Article ID: 1003-6326(2005)05-0957-08
Coating of calcium phosphate on biometallic materials by electrophoretic deposition
ZHANG Er-lin(张二林), YANG Ke(杨 柯)
(Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China)
Abstract:
Although biometallic materials have been used as bone implant materials for a long time, they are still detected as foreign bodies by human immune system. Calcium phosphate coating, especially hydroxyapatite(HA) coating attracts special attention due to its good biocompatibility. Being one of the effective methods used to deposit HA coating onto the metallic implant, the electrophoretic deposition(EPD) was reviewed in detail, including the process of EPD, the advantages and disadvantages, the important processing factors and the microstructure and mechanical properties of the coating. Research results on the processing and the coating show potential application of EPD process to the biomedical materials surface modification. In addition, the nanoparticulate HA coating as a new trend in HA coating was also introduced.
Key words:
hydroxyapatite coating; biomedical application; electrophoretic deposition CLC number: TB39;
Document code: A
1 INTRODUCTION
For a long time the repair of wear, tear and disease on the human bone has involved the use of materials that were not originally designed for such application, e.g. stainless 316 steel, Co-alloy and Ti-6Al-4V alloy. These materials are often detected as foreign bodies by the patients immune system and sometimes interact with the body in an undesirable manner. In the recent years, there is huge development in biomaterials that are specially designed to repair and reconstruct damaged or diseased parts of the human bone[1-3]. On the other hand, calcium phosphate, especially hydroxyapatite, Ca10(PO4)6(OH)2, or HA, has demonstrated high compressive strength, good biocompatibility and osteoconductivity and excellent wear properties. However, a component made of solely HA was found to lack of toughness and tensile strength, and can fail catastrophically. As a result, HA coated titanium components, which combine the advantages of the mechanical strength of titanium metal and the bioactivity of HA, are developed and reckoned to be one of the most promising groups of implant materials in orthopedic and dental fields.
Many processes have been developed to coat HA onto the titanium substrate, such as plasma spray[4, 5], ion beam sputtering[6], sol-gel[7], ion beam dynamic mixing[8], dipping[9], electro-phoretic deposition[10-13], biomimetic[14] and electrochemical deposition[15]. Fig.1 shows the typical thickness of HA coating produced by different processes. The most widely used coating technique for applying HAp on metal substrates is plasma spraying, which has been used as a commercial processing technique. However, the plasma-spraying technique presents several drawbacks[10, 11]. Many researchers are focusing on developing a low cost, high efficiency and low temperature process. As one of the effective methods to deposit HA coating, the electrophoretic deposition has shown more advantages over plasma spray and more potential in bone implant application. Here the electrophoretic deposition, focusing on the application to the HA coating was reviewed.
2 CALCIUM PHOSPHATE COATING
2.1 Calcium phosphate
The most common apatite calcium phosphate ceramic used in medicine is hydroxyapatite(HA), a material with a hexagonal crystal lattice, an ideal chemical formula Ca10(PO4)6(OH)2, ideal mass fraction of 39.9%Ca, 18.5%P and 3.38%OH and ideal calcium/phosphate ratio of 1.67[17]. Most synthetic hydroxyapatites actually contain substitutions for the phosphate and /or hydroxyl groups and vary from the ideal stoichiometry and calcium/phosphorus ratios.
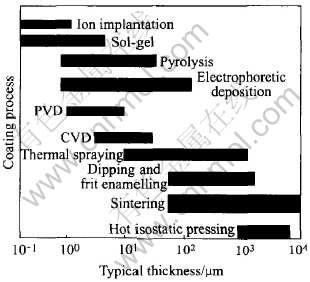
Fig.1 Typical thickness of HA coating produced by different coating processes[16]
2.2 Requirements for apatite coating
Being an interface layer between implant and bone tissue, calcium phosphate apatite coating plays a very important role in the bio-performance of the implant. Not only the mechanical properties of the coating and the interface between the coating and implant, but also the physical properties of the coating show great influence on the final application.
2.2.1 Composition and crystallinity
High crystallinity HA coating is still the most widely used coating composition due to its excellent biocompatibility, although some biphasic apatite coatings have also been reported to show good biocompatibility and osteoconductivity. In general, it appears that calcium phosphate with calcium/phosphate ratios in the range of 1.0-1.67(tricalcium phosphate and hydroxyapatite respectively) yields the most beneficial tissue response.
2.2.2 Stability or biosolubility
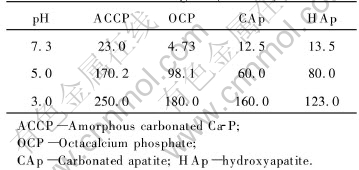
The stability of apatite in bioenvironment or biosolubility of the apatite is another important property in biomedical coating application. The role of coating ends after the initial stages of osseointegration, so this layer does not have to be insoluble[18]. Furthermore, the degradation rate should be adjusted to the bony growth rate to prevent gap formation. Stability and solubility of Ca-P materials are strongly dependent on composition and crystallographic structure. Barrere et al[19] studied the dissolution of various calcium-phosphate coatings on Ti-6Al-4V, as listed in Table 1. In all cases, ACCP coating exhibited the fastest dissolution rate. Coatings with higher ACP/HA ratios will dissolve or biodegrade to a greater extend than coatings with a low ACP/HA ratio or higher crystallinity. Also increased calcium/phosphate ratios, fluorine and carbonate contents, and degree of crystallinity all lead to greater stability of the biological precipitate in calcified tissue.
Table 1 Dissolution rate of various coatings[19](10-6/h)
2.2.3 Thickness
The coating thickness is another important parameter in bio-applications, since thick coatings tend to crack and fail prematurely. As discussed above, the coating function ends after the initial stages of osseointegration, so the coating does not have to be thick. However, as bony growth rate depends on the patient, surgical techniques, and so on, it is very difficult to define ideal thickness. A coating thickness greater than 100μm can potentially introduce fatigue failure under tensile loading[16]. There are some disputes in literatures concerning the optimum thickness of bioceramic coatings that are intend for orthopaedic applications; Groot et al[20] proposed 50μm in thickness whereas Osborn[21] recommended on 200μm in thickness.
3 ELECTROPHORETIC DEPOSITION
3.1 Process
Following several refinements, electrophoretic deposition(EPD) has by now been established as a low cost, simple, less time consuming and flexible coating process. It offers rigid control of film thickness, uniformity and deposition rate. It is especially attractive due to the low cost of equipment and raw materials. Because of the use of an electric field and being a non-line-of-sight process, EPD is particularly suited for the formation of uniform films on substrates of complicated shape, impregnation of porous substrates, and deposition on selected areas of the substrates. Rapid deposition rates are possible, in the orders of seconds to minutes for coating thickness from less than 1μm to more than 100μm, with excellent control over the thickness and morphology of the coating. Recently, EPD has been used to produce multilayer coating or graded coating on metal for biomedical application[22]. Substrates can be titanium, titanium alloy and stainless steel[23]. Fig.2 schematically shows the process of electrophoretic deposition and some important factors.
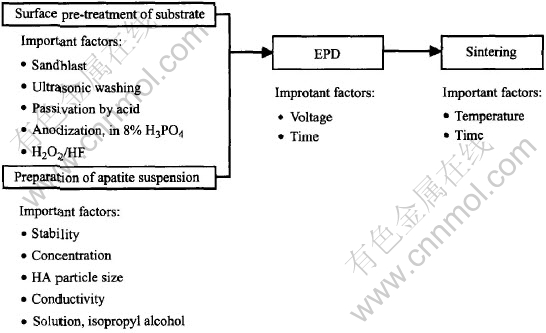
Fig.2 Schematic process of EPD processing
The deposit mass m in the elctrophoretic process can be described by the following equation[13]:
m=CμUt/d
where C and μ are the particle concentration and mobility, respectively.
U=Uap-Udep
where Uapis the applied voltage, Udep is the voltage drop in deposit, t is the deposition time, and d is the distance between electrodes. The electrophoretic mobility can be determined from the Smoluchowski equation:
μ=ζε/4πη
where ζ is the zeta potential, ε is the dielectric constant, and η is the viscosity of the medium.
It was also reported that the change of the deposition mass of HA against deposition time can be simply described by a empirical model[10]
m=m0-(1-e-kt)
where m0 is the initial mass of the powder in the suspension and k is the kinetic constant.
3.2 Advantages and disadvantages
Compared with other coating technologies, the main advantages of electrophoretic deposition (EPD) can be summarized as follows:
1) Low-cost and flexible coating process.
2) A non-line-of-sight coating process. It can be used to deposit even coatings on substrates of complex shape or surface morphology[12, 24].
3) A wide range of coating thickness, from less than 1μm to more than 100μm with a high degree of control over coating thickness and morphology[12].
4) Rapid deposition. In the order of seconds to minutes[24].
5) Wide composition of apatite, and two phases or more apatites can be deposited on to the substrate.
6) Stainless steel, Ti and Ti alloys and Co alloy can be selected as substrate.
However, there still exist some disadvantages:
1) Post-sintering is needed to densify the coating, which may result in crack between the substrate and coating due to the mismatch in coefficient of thermal expansion[24].
2) High-temperature sintering may result in degradation of the metal substrate(oxidation and impaired mechanical properties).
3) Decomposition of HAp to TCP may be catalyzed by the metal substrates.
4) Phase transformation and grain growth cause significant decrease in mechanical properties of substrate.
3.3 Important factors in EPD process
In EPD process, there are many factors which affect the final quality of the coating and its bio-properties, as listed in Table 2. Here only the pretreatment before EPD, and the properties of apatite suspension will be discussed.
3.3.1 Pretreatment
Surface treatments on titanium samples prior to hydroxyapatite deposition are essential to guarantee coating adhesion to metallic substrate. Many methods have been used to treat the surface, including mechanical, chemical and electrochemical methods, such as sand polish and blast, acid etch and electrolytic etch. The chemical or electrochemical method following mechanical treatment not only removes the sand particle but also creates a homogeneous and rough microtopography. Table 3 lists three pretreatment methods used on titanium substrate[18]. It indicates that the abraded or blasted titanium followed by acid etch shows an improved HA deposition and good adhesion. However, no difference was found in the titanium treated by electrolytic etch with H3PO4 solution and blasting followed by H2O2/HF solution etch.
Table 2 Parameters in electrophoretic deposition of ceramic materials[16]
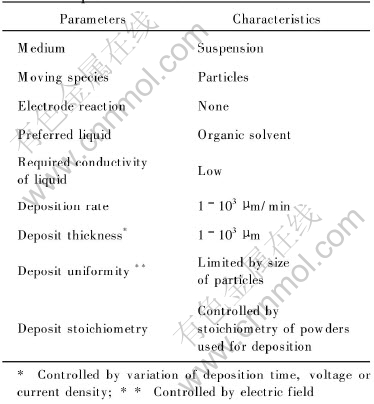
Table 3 Summary of titanium substrate treatment prior to HA deposition[18]

In another investigation[25], a silica or calcium-silica sol pre-coating produced by dip- withdrawing was reported to densify the HAp coating after subsequent annealing and enhance its adhesion to the metal substrate.
3.3.2 Stability of apatite suspension
It is important to note that stable suspensions are necessary for electrophoretic deposition experiments. However, ultrasonically treated HA suspensions exhibited significant settling after several tens of minutes. In contrast, a HA which contained submicrometre particles (55nm in average size and washed by isopropyl alcohol) suspensions were found to be stable for 1-2d[13], but magnetic stirring was found to have a detrimental effect on suspension stability and led to relatively fast settling when stirring was interrupted. The electric field also has an adverse effect on this HA suspension stability. Passing an electric current through the suspension during electrodeposition also brought about significantly faster settling. It is clear that electric-field-induced particle sedimentation decreases the amount of fine particle in the suspension and in the deposit this effect is more distinct in higher electric fields.
It is considered that the pH value of the suspension plays an important role in determining the stability of the dispersed apatite suspension and the size of apatite particles. The pH of 2 provides the most stable dispersed suspension and smallest particle size[10].
The liquid medium used to suspend the particles during electrophoretic deposition is also important for two main reasons[24]: 1) the particles must be colloidally stable in the medium, some medium-particle systems give inherent steric stabilization; 2) the medium must have a dielectric constant that gives effective coating characteristics. Adsorbed water was reported to interfere with electrophoretic transport and therefore electrophoretic deposition of non-calcined HA powders was not achieved[26]. Organic liquids are preferable to water since electrophoretic deposition in water is accompanied by significant gas evolution. However, uncalcined HAp coating was electrophoretically deposited on Ti, Ti6Al4V and 316 stainless steel substrate[11].
4 MICROSTRUCTURE AND MECHANICAL PROPERTIES
HA coatings can be readily deposited on metal substrates by electrophoretic deposition. The surface morphology of the HA coating significantly depends on the processing parameters, such as applied voltage, particle size. Normally, the HA coating is of porous structure and the bonding strength of the HA coating is low, as shown in Fig.3. So post-sintering is necessary to increase the density and the bonding strength of HA coating.
However, subsequent sintering is highly problematic owing to the fact that temperatures in excess of 1100℃ are required for commercial hydroxyapatite powders to achieve high density. For example, it was reported[10] that after sintered at 1000, 1150, 1300℃ for 2h, an interconnected porous structure was observed in all samples,as shown in Fig.4. Although a layer of HA coating as thickness as 400μm has adhered very well onto the titanium substrate and no delamination or crack was observed at both interface and surface after sintered at 1000℃, a porous structure with a porosity of 19% was observed.
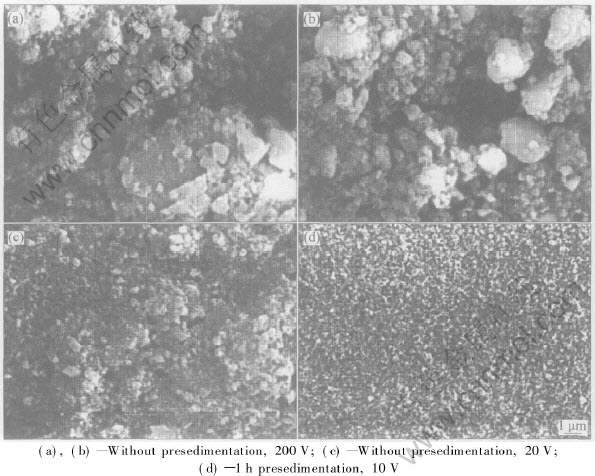
Fig.3 Surface morphologies of HA coating on Ti6Al4V at different applied voltage[13]
In addition, the firing shrinkage of the HA coating on a constraining metal substrate during the high temperature sintering process leads to severe cracking in the coating, as shown in Fig.5, even delamination or crack at the interface between substrate and coating(Fig.6). A dual-HAp coating (first deposition-sintering+the second deposition-sintering)[11, 12] was developed to avoid the crack of the coating. Microstructure observation has indicated that extensive cracking of coatings was observed on the top of coating layer in all substrates and all sintering temperature (range from 875-1000℃), but the evidence of “valley filling” by the second coating in the macrocrack of the first coating was clearly found and there appeared to be no distinct border between the first and the second coating layers, which suggested that a strong seamless bonding formed between these two layers, as shown in Fig.7.
Furthermore, the high temperature sintering damages the metal and induces metal-catalysed decomposition of the hydroxyapatite. Pure HAp decomposes to tricalcium phosphate(TCP) in the temperature range of 1200-1450℃. TCP is biodegradable in vivo[27]. In the presence of the underlying metal substrate, HAp coatings may decompose even at a sintering temperature as low as 1050℃ for titanium substrate and 950℃ for stainless steel substrate due to ion migration from the metal substrates into the HAp coating[11, 28, 29]. A high temperature sintering is also unfavourable for the metal substrates since it can lead to phase transformation and grain growth. This may cause significant deterioration of the mechanical properties of the metal substrates. One way to alleviate the problem of HA decomposition is the formation of titanium oxide layers[13].
The interface adhesive strength of HA coating on the metal substrate is a very important factor during the following service. Table 4 lists the reported adhesive strength of HAp coating on different metal substrate. On average, the interfacial bond strength is 23MPa for stainless steel, 14MPa for Ti and 11MPa for Ti6Al4V substrates, respectively. The shear strength of cortical bone is about 35MPa.
5 NANOPARTICULATE HYDROXYAPATITE
A new wave of interest in electrophoretic technology is concerned with the use of submicrometre or nano ceramic particles. Electrophoretic deposition of submicrometre or nano powders offers advantages in fabrication of ceramic coatings and bodies with dense packing, good sinterability and homogeneous microstructure[13]. One of the important capabilities provided by fine particle electrophoresis is the ability to achieve ceramic particle impregnation into a porous substrate and composite consolidation[30]. Another advantage of fine particle electrophoresis is the agglomerate-free structures made from close-packed fine particles can be densified at lower sintering temperatures, for example, at 1000℃[27].
Table 4 Adhesive strength of HAp coatings on metal substrates[11, 12](MPa)

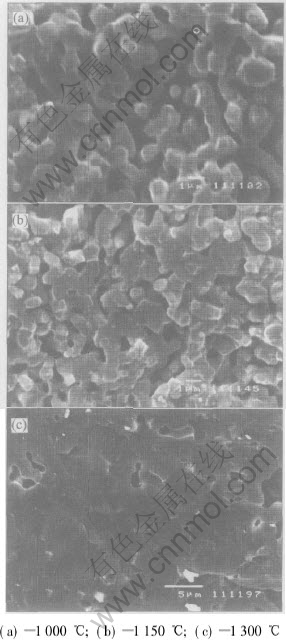
Fig.4 Microstructures of HA deposits at various sintering temperatures[10]
More than two methods have been developed to produce nanometer size HA particles. The particle size ranges from 50-70nm to 600-700nm[31] depending on the processing. Particle distribution in the suspension plays a very important role in the deposition coating quality. Nano particles due to their small size and large surface are prone to be agglomerated. Some methods have been used to optimize the properties of the suspension, especially the particle distribution. An important advantage of electrophoretic processing is that agglomerates can be separated by pre-sedimentation. Moreover, owing to the insulating properties of the deposit, the electric field provides a higher deposition rate in defect regions, resulting in better packing and uniformity of the deposit[13]. Another way is to add some kinds of additives into the suspension in order to obtain stable suspensions and to achieve the desired level of electrophoretic mobility[32]. Furthermore, it was found that ripening of the nanoparticle had a significant effect on the particle size, the agglomeration state and the cracking of the deposition coating[12]. Ripening by ambient aging or boiling transformed the as-precipitated HAp from a gel-like state to a weakly agglomerated nanoparticulate state, in turn eliminated the cracking in the electrophoretic coating.
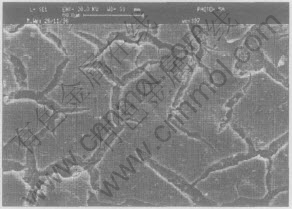
Fig.5 Surface morphology of ELP HA coating on Ti substrate[11]
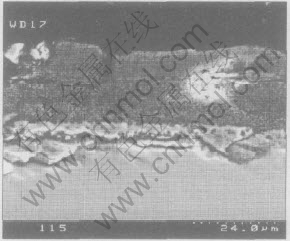
Fig.6 Cross-section morphology of ELP HA coating on Ti-6Al-4V substrate[11]

Fig.7 SEM cross-sectional micrograph of dual-HAp-Ti6Al4V coating[12]
6 CONCLUSIONS
As a surface coating technique, electrophoresis deposition(EPD) has shown significant advantage over the commercial plasma spray coating process on the preparation of calcium phosphate coating onto metal substrate for biomedical application, such as low cost and flexible process, wide range of apatite composition and thickness and non-line-of-sight characteristic. The non-line-of-sight characteristic produces the possibility to cover an even calcium phosphate onto the complex shaped substrate, which has attracted much more attention in the porous implant application. Although the high temperature sintering induces cracks in the coating and metal-catalysed decomposition of hydroxyapatite, and causes deterioration of the mechanical properties of the metal substrate, many methods have been developed to modify the process and the process has been successfully used in calcium phosphate coating. Microstructure of the calcium phosphate coating and the mechanical properties of the coating and metal substrate have shown potential application of EPD process in the biomedical materials surface modification. However, more research is needed to apply this process in the commercial application.
REFERENCES
[1]Noort R V. Titanium: the implant material of today [J]. J Mater Sci, 1987, 22: 3801-3811.
[2]Kokubo T, Kim H M, Kawashita M. Novel bioactive materials with different mechanical properties [J]. Biomaterials, 2003, 24: 2167-2175.
[3]Kuroda D, Niinomi M, Morinaga M, et al. Design and mechanical properties of new ( type titanium alloys for implant materials [J]. Mater Sci Eng, 1998, A243: 244-249.
[4]Tsui Y C, Doyle C, Clyne T W. Plasma sprayed hydroxyapatite coatings on titanium substrates(Part 2): optimization of coating properties [J]. Biomaterials, 1998, 19: 2031-2043.
[5]Guipont V, Espanol M, Borit F, et al. High-pressure plasma spraying of hydroxyapatite powders [J]. Mater Sci and Eng, 2002, A325: 9-18.
[6]WANG C X, CHEN Z Q, GUAN L M, et al. Structural characterization of ion beam sputter deposited calcium phosphate coatings [J]. Surface & Coatings Technology, 2002, 130(1): 39-45.
[7]Metikos-Hukovic M, Tkalcec E, Kwokal A, et al. An in vitro study of Ti and Ti-alloys coated with sol-gel derived hydroxyapatite coatings [J]. Surface and Coatings Technology, 2003, 165: 40-50.
[8]WANG C X, CHEN Z Q, WANG M, et al. Functionally graded calciumphosphate coatings produced by ion beam sputtering/mixing deposition [J]. Biomaterials, 2001, 22: 1619- 1626.
[9]Ploska U, Berger G, Willfahrt M. A new procedure of a calcium-containing coating on implants of titanium alloy [J]. Key Engineering Materials, 2004, 254-2: 411-414.
[10]MA J, LIANG C H, KONG L B, et al. Colloidal characterization and electrophoretic deposition of hydroxyapatite on titanium substrate [J]. J Mater Sci Mater in Med, 2003, 14: 797-801.
[11]WEI M, Ruys A J, Milthorpe B K, et al. Electrophoretic deposition of hydroxyapatite coating on metal substrates: a nanoparticulate dual-coating approach [J]. J Sol-gel Sci Tech, 2001(21): 39-48.
[12]Wei M, Ruys A J, Swain M V, et al. Interfacial bond strength of electrophoretically deposited hydroxyapatite coatings on metals [J]. J Mater Sci Mater Med, 1999, 10(7): 401-409.
[13]Zhitomirsky I, Gal-Or L. Electrophoretic deposition of hydroxyapatite [J]. J Mater Sci Mater Med, 1997, 8: 213-219.
[14]Wang J, Layrolle P, Stigter M, et al. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: physicochemical characteristics and cell attachment [J]. Biomaterials, 2004, 25 : 583-592.
[15]Helen A, Therese G, Vishnu Kamath P, et al. Novel electrosynthetic rout to calcium phosphate coatings [J]. J Mater Chem, 1998(8): 405-408.
[16]Sridhar T M, Eliaz N, Kamachi U F, et al. Electrophoretic deposition of hydroxyapatite coatings and corrosion aspects of metallic implants [J]. Corrosion Reviews, 2002, 20(4-5): 255-293.
[17]Cahn R W, Haasen P, Kramer E J. Materials Science and Technology: A Comprehensive Treatment, Medical and Dental Materials (Vol.14) [M]. New York: VCH.
[18]de Sena L , de Andrade M C, Rossi A M, et al. Hydroxyapatite deposition by electrophoresis on titanium sheets with different surface finishing [J]. J Biomed Mater Res, 2002, 60: 1-7.
[19]Barrere F, Stigter M, Layrolle P, et al. In vitro dissolution of various calcium phosphate coatings on Ti6Al4V [J]. Key Engineering Materials, 2001, 192-195: 67-70.
[20]de Groot K, Geesink R, Klein C P A T, et al. Plasma sprayed coatings of hydroxylapatite [J]. J Biomater Med Res, 1987, 21: 1375-1381.
[21]Osborn J F. Biological behavior of the hydroxyapatite ceramic coating on the femur shaft of a titanium endoprosthesis—initial histologic evaluation of a human explant [J]. Biomed Tech, 1987, 32(7-8): 177-183.
[22]Chen X M, Jiao Y H, Xu C B. Study of preparing hydroxyapatites bioglass coating on Ti6Al4V alloy [J]. Mater Sci Eng, 2002, 20(4): 545-548.
[23]Sridhar U, Kamachi Mudali, Subbaiyan M. Preparation and characterisation of electrophoretically deposited hydroxyapatite coatings on type 316L stainless steel [J]. Corrosion Science, 2003, 45: 237-252.
[24]Wei M, Ruys A J, MIlthorpe B K, et al. Solution ripening of hydroxyapatite nanoparticles: Effects on electrophoretic deposition [J]. J Biomed Mater Res, 1999, 45: 11-19.
[25]Stoch A, Brozek A, Kimita G, et al. Electrophoretic coating of hydroxyapatite on titanium implants [J]. J Molecular Structure, 2001, 596: 191-200.
[26]Ducheyne P, Radin S, Heughebaert M, et al. Calcium phosphate ceramic coatings on porous titanium: effect of structure and composition on electrophoretic deposition, vacuum sintering and in vitro dissolution [J]. Biomaterials, 1990, 11: 244-254.
[27]Ruys A J, Sorrell C C, Brandwood A. Hydroxyapatite sintering characteristics - correlation with powder morphology by high-resolution microscopy [J]. Journal of Material Sci Letters, 1995, 14: 744.
[28]Ducheyne P, Raemdonch W V, Heughebaert J C, et al. Structural analysis of hydroxyapatite coatings on titanium [J]. Biomaterial, 1986, 7: 97-103.
[29]WENG J, LIU X, ZHANG X, et al. Integrity and thermal decomposition of apatite in coatings influenced by underlying titanium during plasma spraying and post-heat-treatment [J]. Journal of Biomedical Materials Research, 1996, 30: 5-11.
[30]Gar-Or L, Liubovich S, Haber S. Deep electrophoretic penetration and deposition of ceramic particles inside porous substrates(Ⅱ)—Experimental model [J]. J Electrochem Soc, 1992, 139: 1078-1081.
[31]Kong L B, Ma J, Boey F. Nanosized hydroxyapatite powders derived from coprecipitation process [J]. J Mater Sci, 2002, 37: 1131-1134.
[32]Mizuguchi J, Sumi K, Muchi T. A highly stable nonaqueous suspension for the electrophoretic deposition of powdered substances [J]. J Electrochem Soc, 1983, 130: 1819-182.
Foundation item: Project(AM07-YC13) support by the Institute of Metal Research(IMR), Chinese Academy of Sciences(CAS), Shen-yang, China
Received date: 2005-03-21; Accepted date:2005-5-31
Correspondence: ZHANG Er-lin, Professor, PhD; Tel: +86-24-23971605; Fax: +86-24-83978616;
E-mail: zhang_erlin 2000@yahoo.com


