
Structures and properties of La-Mg-Ni system hydrogen storage alloys
XIAO Fang-ming(肖方明), WANG Ying(王 英), TANG Ren-heng(唐仁衡),
LU Qi-yun(卢其云), PENG Neng(彭 能)
Research Institute of Rare Metal, Guangzhou Research Institute of Non-ferrous Metals, Guangzhou 510651, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The double-roller rapid quenching technology was successfully used to prepare La-Mg-Ni system hydrogen storage alloys. The effects of magnesium content and heat-treatment process on the alloys properties were studied. When the alloy with 1.09%(mass fraction) Mg is heat treated at 900 ℃ for 4 h , its discharge capacity is more than 380 mA?h/g at 0.2C, and the cyclic life is beyond 500 counts at 2C. By XRD and PCI analyzing, the results show that the alloys are composed of LaNi5 and LaNi3 phase. The hydrogen absorption/desorption pressure of the alloy increases, so does the slope of plateau, and the plateau becomes broad first and narrow again as Mg content increases. This method is simple to be suitable for production on a large scale.
Key words:
La-Mg-Ni; hydrogen storage alloy; structures; properties; double-roller rapid quenching;
1 Introduction
As crucial important negative material of Ni/MH battery, well or poor properties of rare earth hydrogen storage alloy directly have influence on its quality. Because it seems to be difficult to further improve discharge capacity of merchandized AB5 -type alloy, the demands of high capacity and high power batteries cannot be satisfied. Meanwhile, the lower hydrogen storage capacity and high cost also limit its application in fuel battery. But La-Mg-Ni system with AB3-type alloy becomes major research focus[1-5] among some universities, institutes and enterprises since it has superiority in large hydrogen storage capacity, high discharge capacity and quicker activation property, but its poor cyclic life needs to be enhanced further. In the past few years, we obtained a nanocrystalline alloy with well-distributed composition in conventional rapid quenching furnace under a sub-pressure, by adding cover reagent and selecting right smelt process, and the problem that the compositions of alloys are instable because of amount of magnesium volatilizing during smelting process was resolved, and the cyclic stability of the alloy was improved. Based on our earlier research work[6-7], the effects of content of magnesium and heat-treatment process on alloy properties are studied to improve the electrochemical properties of the alloys further in the present work.
2 Experimental2.1 Preparation of alloys
A series of La-Mg-Ni hydrogen storage alloys with different magnesium contents (mass fraction, %) of 0.35, 1.09 and 3.85, respectively, were prepared by rapid quenching furnace in Ar atmosphere, and the cover reagent was added up. When molten alloy liquid flowed across the cobalt roller to be quenched, the alloy plates with thickness from 0.05 mm to 1.5 mm were obtained, then alloy powders were prepared by mechanically ball milling after heat-treatment. The purity of experimental raw materials is more than 99.9%.
2.2 Measurement of structures and properties
The phase structures were measured on RINT-1100 type X-ray diffraction analyzer of Japan with Cu radiation. AMC GAS REACTION CONTROLLER of America was used to test PCI curves for reflecting the hydrogen absorption and desorption properties. The discharge capacity and the cyclic stability of the alloys were determined by the galvanostatic method. The maximum discharge capacities were measured of which the alloy electrodes were charged at 0.2C for 360 min followed by a 5-min break, and then discharged at 0.2C to the cut-off potential of 1.0 V. In order to test the cyclic life of alloy electrodes, it would be activated at 0.7C with 8 cycles, charged at 2C for 36 min, followed by a 5-min break, and then discharged at the same current density to the cut-off potential of 1.0 V.
3 Results and discussion3.1 Phase structure
Fig.1 presents the X-ray diffraction patterns of the alloys with different Mg contents. It is found that these
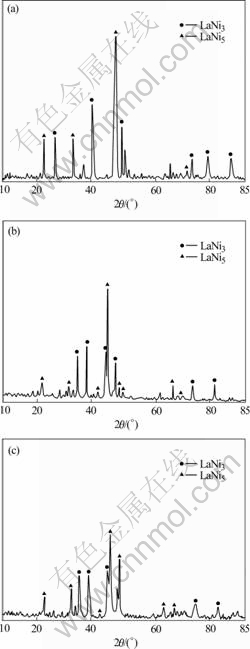
Fig.1 XRD patterns of alloys: (a) 0.35% Mg; (b) 1.09% Mg; (c) 3.85% Mg
alloys are composed of LaNi5 and LaNi3 phase, and the LaNi3 phase increases but LaNi5 phase decreases as the content of Mg increases, which indicates that Mg may facilitate the formation of LaNi3 phase[8].Moreover, while La is replaced by Mg, and the crystal axle be shrunk, the cell volume reduces because the atomic radius of Mg (0.172 nm) is smaller than that of La (0.274 nm), and it can be proved that the diffraction peaks shift towards the larger angle with Mg content increasing.
3.2 Properties of hydrogen absorbing/desorbing
Fig.2 shows that the effects of Mg content on hydrogen absorption/desorption properties at 343 K. It can be seen that not only the plateau pressure of hydrogen absorption/desorption but also the slope of plateau continuously increases when the content of Mg increases from 0.35% to 3.85%, and the regions of plateau become broad first and narrow again. When the pressure of plateau is lower, the region grows narrow since the hydrides formed are too stable to release part of hydrogen, and it causes hydrogen capacity reduces. The more Mg content, the smaller the cell volume and the place which storages hydrogen atom, and the narrower the plateau regions[9]. The hydrogen absorption/ desorption plateau region of the alloy with Mg content of 1.09% is expanded and reversibility is better. By calculating, the equilibrium hydrogen pressure is 8 659 Pa, the hysteresis (lg(Pa/Pd), where Pa and Pd are absorption and desorption equilibrium pressure, respectively) is 0.352 7 (at H/M=2.4 dehydrogenation), and the slope of the plateau (lg(P3/P1.5)/[(H/M)3- (H/M)1.5]) is 0.118 3.
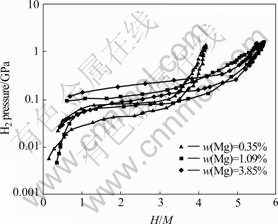
Fig.2 PCI curves of alloys with different magnesium contents at 343 K
3.3 Effects of magnesium content on electrochemical properties
Table 1 lists the results of electrochemical pro- perties of the alloys prepared with different Mg contents.
Table 1 Effects of Mg content on alloy properties
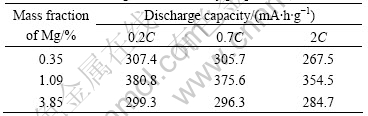
Whether high or low content of Mg, the discharge capacity and cyclic life are poor. These alloys are all complexes measured by phase structure analysis, in which LaNi3 phase is absorbing hydrogen phase, LaNi5 phase is catalytic phase, and the discharge capacity directly depends on the abundance of these two phases. Because there is few LaNi3 phase but many LaNi5 phases in the alloy with 0.35% Mg, its small hydrogen storage capacity leads to low discharge capacity. As the content of Mg is larger, the abundance of LaNi3 phase enhances, at the same time, the alloy has not enough LaNi5 phase to catalyze LaNi5 phase. As a result, the process of hydrogen absorption/ desorption is carried on slowly to make discharge capacity decrease. The discharge capacity of the alloy with 1.09% Mg can reach 380.8 mA?h/g at 0.2C as the abundance of LaNi5 phase is a match for that of LaNi3 phase.
3.4 Effects of heat-treatment process on electro- chemical properties
The effects of heat-treatment process on alloy properties with 1.09% Mg was investigated, the results are listed in Table 2.
It can be seen that the discharge capacity of the alloy without suffering from heat-treatment is lower and it is no more than 331.5 mA?h/g. When the temperature is beyond 900 ℃, the discharge capacity of the alloy decreases. It produces amount of lattice defects in the alloy that lead to poor arrangement and make inner stress increase, while the rapid quenching technology greatly
decreases grain size. With the temperature increasing, the electrochemical property of the alloy is improved by means of releasing stress, poor arrangement as well as lattice defects. But excessively high temperature causes the second phase to be easily formed when dropping in temperature, so that the electrochemical property of the alloy decreases. In addition, long time also makes the discharge capacity reduce because of Mg volatilizing during heat-treatment process[10], so the experimental results indicate that the better heat-treatment technology is at 900 ℃ for 4 h.
3.5 Electrochemical characteristics
The discharge curve of alloy at 0.2C and cyclic curve at 2C are shown in Figs.3 and 4.The mid-point voltage is 1.298 V. After 556 cycles, the capacity loss rate of the alloy is only 19.7%. The important reasons of improving cyclic property of the alloy is that rapid quenching technology not only enhances the anti- pulverizibility, but also strengthens its toughness and strength.
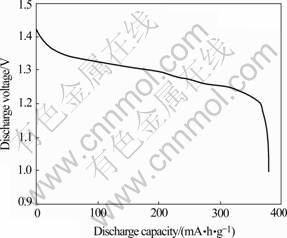
Fig.3 Discharge curve of alloy at 0.2C
Table 2 Effects of heat-treatment on alloy properties
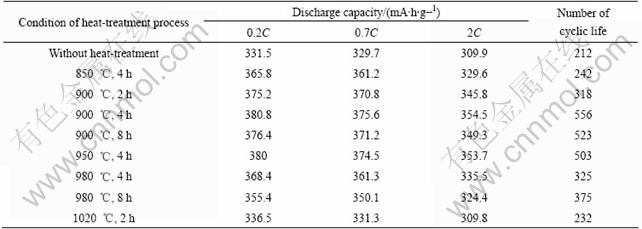
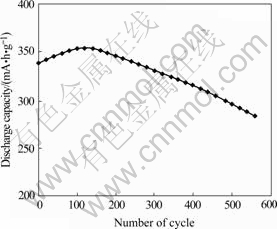
Fig.4 Cyclic curve of alloy at 2C
4 Conclusions1) When the alloy with 1.09%(mass fraction) Mg is heat treated at 900 ℃ for 4 h, the discharge capacity of the alloy is more than 380 mA?h/g at 0.2C, and it has better cyclic stability.
2) The hydrogen absorbing/desorbing pressure of the alloys increases, so does the slope of plateau, and the plateau becomes broad first and narrow again as Mg content increases from 0.35% to 3.85%.
References[1] ZHANG Yang-huan, DONG Xiao-ping, WANG Guo-qing, GUO Shi-hai, REN Jiang-yuan, WANG Xin-lin. Effects of rapid quenching on microstructures and electrochemical properties of La0.7Mg0.3Ni2.55Co0.45Bx (x=0-0.2) hydrogen storage alloy [J]. Trans Nonferrous Met Soc China, 2006, 16(4): 800-807.
[2] LIU Yong-feng, PAN Hong-ge, YUE Yuan-jian, WU Xue-feng, CHEN Ni, LEI Yong-quan. Cycling durability and degradation behavior of La-Mg-Ni-Co-type metal hydride electrodes [J]. Journal of Alloys and Compounds, 2005, 395: 291-299.
[3] PAN Hong-ge, CHEN Ni, GAO Ming-xia, LI Rui, LEI Yong-quan, WANG Qi-dong. Effects of annealing temperature on structure and the electrochemical properties of La0.7Mg0.3Ni2.45Co0.75Mn0.1Al0.2 hydrogen storage alloy [J]. Journal of Alloys and Compounds, 2005, 397: 306-312.
[4] LIU Yong-feng, PAN Hong-ge, GAO Ming-xia, LI Rui, SUN Xian-dong, LEI Yong-quan. Investigation on characteristics of La0.7Mg0.3Ni2.65 Mn0.1Co0.75+x (x=0.00-0.85) metal hydride electrode alloys for Ni/MH batteries (Ⅱ): Electrochemical performances [J]. Journal of Alloys and Compounds, 2005, 388: 109-117.
[5] PAN Hong-ge, LIU Yong-feng, GAO Ming-xia, ZHU Yun-feng, LEI Yong-quan. The structure and electrochemical properties of La0.7Mg0.3(Ni0.85Co0.15)x (x=3.0-3.5) hydrogen storage alloys [J]. International Journal of Hydrogen Energy, 2003, 28: 1219-1228.
[6] WANG Ying, LU Qi-yun, PENG Neng, XIAO Fang-ming, TANG Ren-heng. Effect of heat-treatment process on properties of rare earth Mg-based system hydrogen storage alloys with AB3-type [J]. Journal of Rare Earths, 2006, 24(s): 340-342.
[7] TANG Ren-heng, LU Qi-yun, XIAO Fang-ming, PENG Neng, WANG Ying. Study on nanocrystalline of rare earth Mg-based system hydrogen storage alloys with AB3-type [J]. Journal of Rare Earths, 2006, 24(s): 343-346.
[8] ZHANG Zhong, HAN Shu-min, LI Yuan, JING Tian-fu. Electrochemical properties of Ml1-xMgxNi3.0Mn0.10Co0.55Al0.10 (x=0.05-0.30) hydrogen storage alloys [J]. Journal of Alloys and Compounds, 2007, 431: 208-211.
[9] ZHOU Zeng-lin, SONG Yue-qing, CUI Shun. Development of rare-earth Mg-based AB3-type hydrogen-storage alloys [J]. Materials Review, 2005, 19(16): 107-112.
[10] ZHANG Fa-liang, LUO Yong-chun, YAN Ru-xu,KANG Long, CHEN Jian-hong. Study of the structure and electrochemical properties of La2-xMgxNi7.0 (x= 0.4, 0.5, 0.6, 0.7, 0.8) alloy [J]. Journal of Functional Materials, 2004, 35(s): 1900-1904.
Foundation item: Project(06026152) supported by the Natural Science Foundation of Guangdong Province, China
Corresponding author: XIAO Fang-ming; Tel: +86-20-37238406(O); Fax: +86-20-37238523; E-mail: xfmworld@163.com


