
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1760-1765
Solvent extraction of hafnium from thiocyanic acid medium in DIBK-TBP mixed system
XU Zhi-gao1,2, WANG Li-jun2, WU Yan-ke2, CHI Ru-an1, ZHANG Li2, WU Ming1
1. Key Laboratory for Green Chemical Process of Ministry of Education,
Wuhan Institute of Technology, Wuhan 430073, China;
2. Division of Mineral Resources, Metallurgy and Materials,
General Research Institute for Nonferrous Metals, Beijing 100088, China
Received 25 July 2011; accepted 13 January 2012
Abstract:
A novel process for the separation of hafnium from thiocyanic acid medium using the mixture of diisobutyl ketone (DIBK) and tributyl phosphate (TBP) as the extractant was developed. This extraction process was investigated experimentally as a function of the amount of TBP added, acidity, zirconium and hafnium concentrations, salting-out agent, temperature, duration, respectively. The results show that hafnium is enriched in the organic layer and zirconium is in aqueous layer in DIBK-TBP system. Under the optimal technological conditions: TBP addition 20% (v/v), aqueous phase acidity 3.0 mol/L, ammonium sulfate addition 0.8-1.25 mol/L, room temperature and extraction time 10 min, the separation factor of hafnium from zirconium is 9.3.
Key words:
diisobutyl ketone (DIBK); zirconium; hafnium; extraction; separation;
1 Introduction
The technology for separation of zirconium and hafnium is the key to prepare nuclear grade productions of zirconium and hafnium. Zirconium and hafnium are normally associated together in nature (m(Hf)/m(Zr+Hf) is 1%-3%) and difficult to separate from each other. With the development of nuclear power, technologies for the separation between hafnium and zirconium, such as molten salt distillation [1], ion exchange [2-4] and solvent extraction [5,6], have become very important because of the increasing demands for the production of nuclear grade hafnium and zirconium metals and compounds. A survey of the literature showed that a limited number of extractants and extraction systems have been used for the extraction and separation between hafnium and zirconium, such as methyl isobutyl ketone (MIBK) [7], Cyanex 302 [8], Cyanex 923 [9], Aliquat 336 [10], LIX 860N-1C [11], Cyanex 301 [12], 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (P507) [13], Cyanex 925 [14], Cyanex 272 [15,16] and 3-phenyl-4-benzoyl-5-isoxazolone (HPBI) [17]. These extractants and extraction systems are most used to extract zirconium selectively, except for the MIBK, Cyanex 301 and Cyanex 302 which are used to extract hafnium selectively.
Nevertheless, conventional extractant MIBK is limited in industrial application because it is easy to burn and volatilize and it has high water solubility, while diisobutyl ketone (DIBK) is well employed in the extraction analysis and separation of precious metal due to its low solubility, high flash point and high boiling point [18]. However, DIBK has not been used for the solvent extraction of hafnium just for precious metals. LIU et al [19] reported the experimental results on the extraction of gold with diisobutyl ketone in Jinchuan, China, and the extraction rate of gold was found to be 99% and the purity of gold product was about 99.99%. GRANT and DAVID [20] reported a rapid single extraction procedure using dithiocarbamate complexing agent, DIBK organic phase and Hg exchange back-extraction for the simultaneous quantitative pre- concentration of Cd, Co, Cu, Fe, Ni, Pb and Zn in seawater.
The extraction mechanism of DIBK is almost similar to MIBK, although the ability for noble metal extraction with DIBK is less than that with MIBK. It can be presumed that as an extractant for extracting minor metal (hafnium) selectively over major metal (zirconium), DIBK with lower solubility in water, higher flash point and higher boiling point, is superior to MIBK in industry. In order to increase the extraction ability of the DIBK, a certain amount of TBP will be added. There is little report on separating between zirconium and hafnium using the mixture of DIBK and TBP at present. This study is carried out to optimize a suitable solvent extraction system consisting of DIBK and TBP for the separation between hafnium and zirconium.
2 Experimental
2.1 Materials
The DIBK was provided by Dow Chemical Company, USA. Zirconium oxychloride (purity 99.9%) was obtained from Zhejiang Shenghua Biok Biology Co., Ltd., China. TBP, ammonium thiocyanate, hydrochloric acid, sulfuric acid, ammonium sulfate and ammonia were used at analytical reagent grade. De-ionized water was used for the whole process. The feed mixtures, namely feed solutions, were prepared by dissolving zirconium oxychloride and ammonium thiocyanate in appropriate amounts of de-ionized water.
2.2 Experimental procedures
All extraction experiments were carried out in separatory funnels of suitable volume and separated at room temperature. Batch contacts with phase ratio V(O)/V(A)=2:1 were performed for 10 min and were taken for analysis after phase separation. The total concentrations of zirconium and hafnium in water phase were titrated by EDTA using xylenol orange as indicator. The acidity of filter liquor was titrated by standard sodium hydroxide using phenolphthalein as the indicator and sodium citrate as masking agent. The concentration of hafnium in filter liquor was determined by ICP-AES or ICP-MS.
3 Results and discussion
3.1 Effects of aqueous solution acidity
The reaction kinetics of micro and large quantity of hafnium and zirconium extraction are quite different because zirconium and hafnium have large complexation, hydrolysis and polymerization tendency in aqueous solution. When a large quantity of zirconium and hafnium hydrolyzate is extracted, the separation effect is reduced for their possible polymerization is difficult to extract into organic phase. So, the increased acidity of water phase is beneficial for extraction. Therefore, acidity has great influence on the separation between zirconium and hafnium.
The effects of aqueous solution acidity ([H+]aq) on the distribution ratio (D) of zirconium and hafnium in the DIBK-TBP system were investigated under the conditions of 10% TBP and 90% DIBK (volume fraction) as organic phase, 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L NH4SCN and 10 min extraction time.
In Fig. 1, the distribution ratios of zirconium and hafnium decrease with the increase of the acidity. The distribution ratio of zirconium (DZr) decreases quickly when [H+]aq≤3.0 mol/L, stays unchanging when 3.0 mol/L<[H+]aq≤5.5 mol/L and decreases when [H+]aq>5.5 mol/L. However, the distribution ratio of hafnium (DHf) reduces gradually with increasing the acidity. The separation factor of hafnium and zirconium (β) first increases, then decreases, and comes to a maximum value at [H+]aq=3.0 mol/L. The reason is that organic solution relative to extraction capacity of zirconium thiocyanate and hafnium thiocyanate is more conducive than non-hydrolyzed compound. When acidity increases, hydrolysis is suppressed, so it is not conducive to the extraction of zirconium and hafnium. However, this strong inhibition is more effective to zirconium than to hafnium and it is conducive to the separation between zirconium and hafnium.
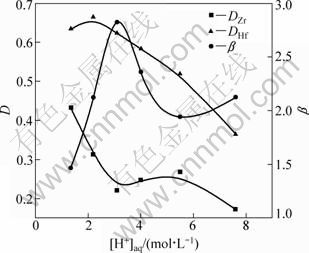
Fig. 1 Effects of aqueous solution acidity on distribution ratio (D) and separation factor (β) of Zr and Hf
3.2 Effects of extraction time
The effects of extraction time on the distribution ratio and separation factor of zirconium and hafnium were investigated under the conditions of 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L acidity and 3.0 mol/L ammonium thiocyanate in aqueous phase and 10% TBP and 90% DIBK (volume fraction) in organic phase. The results of the distribution ratios, the separation factors and the zirconium and hafnium are shown in Fig. 2.
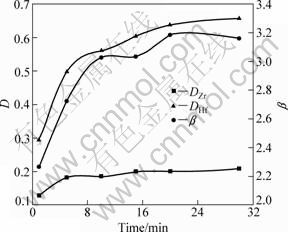
Fig. 2 Effects of extraction time on distribution ratio (D) and separation factor (β) of Zr and Hf
It can be found that DIBK-TBP extraction system for zirconium and hafnium responses rapidly. When the extraction time is 2 min, the separation factor is up to 2.3, indicating that the extraction rate increases rapidly at beginning. The extraction rate changes little over 10 min. It means that the extracted complex goes fast into organic phase under the experimental conditions, but it needs 10 min to achieve extraction equilibrium of zirconium and hafnium. This may be directly related to the zirconium and hafnium with high valence state which may be easy to hydrolyze into multicore hydroxy compounds [21]. Therefore, the extraction equilibrium time is chosen as 10 min.
3.3 Extraction equilibrium isotherms of zirconium and hafnium
The extraction equilibrium isotherms of zirconium and hafnium were obtained under the conditions of 3.0 mol/L acidity, 3.0 mol/L ammonium thiocyanate, 10% TBP and 90% DIBK. The results are shown in Fig. 3 and Fig. 4, respectively.

Fig. 3 Extraction equilibrium isotherm of zirconium

Fig. 4 Extraction equilibrium isotherm of hafnium
With increasing the zirconium and hafnium concentrations in the balance aqueous phase, the tangent slopes of the zirconium and hafnium balance isotherms keep decreasing and the curve of hafnium decreases faster, because when more metal is extracted into the organic phase, the concentration of free extractant decreases, and the distribution ratio decreases. Faster reduction of the slope of the hafnium indicates that the effect of hafnium concentration on the distribution ratio is greater than zirconium concentration. Therefore, low metal concentrations are conducive to the separation of hafnium from zirconium.
3.4 Effects of extraction temperature
The effects of extraction temperature on the extraction of zirconium and hafnium were investigated at different temperatures of 20 ℃, 30 ℃, 40 ℃, 50 ℃ and 60 ℃ for 10 min under the following conditions: 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L acidity, 3.0 mol/L ammonium thiocyanate, 10% TBP and 90% DIBK.
In Fig. 5, with increasing the temperature, the distribution ratios of zirconium and hafnium continue to decrease, but the separation factor continues to increase. Therefore, low-temperature operation should be chosen in the extraction of zirconium and hafnium [22,23].
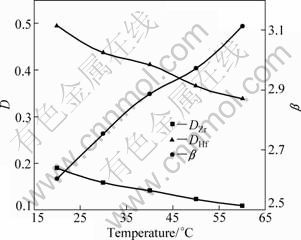
Fig. 5 Effects of temperature on distribution ratio (D) and separation factor (β) of Zr and Hf
3.5 Effects of NH4SCN concentration
The extractions of zirconium and hafnium are given in Fig. 6 as a function of NH4SCN concentration at room temperature for 10 min under the following conditions of 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L acidity, 10% TBP and 90% DIBK.

Fig. 6 Effects of NH4SCN concentration on distribution ratio (D) and separation factor (β) of Zr and Hf
As the NH4SCN concentration increases, the distribution ratios of zirconium and hafnium first increase and then decrease, both of them get a maximum value when the concentration of NH4SCN is 4 mol/L. While the separation factor of zirconium and hafnium increases gradually. These results are consistent with results of DA SILVA and DISTIN [24]. When the addition of NH4SCN exceeds 5 mol/L, NH4SCN cannot be dissolved completely in the water phase. It can be concluded from the above results that the suitable NH4SCN concentration is 4 mol/L.
3.6 Effects of (NH4)2SO4 concentration
The extraction of zirconium and hafnium is given in Fig. 7 as a function of (NH4)2SO4 concentration at room temperature for 10 min under the following conditions: 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L acidity, 3.0 mol/L NH4SCN, 10% TBP and 90% DIBK.
When the amount of (NH4)2SO4 increases, the distribution ratio of zirconium decreases, while the distribution ratio of hafnium first increases and then decrease slightly. When (NH4)2SO4 is added to 0.8 mol/L, the distribution ratio of hafnium reaches the maximum of 0.75; while the distribution ratio of zirconium remains at 0.15. When the amount of (NH4)2SO4 increases, the distribution ratio of hafnium decreases slightly, while the distribution ratio of zirconium continues to decrease. The zirconium and hafnium separation coefficient is up to 9.3 as the amount of (NH4)2SO4 is 1.25 mol/L. It shows that it is conducive for hafnium to extract into the organic phase by adding (NH4)2SO4, and it significantly improves the separation between zirconium and hafnium. As the feed is added with SO42-, the products of the reaction of ZrO2+ and SO42- are more stable than those of HfO2+ and SO42-, while the products of the reaction of HfO2+ and SCN- are more stable than those of ZrO2+ and SCN-. So the extraction ability of DIBK to HfO(SCN)2 is enhanced while the extraction ability to ZrO(SCN)2 is reduced. The zirconium and hafnium separation coefficient with DIBK is slightly lower than that with MIBK (up to 14) [25,26]. However, the minor metal (hafnium) is the extracted species with DIBK in the parallel MIBK-HSCN system.
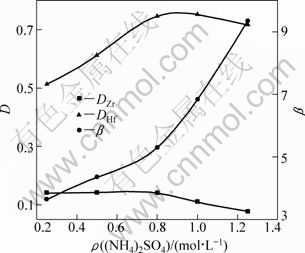
Fig. 7 Effects of (NH4)2SO4 concentration on distribution ratio (D) and separation factor (β) of Zr and Hf
In addition, the quantity of free water molecules in the aqueous phase is reduced because of the salt hydration, which results in lower effective concentration of water and higher effective concentration extracted. Both of them are helpful to extraction. It is found that white precipitate appears in the aqueous solution when the dosage of (NH4)2SO4 added is more than 1.25 mol/L. So, the suitable dosage of (NH4)2SO4 in extraction is 0.8-1.25 mol/L.
3.7 Effects of NaCl concentration
The extraction of zirconium and hafnium is given in Fig. 8 as a function of NaCl concentration at room temperature for 10 min under the following conditions: 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L acidity, 3.0 mol/L ammonium thiocyanate, 10% TBP and 90% DIBK.
With increasing the sodium chloride, the distribution ratios of zirconium and hafnium keep increasing, while the distribution ratio of hafnium increases more greatly than that of zirconium. The separation factor of zirconium and hafnium remains at 3.6 when sodium chloride concentration ≤2 mol/L, the separation factor increases rapidly when ρ(NaCl)>2 mol/L, and the separation factor is up to 4.2 when ρ(NaCl)=4 mol/L. It is because chloride ion suppresses the hydrolysis and condensation of zirconium ion and hafnium ion, and it is conducive for the complexes to extract into the organic phase. However, there is a little increase of hafnium and zirconium extractions as sodium chloride concentration is further added, especially when its dosage is up to 5 mol/L, it does not conducive to the extraction process because sodium chloride does not dissolve completely.

Fig. 8 Effects of NaCl concentration on distribution ratio (D) and separation factor (β) of Zr and Hf
3.8 Effects of TBP concentration
The effects of TBP on the distribution ratios and separation factor of zirconium and hafnium are shown in Fig. 9. For these tests, the extractions were conducted using the mixture of organic phase with different quantity of TBP and DIBK at room temperature for 10 min under the conditions of 120 g/L zirconium and hafnium (2.89 g/L hafnium), 3.0 mol/L acidity and 3.0 mol/L ammonium thiocyanate.
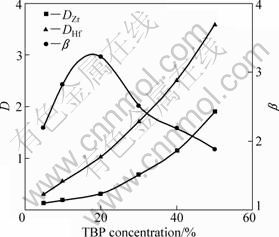
Fig. 9 Effects of TBP concentration on distribution ratio (D) and separation factor (β) of Zr and Hf
With increasing the concentrations of TBP in organic phase, the distribution ratios of zirconium and hafnium increase correspondingly, while the separation factor of zirconium and hafnium first increases, and then decreases. The separation factor reaches a maximum with 20% ??TBP. Therefore, in DIBK-TBP system, the content of TBP is 20%.
4 Conclusions
1) The mixture of DIBK and TBP was studied as a possible alternative for hafnium/zirconium separation. DIBK selectively extracts the minor metal (hafnium, 1%-3%) over the major metal (zirconium, 97%-99%) from thiocyanic acid solutions, and both metals can be produced to meet high grade specifications in the conventional technology using MIBK. The zirconium and hafnium separation coefficient with DIBK is slightly lower than that with MIBK.
2) The optimal technological conditions are as follows: TBP concentration 20%, aqueous phase acidity 3.0 mol/L, ammonium sulfate addition 0.8-1.25 mol/L, room temperature and extraction time 10 min. Under these conditions, the separation factor of hafnium from zirconium is 9.3.
References
[1] NISELSON L A, EGOROV E A, CHUVILINA E L ARZHATKINA O A, FEDOROV V D. Solid-liquid and liquid-vapor equilibria in the Zr(Hf)Cl4-KAlCl4 systems: A basis for the extractive distillation separation of zirconium and hafnium tetrachlorides [J]. Journal of Chemical & Engineering Data, 2009, 54(3): 726-729.
[2] FAVRE-REGUILLON A, FIATY K, LAURENT P, PORIEL L, PELLET-ROSTAING S, LEMAIRE M. Solid/liquid extraction of zirconium and hafnium in hydrochloric acid aqueous solution with anion exchange resin—Kinetic study and equilibrium analyses [J]. Industrial and Engineering Chemistry Research, 2007, 46(4): 1286-1291.
[3] SMOLIK M, JAK?BIK-KOLON A, PORA?SKI M. Separation of zirconium and hafnium using Diphonix? chelating ion-exchange resin [J]. Hydrometallurgy, 2009, 95(3-4): 350-353.
[4] XU Zhi-gao, WU Yan-ke, ZHANG Jian-dong, ZHANG Li, WANG Li-jun. Equilibrium and kinetic data of adsorption and separation for zirconium and hafnium onto MIBK extraction resin [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8):1527-1533.
[5] XIE Xi-jing, LIU Song, WANG Jing-xin. Progress in solvent extraction and separation of zirconium and hafnium [J]. Chinese Journal of Rare Metals, 2009, 33(3): 426-433. (in Chinese)
[6] XU Zhi-gao, WU Yan-ke, ZHANG Jian-dong, WANG Xin, ZHANG Li, WANG Li-jun. Research progress in separation technique of zirconium and hafnium [J]. Chinese Journal of Rare Metals, 2010, 34(3): 444-454. (in Chinese)
[7] FISCHER W, DEIERLING B, HEITSCH H. The separation of zirconium and hafnium by liquid-liquid partition of their thiocyanates [J]. Angewandte Chemie International Edition in English, 1966, 5: 15-23.
[8] REDDY B R, KUMAR J R, RAJA K P, REDDY A V. Solvent extraction of Hf (IV) from acidic chloride solutions using Cyanex 302 [J]. Minerals Engineering, 2004, 17: 939-942.
[9] BINA G, POONMA M, NITI M. Extraction and recovery of zirconium from zircon using Cyanex 923 [J]. Solvent Extraction and Ion Exchange, 2005, 23(3): 345-357.
[10] PORIEL L, FAVRE-RGUILLON A, PELLET-ROSTAING S, Lemaire M. Zirconium and hafnium separation, Part 1. Liquid/liquid extraction in hydrochloric acid aqueous solution with aliquat 336 [J]. Separation Science and Technology, 2006, 41(9): 1927-1940.
[11] REDDY B R, KUMAR I R. Liquid-liquid extraction of tetravalent zirconium and hafnium from acidic chloride solutions using 2-hydroxy-5-nonylsalysildehy-deoxime LIX 860N-IC [J]. Solvent Extraction Research and Development, 2006, 13: 37-49.
[12] SABERYAN K, MEYSAMI A H, RASHCHI F, ZOLFONOUN E. Proposal of a new Hf (IV)/Zr (IV) separation system by the solvent extraction method [J]. Chinese Journal of Chemistry, 2008, 26(11): 2067-2072.
[13] XU Xin, YANG Xin-wei, WU Yuan-xu, ZHONG Yue-ming, LI Yong-xiu. Extraction and separation properties of zirconium (IV) hafnium (IV) [J]. Chinese Journal of Rare Metals, 2008, 32(3): 355-359. (in Chinese)
[14] NAVL A A, EL-NADI Y A, DAOUD J. Extraction and separation of Zr (IV) and Hf (IV) from nitrate medium by some CYANEX extractants [J]. Separation Science and Technology, 2009, 44(12): 2956-2970.
[15] TAGHIZADEHA M, GHASEMZADEHA R, ASHRAFIZADEHC S N. Determination of optimum process conditions for the extraction and separation of zirconium and hafnium by solvent extraction [J]. Hydrometallurgy, 2008, 90(2-4): 115-120.
[16] TAGHIZADEHA M, GHASEMZADEHA R, ASHRAFIZADEHC S N, GHANNADI M. Stoichiometric relation for extraction of zirconium and hafnium from acidic nitrate solutions with Cyanex272 [J]. Hydrometallurgy, 2009, 96(1-2): 77-80.
[17] REDDY K J, KUMAR J R, REDDY M L P. Synergistic enhancement and separation of zirconium (IV) and gafnium (IV) with 3-phenyl-4-benzoyl-5-isoxazolone in the presence of crown ethers [J]. Separation Science and Technology, 2009, 44(9): 2022-2040.
[18] LI Hua-chang. Practical handbook of chemistry [M]. Beijing: Chemical Industry Press, 2006: 76-166. (in Chinese)
[19] LIU Mo-xi, MAO Jian-jun, ZHONG Xiang. A study on the extraction of gold with diisobutyl ketone [J]. Mining and Metallurgical Engineering, 1986, 6(3): 54-58. (in Chinese)
[20] GRANT J B, DAVID L P. Improved dithiocarbamate/oxine solvent extraction method for the preconcentration of trace metals from seawater using metal exchange back-extraction [J]. Marine Chemistry, 1996, 55: 381-388.
[21] XIONG Bing-kun, WEN Wang-guang, YANG Xin-ming, LI Hui-yuan, LUO Fang-cheng, ZHANG Wei, GUO Jing-mao. Zirconium and hafnium metallurgy [M]. Beijing: Metallurgical Industry Press, 2006: 146-199. (in Chinese)
[22] XIE F, DREISINGER D B. Copper solvent extraction from alkaline cyanide solution with guanidine extractant LIX 7950 [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1136-1140.
[23] XU Zhi-gao, WANG Li-jun, CHI Ru-an, ZHANG Li. Thermodynamics of extraction and separation of zirconium and hafnium using mixtures of DIBK and TBP [J]. Chinese Journal of Rare Metals, 2012, 36(1): 142-148. (in Chinese)
[24] DA SILVA A B V, DISTIN P A. Zirconium and hafnium separation without waste generation [J]. CIM Bulletin, 1998, 91: 221-224.
[25] L? Biao-qi, XU Zhi-gao, ZHANG Li, WANG Li-jun. Influence parameters of separation of zirconium and hafnium in MIBK-HSCN system [J]. Chinese Journal of Rare Metals, 2008, 32(6): 754-757. (in Chinese)
[26] XU Zhi-gao, WANG Li-jun, CHI Ru-an, ZHANG Li. Solvent extraction and separation of hafnium from zirconium with DIBK [J]. Nonferrous Metals: Extractive Metallurgy, 2012(3): 35-38. (in Chinese)
DIBK-TBP体系萃取分离锆铪
徐志高1,2,王力军2,吴延科2,池汝安1,张 力2,吴 明1
1. 武汉工程大学 绿色化工过程教育部重点实验室,武汉 430073;
2. 北京有色金属研究总院 矿物资源与冶金材料研究所,北京100088
摘 要:采用DIBK和TBP混合萃取剂在硫氰酸盐介质中对锆铪进行萃取分离,考察TBP的加入量、水相酸度、料液中锆铪浓度、盐析剂加入量、萃取温度和萃取时间等因素对锆铪分离的影响。结果表明:DIBK-TBP体系优先萃取铪;在优化工艺条件下,即20%的TBP、水相酸度3.0 mol/L、硫酸铵加入量0.8~1.25 mol/L、室温、萃取时间10 min,锆铪的分离系数可达到9.3。
关键词:DIBK;锆;铪;萃取;分离
(Edited by YANG Hua)
Foundation item: Project (2012BAB10B10) supported by the National Key Technology R&D Program during the 12th Five-year Plan of China; Project (51174146) supported by the National Natural Science Foundation of China; Project (212110) supported by the Foundation for Key Program of Ministry of Education, China; Project (Q20111509) supported by the Program for Excellent Talents of the Education Department of Hubei Province, China; Project (10125042) supported by the Scientific Research Foundation of Wuhan Institute of Technology, China
Corresponding author: WANG Li-jun; Tel: +86-10-82241308; Fax: +86-10-62355399; E-mail: gold@grinm.cn
DOI: 10.1016/S1003-6326(11)61384-8
Abstract: A novel process for the separation of hafnium from thiocyanic acid medium using the mixture of diisobutyl ketone (DIBK) and tributyl phosphate (TBP) as the extractant was developed. This extraction process was investigated experimentally as a function of the amount of TBP added, acidity, zirconium and hafnium concentrations, salting-out agent, temperature, duration, respectively. The results show that hafnium is enriched in the organic layer and zirconium is in aqueous layer in DIBK-TBP system. Under the optimal technological conditions: TBP addition 20% (v/v), aqueous phase acidity 3.0 mol/L, ammonium sulfate addition 0.8-1.25 mol/L, room temperature and extraction time 10 min, the separation factor of hafnium from zirconium is 9.3.


