stress and disperse load and inhibit crack propagation increase in PA6, so the tensile strength increases. Meanwhile, the tensile strength decreases with the increase of PTFE content. This may be due to the lower tensile strength of PTFE itself. While using PTFE and Gr as combined solid lubricants, the tensile strength of the composite is higher than the composite which contains 10 parts PTFE alone and lower than that of which contains 5 parts Gr alone.
The effects of flame retardants on the tensile strength of WRPA 6 are presented in Figure 6. The results show that the tensile strength of WRPA 6 firstly increases and then decreases with the increase of RP dosages, which is similar to the research of SI et al [27]. This may be due to the nano-effect of RP which is at nanoscale. While RP is replaced by equal amounts of ZB, the variation of the tensile strength is within the error range. On the one hand, this is because the RP used here is coated with PA6. After RP is replaced by ZB, the content of the fillers increases, which may make the interface adhesion between matrix and fillers weak, then it weakens the tensile strength. On the other hand, the rigid ZB has an enhanced effect which can increase the tensile strength. The combination of the two reasons makes the tensile strength change little. This is because the ability of WRPA 6 to withstand stress is improved by ZB.

Figure 6 Effect of flame retardants on tensile strength of WRPA 6:
3.4 UL94 rating and LOI
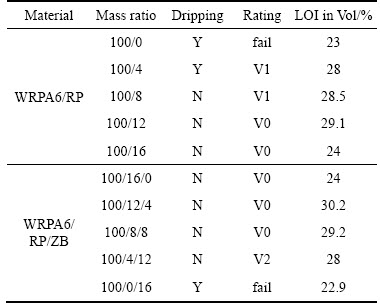
The effects of RP and RP/ZB on LOI as well as flame retardant grade of WRPA6 are shown in Table 1, from which we can see that WRPA6 without fire retardants has a very low LOI of just 23 vol%. With increasing the content of RP, the LOI increases first and then decreases, and the maximum LOI (29.1 vol%) reaches when the content of RP is 12 parts, above which a worsening of flame resistance observed. The composites containing greater than or equal to 12 parts of RP also can achieve a UL94 V-0 rating in vertical firing test. During burning, RP can act both in the gas phase and condensed phase. In gas phase, the PO radical produced by RP plays a major role. In this case, ·H and ·OH radicals can react with PO radical, then they will transform to less effective radicals. So the oxidation of PA6 in the gas phase is slowed down or suspended [28]. In the condensed phase, RP can enhance the charring of PA6 or promote the formation of inorganic glasses, so the fire risks are reduced. But instead of improving, too much RP worsens the flame retardant performance of WRPA6. Maybe on the one hand the quality of the char is deteriorated; on the other hand, it is due to the flammability of RP itself, and the combustion heat of RP will be favorable to combustion. Therefore, 12 parts of RP is the optimum quantity.
During burning, zinc borate (ZB) can be translated into crystalline borophosphate, which acts as residue and forms a protective layer [29, 30]; hence ZB was used as an adjuvant in this study. The effect of RP/ZB on combustion performance of WRPA6 is shown in Table 1, and the total content of RP and ZB is fixed on 16 parts. As seen from Table 1, when using ZB to substitute equivalent RP, the LOI increases first and then decreases with the increasing of ZB content; the composite has the best flame resistance when the mass ratio of RP/ZB is 12/4, and in this case, the LOI is 30.2 vol% and the flame retardant grade is UL94 V-0, which is better than the composite just used 12 parts of RP as flame retardant. During burning, on the one hand, the water in ZB is evaporated to water vapor which can dilute the oxygen; and in condense phase, ZB translates to a glassy boric acid film that can enhance the structure of char layer and retard the decomposition of PA 6. On the other hand, the water vapor will be absorbed by P2O5 produced by RP, resulting in the formation of polyphosphoric acid films, and these polyphosphoric acid derivatives will react with the decomposition of PA 6 to form a protective layer on burning surface, so the decomposed product inside and the oxygen outside are insulated, and the transfer of heat is interrupted. Consequently, the synergistic effect of RP and ZB is obtained.
Table 1 Flammability of wear-resistant PA6 composite (WRPA6) with RP or RP/ZB
3.5 Thermal degradation behaviors
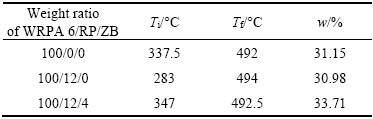
The mechanical, friction and flame retardant properties of the composite are greatly affected by thermal property [31]. So the thermogravimetry curves and thermal analysis of flame resistant material in comparison to WRPA6 are shown in Figure 7 and Table 2. For the formula with 12 parts of RP, the thermal decomposition presents a two-step decomposition characteristic. And the onset decomposition process is shifted to lower temperatures comparing to WRPA6. The results presented here correspond to the data published for PA 66 [20]. It may be due to the fact that the H3PO4 produced by RP at the onset decomposition temperature can catalyse the thermal decomposition of PA6. But the further decomposition at higher temperature is restrained, which is embodied in the phenomenon that the formula with RP exhibits less mass loss instead after 469 °C. This may be attributed to the formation of polyphosphoric acid, which can not only block the transfer of heat or oxygen, but also eliminate some kinds of volatile small molecules by accelerating the reactions such as esterification, dehydration and carbonization between adjacent chains. WRPA6 with 12 parts of RP and 4 parts of ZB decomposes in a relatively narrow range at temperatures between 347 °C and 492.5 °C; And its decomposition curve is steeper than that of WRPA6, which indicates that the combustion time is shorter. It may be because more carbonaceous char is produced and this viewpoint can be verified by its highest char residue of 33.71 wt% among all samples.

Figure 7 TG curves of WRPA6 with and without flame retardant under nitrogen (heating rate 10 °C/min)
Table 2 Thermal analysis of WRPA6 with and without flame retardants
3.6 Flame retardant mechanism
To further understand the flame retardant mechanism, the SEM photos of residues are shown in Figure 8. The char residue surface of WRPA 6 (Figure 8(a)) is very smooth and covered with some inorganic particles as well as some glass fiber with little char burring on it, and the WRPA6 with 12 parts of RP shows a similar morphology (Figure 8(b)). Those incontinuous barriers can hardly endow the materials with high flame resistance, so in this case, the RP may work mainly in gas phase. On the contrast, WRPA6 with 12 parts of RP and 4 parts of ZB exhibits a thick and continuous glass like char residue (Figure 8(c)), and this is accordance with the TG test. In this time, the flame retardant mechanism maybe turn to gas phase mechanism along with condensed phase: part of RP translates into phosphorus compounds and then release into the gas phase, and the other part of RP interacts with ZB to form inorganic phosphoric salts in the condensed phase, so the synergistic effect of RP and ZB is gained.
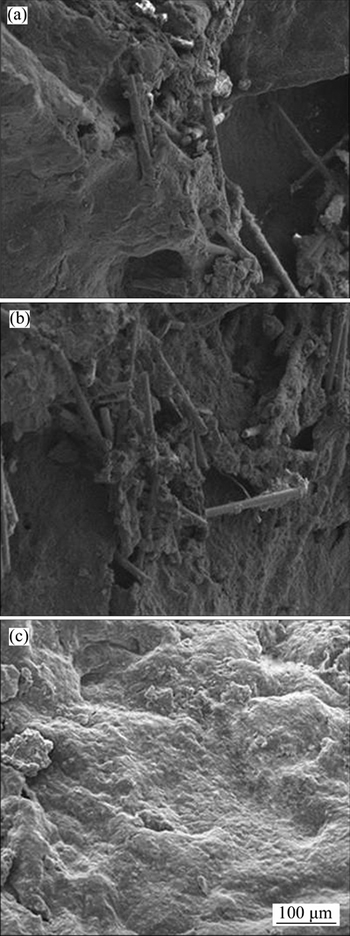
Figure 8 Photographs of residual char of WRPA6 (a), WRPA6 with 12 parts of RP (b) and WRPA6 with 12 parts of RP and 4 parts of ZB (c)
4 Conclusions
The wear and flame resistant PA6 composites were successfully prepared and the major conclusions can be drew as follows:
1) The optimum tribological property of PA6/GF/T/PTFE/Gr composite is obtained when the ratio of it is 100/15/5/10/5. The wear mass loss of this composite can not be detected, and the friction coefficient is only 0.1429, and its tensile strength is higher than that of PA6.
2) The optimum formula of flame retardant material is WRPA6 (PA6/GF/T/PTFE/Gr, 100/15/5/10/5 in weight) 100 parts, RP 12 parts and ZB 4 parts. In this case, the synergistic effect of RP and ZB on flame performance of WRPA6 is obtained by forming a compact and homogeneous char on the surface. Consequently, the highest LOI (30.2 vol%) and a UL94 V-0 rating are received. In addition, the flame retardant mechanism may be gas phase mechanism along with condensed phase mechanism. The friction coefficient and wear loss of this composite is 0.2387 and 3.5 mg respectively, which is lower than 0.34 and 3.8 mg of pure PA6.
References
[1] ALAJMI M, SHALWAN A. Correlation between mechanical properties with specific wear rate and the coefficient of friction of graphite/epoxy composites [J]. Materials, 2015, 8(7): 4162–4175.
[2] SHEN J T, TOP M, PEI Y T, JTMD Hosson. Wear and friction performance of PTFE filled epoxy composites with a high concentration of SiO2 particles [J]. Wear, 2014, 322–323: 171–180.
[3] YOU Yi-lan, LI Du-xin, SI Gao-jie, DENG Xin. Investigation of the influence of solid lubricants on the tribological properties of polyamide 6 nanocomposite [J]. Wear, 2014, 311(1, 2): 57–64.
[4] ZALAZNIK M, KALIN M, NOVAK S, JAKSA G. Effect of the type, size and concentration of solid lubricants on the tribological properties of the polymer PEEK [J]. Wear, 2016, 364–365: 31–39.
[5] YOU Yi-lan, LI Du-xin, DENG Xin, LI Wen-juan, XIE Ying. Effect of solid lubricants on tribological behavior of glass fiber reinforced polyamide 6 [J]. Polymer Composites, 2013, 34: 1783–1793.
[6] CAO W H, GONG J, YANG D Y, GAO Gui, WANG Hong-gang, REN Jun-fang, CHEN Sheng-sheng. Tribological behavior and energy dissipation characteristics of nano- Al2O3-reinforced PTFE-PPS composites in sliding system [J]. Journal of Central South University, 2017, 24(9): 2001–2009.
[7] SUN L H, YANG Z G, LI X H. Mechanical and tribological properties of polyoxymethylene modified with nanoparticles and solid lubricants [J]. Polymer Engineering Science, 2008, 48: 1824–1832.
[8] SCHROEDER R, TORRES F W, BINDE C, KLEIN A N, MELLO JDBD. Failure mode in sliding wear of PEEK based composites [J]. Wear, 2013, 301(1, 2): 717–726.
[9] HASHMI S A R, DWIVEDI U K, CHAND N. Friction and sliding wear of UHMWPE modified cotton fibre reinforced polyester composites [J]. Tribology Letters, 2006, 21: 79–87.
[10] ZHANG Z, BREIDT C, CHANG L, HAUPERT F, FRIEDRICH K. Enhancement of the wear resistance of epoxy: short carbon fibre, graphite, PTFE and nano-TiO2 [J]. Composites Part A Applied Science & Manufacturing, 2004, 35: 1385–1392.
[11] BIJWE J, SHARMA S, SHARMA M, PARIDA T, TRIVEDI P. Exploration of potential of solid lubricants and short fibers in Polyetherketone (PEK) composites [J]. Wear, 2013, 301: 810–819.
[12] PERRET B, PAWLOWSKI K H, SCHARTEL B. Fire retardancy mechanisms of arylphosphates in polycarbonate (PC) and PC/acrylonitrile-butadiene-styrene [J]. Journal of Thermal Analysis & Calorimetry, 2009, 97(3): 949–958.
[13] SHEN K K, KOCHESFAHANI S, JOUFFRET F. Zinc borates as multifunctional polymer additives [J]. Polymers for Advanced Technologies, 2010, 19(6): 469–474.
[14] TANG S, QIAN L J, QIU Y, SUN N. The effect of morphology on the flame-retardant behaviors of melamine cyanurate in PA6 composites [J]. Journal of Applied Polymer Science, 2014, 131(15): 338–347.
[15] CHOI Y S, CHOI S K, MOON S C, JO B W. Halogen-free flame retarding NBR/GTR foams [J]. Journal of Industrial & Engineering Chemistry, 2008, 14(3): 387–395.
[16] SAHYOUN J, BOUNOR-LEGARE V, FERRY L, SONNIER R, BONHOMME A. Influence of organophosphorous silica precursor on the thermal and fire behaviour of a PA66/PA6 copolymer [J]. Polymer Degradation & Stability, 2015, 115: 117–128.
[17] LI J, CHEN X, WANG Y, SHI Y, SHANG J. Burning and radiance properties of red phosphorus in magnesium/ PTFE/viton (MTV)-based compositions [J]. Infrared Physics & Technology, 2017, 85: 109–113.
[18] MOSTASHARI S M. The superiority of red phosphorus over polymetaphosphate as flame-retardants on cellulosic substrates [J]. Cellulose Chemistry & Technology, 2009, 43(4): 199–204.
[19] LI L, QIAN Y, JIAO C M. Influence of red phosphorus on the flame-retardant properties of ethylene vinyl acetate/ layered double hydroxides composites [J]. Advanced Materials Research, 2012, 21(9): 557–568.
[20] SCHARTEL B, KUNZE R, NEUBERT D. Red phosphorus- controlled decomposition for fire retardant PA 66 [J]. Journal of Applied Polymer Science, 2001, 83(10): 2060–2071.
[21] NING Y, GUO S. Flame-retardant and smoke-suppressant properties of zinc borate and aluminum trihydrate-filled rigid PVC [J]. Journal of Applied Polymer Science, 2015, 77(14): 3119–3127.
[22] FANG K Y, LI J, KE C H, ZHU Q L, ZHU J. Synergistic effect between a novel hyperbranched flame retardant and melamine pyrophosphate on the char forming of polyamide 6 [J]. Polymer-Plastics Technology and Engineering, 2010, 49(14): 1489–1497.
[23] SONG L, HU Y, LIN Z H, XUAN S Y, WANG S F. Preparation and properties of halogen-free flame-retarded polyamide 6/organoclay nanocomposite [J]. Polymer Degradation & Stability, 2004, 86(3): 535–540.
[24] HAO X Y, GAI G S, LIU J P, YANG Y F, ZHAGN Y H, NAN C W. Flame retardancy and antidripping effect of OMT/PA nanocomposites [J]. Materials Chemistry & Physics, 2006, 96(1): 34–41.
[25] FEI G, LIU Y, WANG Q. Synergistic effects of novolac- based char former with magnesium hydroxide in flame retardant polyamide-6 [J]. Polymer Degradation & Stability, 2008, 93(7): 1351–1356.
[26] LU C, WANG J, CHEN L, FU Q, CAI X. The effect of adjuvant on the halogen-free intumescent flame retardant ABS/PA6/SMA/APP blend [J]. Journal of Applied Polymer Science, 2010, 118(3): 1552–1560.
[27] SI Gao-Jie, LI Du-xin, YOU Yi-lan, HU Xi. Investigation of the influence of red phosphorus, expansible graphite and zinc borate on flame retardancy and wear performance of glass fiber reinforced PA6 composites [J]. Polymer Composites, 2017, 38(10): 2090–2097.
[28] BERNHARD S. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development [J]. Materials, 2010, 3: 4710–4745.
[29] SUT A, GREISER S, JAGER C, BCHARTEL B. Interactions in multicomponent flame-retardant polymers: Solid-state NMR identifying the chemistry behind it [J]. Polymer Degradation & Stability, 2015, 121: 116–125.
[30] FONTAINE G, BOURBIGOT S, DUQUESNE S. Neutralized flame retardant phosphorus agent: Facile synthesis, reaction to fire in PP and synergy with zinc borate [J]. Polymer Degradation & Stability, 2008, 93(1): 68–76.
[31] GOLBAKHSHI H, NAMJOO M. Thermo-structural analysis on evaluating effects of friction and transient heat transfer on performance of gears in high-precision assemblies [J]. Journal of Central South University, 2017, 24(1): 71–80.
(Edited by HE Yun-bin)
中文导读
酰胺6的摩擦与阻燃性能改性研究
摘要:采用玻璃纤维(GF)、滑石粉(T)为增强体,聚四氟乙烯(PTFE)和石墨(Gr)为固体润滑剂,红磷(RP)和硼酸锌(ZB)为阻燃剂制备了一系列耐磨阻燃的聚酰胺6(PA6)复合材料。研究了复合材料的摩擦磨损性能、力学性能、阻燃性能及阻燃机理。结果表明,耐磨材料(WRPA6)配方是质量比为100/15/10/5的PA6/GF/T/PTFE/Gr复合材料,该材料的摩擦因数为0.1429,且没有检测到质量损失。RP和ZB可以提高WRPA6的阻燃性能,RP和ZB有协同作用。当WRPA6含有4份ZB和12份RP时达到最大极限氧指数30.2 vol%和UL94 V-0级,阻燃机理为气相阻燃和凝聚相阻燃。
关键词:耐磨性;阻燃;PA6复合材料
Foundation item: Project(149929) supported by the Postdoctoral Fund of Central South University, China; Project(16C0292) supported by the Hunan Education Department, China; Project(2016TP1022) supported by the Hunan Provincial Key Lab of Dark Tea and Jin-hua, China
Received date: 2017-11-22; Accepted date: 2018-05-21
Corresponding author: LI Du-xin, PhD, Professor; Tel: +86-13874882497; E-mail: liduxin@csu.edu.cn; ORCID: 0000-0003- 1075-8096
Abstract: A series of wear and flame resistant polyamide 6 (PA6) composites were prepared using glass fiber (GF) and talc (T) as reinforcer, polytetrafluoroethylene (PTFE) and graphite (Gr) as solid lubricants, red phosphorus (RP) and zinc borate (ZB) as flame retardant. The tribological property, mechanical property, flame retardant property and the flame retardant mechanism were investigated. The tests show that the formula of the wear resistant PA6 composite (WRPA 6) is PA6/GF/T/PTFE/Gr in the ratio of 100/15/5/10/5 by mass. Because this composite exhibits the lowest friction coefficient (0.1429) and no wear mass loss, the introduction of RP and ZB can increase the flame resistance of WRPA6, and the synergistic effect of RP and ZB is obtained. Detailedly, the composite with 4 parts of ZB and 12 parts of RP shows the best flame retardant property, achieving the highest limiting oxygen index (LOI) (30.2 vol%) and a UL94 V-0 rating, and the flame retardant mechanisms may be gas phase along with condense phase mechanism.


