
Effect of cold rolling on glass transition of Zr55Al10Ni5Cu30 bulk metallic glass
HU Yong(胡 勇)1, 2, LI Jin-fu(李金富)1, ZHANG Peng-na(张朋娜)1, ZHOU Yao-he(周尧和)1
1. State Key Laboratory of Metal Matrix Composites, School of Materials Science and Engineering,
Shanghai Jiao Tong University, Shanghai 200240, China;
2. School of Materials Science and Engineering, Taiyuan University of Science and Technology,Taiyuan 030024, China
Received 17 November 2008; accepted 6 March 2009
Abstract:
Zr55Al10Ni5Cu30 bulk metallic glass was prepared through water-cooled copper mold suction casting, and was rolled up to 95% in thickness reduction. The structures and thermal stabilities of the as-cast and as-rolled specimens were examined by X-ray diffractometer and differential scanning calorimeter. As the thickness reduction increases, the crystallization onset temperature, peak temperature and the apparent activation energy of crystallization almost keep constant, while the glass transition temperature decreases from 681 to 671 K and the apparent activation energy of glass transition increases from (404±26) to (471±29) kJ/mol. The glass transition process is markedly affected by the rolling induced changes of microstructure and structural relaxation.
Key words:
bulk metallic glass; cold rolling; glass transition;
1 Introduction
In many theoretical approaches, when a liquid is supercooled to a critical temperature where entropies of the liquid and the crystalline solid are identical, a phase transition will take place from the supercooled liquid to a glassy phase if crystallization is suppressed, namely a glass transition[1]. In terms of the free volume model, glass transition is regarded as a kinetic phenomenon depending on competition between the creation and annihilation of the free volume in glasses[2]. Glass transition, one of the important aspects to be considered in applications of amorphous alloys, is affected not only by composition[3-4], mixture enthalpy[5], heat treatment[6-9], and heating rate[10-12], but also by mechanical treatments. An instance is the Zr46.75Ti8.25Cu7.5Ni10Be27.5 bulk metallic glass(BMG). Its glass transition temperature Tg slightly increases by 5.6 K/GPa under hydrostatic pressure[13], but drastically decreases by 0.08 K/MPa induced by shear stress during the compression[14]. These phenomena were attributed to the different activation volume of relaxation. As both experiments were performed near Tg, it is difficult to eliminate the effect of phase transformation. In addition, when some BMGs were rolled in the supercooled liquid region[15-16] or at room temperature[17], Tg kept constant or shifted to lower temperature with the thickness reduction increasing, although nanocrystals were observed in all the specimens after deformation. So, the relation between deformation and glass transition is still a matter of debate up to now.
Zr55Al10Ni5Cu30 BMG possesses good stability in structure. Our previous investigation showed that no phase transformations such as crystallization and phase separation occur while it was rolled at the strain rate of 3.0×10-1 s-1 up to a deformation degree of 95%[18]. Therefore, in order to eliminate the effect of phase transformation on the results, Zr55Al10Ni5Cu30 BMG is chosen to investigate the effect of cold rolling on glass transition.
2 Experimental
The master alloy ingot of Zr55Al10Ni5Cu30 (molar fraction, %) was prepared by arc melting a mixture of pure Zr(99.9%), Al(99.99%), Ni(99.98%) and Cu(99.98%) in a water-cooled copper crucible in a Ti- gettered argon atmosphere. The alloy ingot was remelted several times to ensure compositional homogeneity.
Samples were produced by suction casting in a water- cooled copper mold, and had a plate-like shape with a cross-section of 1 mm×10 mm and a length of 60 mm. The plates were cut into several segments of 1 mm× 3 mm×10 mm for rolling. The rolling apparatus consists of two rollers of 100 mm in diameter. Covered by two steel plates of 1 mm in original thickness, the specimen was repeatedly rolled in one direction until the desired deformation was obtained. The degree of deformation was denoted by the reduction in thickness, e=(h0-h)/h0, where h0 and h represent the specimen thicknesses before and after rolling, respectively. Many small deformation passes were used with a progressively narrowing gap between the two rollers. The decrease of the gap during deformation was carefully controlled so that the strain rate was about 3.0×10-1 s-1.
The structural natures of the as-cast and as-rolled specimens were examined using a Thermo ARL X-ray diffractometer(XRD) with Cu Ka radiation. The thermal analyses were performed using a Perkin-Elmer Pyris Diamond differential scanning calorimeter(DSC) in a heating rate range from 10 to 80 K/min under a flow of high-purity Ar atmosphere.
3 Results and discussion
Fig.1 shows the continuous DSC traces of the as-cast Zr55Al10Ni5Cu30 specimens and those rolled up to different strains at a heating rate of 20 K/min. Each DSC trace exhibits the endothermic characteristic of glass transition followed by a supercooled liquid region and an exothermic reaction due to crystallization at higher temperature. The values of parameters, such as the glass transition temperature Tg, crystallization onset temperature Tx, crystallization peak temperature Tp and supercooled liquid region DTx, are listed in Table 1. The Tg, Tx, Tp and DTx for the as-cast specimen are 681, 764, 767.5 and 83 K, respectively. After cold rolling, Tg obviously decreases, while Tx and Tp remain almost unchanged.
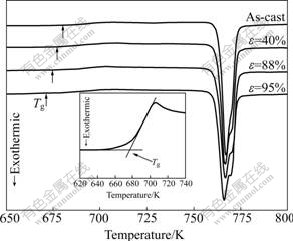
Fig.1 DSC curves of as-cast Zr55Al10Ni5Cu30 specimens and those rolled up to different strains at heating rate of 20 K/min (Inset is definition of glass transition temperature)
Table 1 Thermodynamic and kinetic parameters of as-cast Zr55Al10Ni5Cu30 specimens and those rolled up to different strains (Tg, Tx, Tgp and Tp are measured at heating rate of 20 K/min)
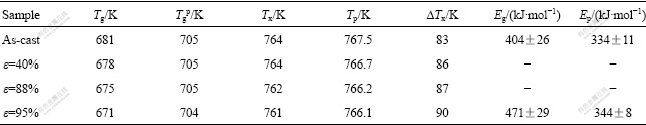
Fig.2 displays the XRD patterns of the as-cast specimen and that rolled up to e=95%. Typical peaks of amorphous phase are observed; meanwhile, no clear difference can be identified between the two specimens. The HRTEM observations also confirm that the two specimens are completely amorphous (the result is not shown here).
Fig.2 XRD patterns of as-cast Zr55Al10Ni5Cu30 specimen and that rolled up to e=95%
Fig.3 shows the continuous DSC curves of the as-cast Zr55Al10Ni5Cu30 BMG at a heating rate of 10, 20, 40 and 80 K/min, respectively. Tg, Tx and Tp increase with increasing the heating rate. Similar trends exist on the DSC curves of the specimen rolled up to e=95%. The apparent activation energy Ei for a reaction can be calculated by Kissinger’s equation[19]:
![]() (1)
(1)
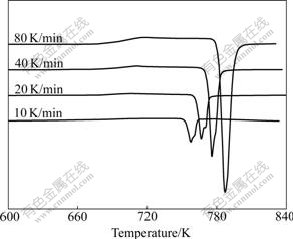
Fig.3 DSC traces for as-cast Zr55Al10Ni5Cu30 specimen at different heating rates
where b is the heating rate; Ti is the characteristic temperature of the reaction; R is the gas constant; and Ci is a constant for the reaction. The Kissinger plots of the glass transition and crystallization for the as-cast specimen and the rolled specimen with e=95% are shown in Fig.4. The apparent activation energies of the glass transition Eg and the crystallization Ep derived from the slope of the Kissinger plots are listed in Table 1. Eg and Ep of the as-cast BMG are (404±26) and (334±11) kJ/mol, respectively. After the specimen is rolled up to 95%, Ep remains almost unchanged considering the experimental error, while Eg increases to (471±29) kJ/mol.
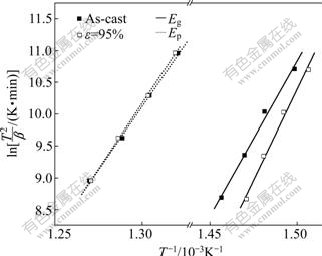
Fig.4 Kissinger plots of glass transition and crystallization for as-cast Zr55Al10Ni5Cu30 specimen and that rolled up to e=95%
From Table 1, it is known that Tx, Tp and Ep are almost invariable even when the thickness reduction is up to 95%, indicating that cold rolling has a little effect on the crystallization of the BMG. However, cold rolling has a considerable influence on the glass transition.
According to the model proposed by COHEN and GREST[20] and WANG et al[21], a metallic glass is heterogeneous in structure, consisting of liquid-like regions with large free volume or high local free energy, and solid-like regions with small free volume or low free energy. In contrast to solid-like regions, the transition from liquid-like regions to supercooled liquid state would occur earlier, and needs less energy. That is to say, the glass transition of liquid-like regions can take place at lower temperature than solid-like regions. The deformation mode of metallic glasses comprises homogeneous deformation that occurs at low strain rate and high temperature, and inhomogeneous deformation that occurs at high strain rate and low temperature. During inhomogeneous deformation, the strain is highly localized in narrow deformation regions, i.e. shear bands [22]. These bands contain more free volume than the matrix[23], which means that shear bands contain more liquid-like region than matrix. The deformed metallic glass, without phase transition, can be treated as a composite of hard undeformed amorphous grains surrounded by soft shear band boundaries. The increase of shear-band density would improve the liquid-like region content of the “composite”. There is a linear relation between the shear-band density and the plastic strain[23], i.e., the shear-band density increases with the increasing thickness reduction during the cold rolling process, which means that the fraction of liquid-like region increases in the BMG. As a result, Tg shifts to a lower temperature for the as-rolled specimens.
Metallic glasses undergo phase separation, structural relaxation, glass transition, nucleation and growth of nuclei during a continuous heating process. As done in other work[11], Tg, in the present paper, is defined on the DSC curves as the point of intersection between the linearly extrapolation curve below the glass transition and the steepest tangent of the rise in the heat flow signal (see inset in Fig.1). Thus, the apparent glass transition activation energy, Eg, should include the activation energies of phase separation, structural relaxation and “real” glass transition. In the isothermal annealing of the Zr41Ti14Cu12.5Ni10Be22.5 BMG in the glass transition region, phase separation and structural relaxation occur prior to glass transition, so Eg decreases[8]. In contrary to the isothermal annealing, the cold rolling is helpful to enhancing the structural relaxation enthalpy, as found in the rolled Cu60Zr20Ti20 BMG[24]. In the present work, no phase transformation is detected in the rolled Zr55Al10Ni5Cu30 BMG, but the structural relaxation enthalpy becomes more obvious (Fig.5). From the enthalpy recovery method(ERM)[14], the structural relaxation enthalpy is strongly dependent on the initial state of the glassy sample before the DSC measurement, while the “real” glass transition is not sensitive to the initial glassy state. Table 1 shows that the overshoot peak temperature, Tgp, during glass transition, which could be used to characterize approximately the “real” glass transition process, keeps almost invariable with increasing the thickness reduction. So, it is speculated that the cold rolling has no effect on the “real” glass transition. The rolling induced increase of Eg might come from the enlarged structural relaxation enthalpy.
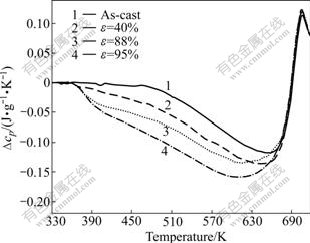
Fig.5 Specific heat capacity of as-cast Zr55Al10Ni5Cu30 specimens and those rolled up to different strains at heating rate of 20 K/min
4 Conclusions
1) Zr55Al10Ni5Cu30 BMG was rolled at a strain rate of 3.0×10-1 s-1 up to a deformation degree of 95% at room temperature. With increasing the thickness reduction, Tg decreases from 681 to 671 K, and Eg increases from (404±26) to (471±29) kJ/mol.
2) The decrease of Tg is due to the presence of many shear bands. The rise of Eg can be ascribed to the enlarged structural relaxation enthalpy.
References
[1] ANGELL C A. Formation of glasses from liquids and biopolymers [J]. Science, 1995, 267: 1924-1935.
[2] van den BEUKEL A, SIETSMA J. The glass transition as a free volume related kinetic phenomenon [J]. Acta Metallurgica et Materialia, 1990, 38: 383-389.
[3] CHEN H S. The glass transition temperature in glassy alloys: Effects of atomic sizes and the heats of mixing [J]. Acta Metallurgica, 1974, 22: 897-900.
[4] INOUE A, SHIBATA T, ZHANG T. Effect of additional elements on glass transition behavior and glass formation tendency of Zr-Al-Cu-Ni alloys [J]. Materials Transactions JIM, 1995, 36: 1420-1426.
[5] LI Xue-lian, BIAN Xiu-fang, HU Li-na, WU Yu-qin, GUO Jing, ZHANG Jun-yan. Glass transition temperature of bulk metallic glasses: A linear connection with the mixing enthalpy [J]. Journal of Applied Physics, 2007, 101: 103540.
[6] VENKATARAMAN S, ECKERT J, SCHULTZ L, SORDELET D J. Effect of preannealing on glass transition and crystallization of gas atomized Cu47Ti33Zr11Ni8Si1 metallic glass powders [J]. Intermetallics, 2006, 14: 1085-1090.
[7] SLIPENYUK A, ECKERT J. Correlation between enthalpy change and free volume reduction during structural relaxation of Zr55Cu30Al10Ni5 metallic glass [J]. Scripta Materialia, 2004, 50: 39-44.
[8] ZHUANG Yan-xin, WANG Wei-hua. Effects of relaxation on glass transition and crystallization of ZrTiCuNiBe bulk metallic glass [J]. Journal of Applied Physics, 2000, 87: 8209-8211.
[9] ZOU Hui, WANG Jing-feng, LIU Lin. Effect of pre-annealing time on glass transition and crystallization of Pd40Cu30Ni10P20 [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 996-1001. (in Chinese)
[10] GREST G S, COHEN M H. Liquid-glass transition: Dependence of the glass transition on heating and cooling rates [J]. Physical Review B, 1980, 21: 4113-4117.
[11] HIKI Y, TAKAHASHI H. Calorimetric study of kinetic glass transition in metallic glasses [C]//TOKUYAMA M, OPPENHEIM I, NISHIYAMA H. AIP Conference Proceedings of 5th International Workshop on Complex Systems. NY: American Institute of Physics, 2008, 982: 177-179.
[12] WANG Wei-hua, ZHUANG Yan-xin, PAN Ming-xiang, YAO Yu-su. Glass transition behavior, crystallization kinetics, and microstructure change of Zr41Ti14Cu12.5Ni10Be22.5 bulk metallic glass under high pressure [J]. Journal of Applied Physics, 2000, 88: 3914-3918.
[13] WEN Ping, WANG Wei-hua, ZHAO Yan-hui, ZHAO De-qian, PAN Ming-xiang, LI Feng-ying, JIN Chang-qing. Glass transition in Zr46.75Ti8.25Cu7.5Ni10Be27.5 metallic glass under high pressure [J]. Physical Review B—Condensed Matter and Materials Physics, 2004, 69: 092201.
[14] JIN H J, WEN J, LU K. Shear stress induced reduction of glass transition temperature in a bulk metallic glass [J]. Acta Materialia, 2005, 53: 3013-3020.
[15] XIAO Xue-shan, FANG Shou-shi, WANG Qing, WANG Guo-ming, HUA Qin, DONG Yuan-da. Effect of hot rolling on thermal stability and microstructure of Zr52.5Al10Ni10Cu15Be12.5 bulk metallic glass [J]. Materials Letters, 2004, 58: 2357-2360.
[16] WANG Guo-ming, FANG Shou-shi, XIAO Xue-shan, HUA Qin, GU Jian-zhong, DONG Yuan-da. Microstructure and properties of Zr65Al10Ni10Cu15 amorphous plates rolled in the supercooled liquid region [J]. Materials Science and Engineering A, 2004, 373: 217-220.
[17] PARK J S, LIM H K, KIM J H, CHANG H J, KIM W T, KIMD H, FLEURY E. In situ crystallization and enhanced mechanical properties of the Zr41.2Ti13.8Cu12.5Ni10Be22.5 alloy by cold rolling [J]. Journal of Non-Crystalline Solids, 2005, 351: 2142-2146.
[18] HU Yong, LI Jin-fu, ZHANG Peng-na, ZHOU Yao-he. Crystallization behavior of Zr55Al10Cu30Ni5 bulk metallic glass rolled at room temperature [J]. Journal of Materials Science and Technology, in press.
[19] KISSINGER H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29: 1702-1706.
[20] COHEN M H, GREST G S. Liquid-glass transition, a free-volume approach [J]. Physical Review B, 1979, 20: 1077-1098.
[21] WANG J F, LIU L, XIAO J Z, ZHANG T, WANG B Y, ZHOU C L, LONG W. Ageing behaviour of Pd40Cu30Ni10P20 bulk metallic glass during long-time isothermal annealing [J]. Journal of Physics D: Applied Physics, 2005, 38: 946-949.
[22] SPAEPEN F. A microscopic mechanism for steady state inhomogeneous flow in metallic glasses [J]. Acta Metallurgica, 1977, 25: 407-415.
[23] BEI H, XIE S, GEORGE E P. Softening caused by profuse shear banding in a bulk metallic glass [J]. Physical Review Letters, 2006, 96: 105503.
[24] CAO Qing-ping, LI Jin-fu, ZHOU Yao-he, HORSEWELL A, JIANG Jian-zhong. Free-volume evolution and its temperature dependence during rolling of Cu60Zr20Ti20 bulk metallic glass [J]. Applied Physics Letters, 2005, 87: 101901.
Foundation item: Project(50671066) supported by the National Natural Science Foundation of China
Corresponding author: LI Jin-fu; Tel: +86-21-54748530; Fax: +86-21-54748530; E-mail: jfli@sjtu.edu.cn
DOI: 10.1016/S1003-6326(09)60100-X


