
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 842-846
Effects of Y addition on structural and mechanical properties of CuZrAl bulk metallic glass
XU Hong-wei1, 2, DU Yu-lei1, 2, DENG Yu3
1. Engineering Research Center of Materials Behavior and Design of Ministry of Education,
School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing 210094, China;
2. Jiangsu Institute of Advanced Materials, Danyang 212300, China;
3. National Laboratory of Solid State Microstructures, Center for Materials Analysis,Nanjing University, Nanjing 210093, China
Received 9 September 2011; accepted 12 January 2012
Abstract:
The effects of Y addition on the structural and mechanical properties of CuZrAl bulk metallic glass (BMG) were studied. The results show that the glass forming ability of CuZrAl system is improved by the addition of Y and the fracture strength decreases with Y addition due to the reduction of binding energy induced by Y. The fracture surface is dominated by vein-like patterns in Cu45Zr48Al7 bulk metallic glass, and changes to smooth regions in Cu46Zr42Al7Y5 BMG. TEM observation shows that Cu45Zr48Al7 BMG has a composite microstructure of nanocrystalline phases dispersed in amorphous matrix. However, the Cu46Zr42Al7Y5 BMG shows a fully amorphous structure.
Key words:
bulk metallic glass; Cu-based alloy; Y addition;
1 Introduction
Bulk metallic glasses (BMGs) are considered new structural materials due to their unique mechanical, physical and chemical properties [1-5]. It is reported that the glass forming ability (GFA), physical, chemical and mechanical properties of BMGs can be greatly affected by the addition of rare earth metals [6, 7]. For example, it is found that the GFA of Fe-, Zr- and Ti-based BMGs can be enhanced by minor addition of Y [8-10]. The reason for the improvement is that the addition of Y can restrain the formation of crystalline phases and scavenge oxygen from the undercooled liquid [11]. Recently, the excellent enhancement in GFA by the addition of Y has been also found in Cu-based BMGs [12]. As reported, the fully amorphous Cu46Zr47-xAl7Yx (0≤x≤10) BMGs with diameter from 4 up to 10 mm could be easily prepared by an injection mold casting.
Compared to Zr-, Pd-, Fe- and Ti-based BMGs [1,2, 13-15], Cu-based BMGs with high GFA are more promising for industrial applications because of their relatively low cost and no toxic element like Be or Ni [16-20]. To promote their applications, the combination of high GFA and excellent mechanical properties of Cu-based BMGs is expected. However, in the previous work, it was found that the fracture strength of a Zr-based BMG decreased with increasing Y content [21]. Up to now, the effects of Y on the mechanical properties of Cu-based BMGs have not been clearly clarified. Therefore, it is valuable to reveal the effects of Y on the structural and mechanical properties of CuZrAl BMGs. In the present work, Cu45Zr48Al7 and Cu46Zr42Al7Y5 BMGs were prepared. The effects of Y on the GFA, thermal stability and fracture strength were studied. The difference in the fracture surface and local-order microstructure induced by Y was also studied by SEM and HRTEM.
2 Experimental
The alloy ingots of Cu45Zr48Al7 and Cu46Zr42Al7Y5 were prepared by arc melting the mixtures of Cu, Zr, Al and Y metals in a Ti-gettered argon atmosphere. All elements used are of >99.95% purity. Each ingot was melted at least five times to ensure the homogeneity. The alloy ingots were remelted in a fused glass tube under a vacuum level of about 5×10-3 Pa and then injection cast with ultrahigh purity argon into a copper mold to prepare cylindrical rods with size of d3 mm×100 mm. X-ray diffraction (XRD) with Cu Kα radiation for phase identification was performed on the as-cast samples via θ-2θ scans. The thermal stability of the as-cast samples was examined by differential scanning calorimetry (DSC) at a constant heating rate of 20 K/min in argon atmosphere using a Netzsch STA 449C device. Room temperature compression tests were carried out with a WDW-200D machine under a maximum load of 200 kN at an engineering strain rate of 5×10-4 s-1. The cross-sectional surfaces of the Cu45Zr48Al7 and Cu46Zr42Al7Y5 samples were examined using a S-3400N II scanning electron microscope (SEM). Specimens for transmission electron microscopy (TEM) were prepared by standard twin-jet electrolytic thinning with a HNO3-CH3OH electrolyte (volume ratio of 7:3). The microstructure was observed by TEM on a JEM-200CX instrument.
3 Results and discussion
Figure 1 shows the XRD patterns of the as-cast Cu45Zr48Al7 and Cu46Zr42Al7Y5 alloys. It can be seen that the patterns of the two alloys exhibit only a broad diffraction maximum without any observable crystalline peaks, demonstrating the formation of a fully amorphous structure.
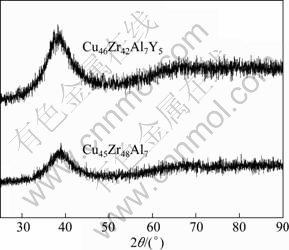
Fig. 1 XRD patterns of as-cast Cu45Zr48Al7 and Cu46Zr42Al7Y5 alloys
The DSC curves of the as-cast Cu45Zr48Al7 and Cu46Zr42Al7Y5 samples are shown in Fig. 2. During heating, both of the samples exhibit a distinct endothermic peak due to glass transition, followed by a supercooled liquid region, and then a sharp exothermic peak due to crystallization. The glass transition temperature (Tg) and the onset temperature of crystallization (Tx) are measured to be 700 and 761 K, 678 and 759 K for Cu45Zr48Al7 and Cu46Zr42Al7Y5 alloys, respectively. The corresponding supercooled liquid region ΔT(Tx-Tg) are calculated to be 61 and 81 K, respectively. Obviously, the addition of Y expands the supercooled liquid region, indicating the improvement of the glass forming ability of CuZrAl bulk metallic glass. As shown in Fig. 2, the exothermic peaks tend to be broadened with Y addition, indicating a possible slow-down in the kinetics of crystal nucleation and growth. It is known that the glass forming ability can be strongly affected by the large negative heat of mixing among the constituent elements. The mixture heat of Y-Cu, Y-Al and Zr-Cu are -22, -31 and -23 kJ/mol, respectively [22]. Therefore, the formation of an inhomogeneous structure, such as short- or medium-range ordering clusters in the amorphous phase can be hindered by the addition of Y in CuZrAl system, which leads to the improvement of the glass forming ability of CuZrAl BMGs.
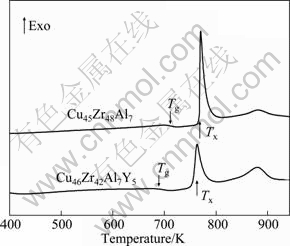
Fig. 2 DSC curves of as-cast Cu45Zr48Al7 and Cu46Zr42Al7Y5 rods at heating rate of 20 K/min
Figure 3 illustrates the room temperature compressive engineering stress—strain curves of the as-cast Cu45Zr48Al7 and Cu46Zr42Al7Y5 rods with dimensions of d3 mm×6 mm. As can be seen, both of the two samples exhibit brittle fracture without any macroscopic plastic deformation. It is seen that the ductility of CuZrAl BMGs is not improved by the addition of Y. The compressive fracture strength of Cu45Zr48Al7 and Cu46Zr48Al7Y5 samples is measured to be about 1892 and 1465 MPa, respectively. Obviously, the strength of the CuZrAl bulk metallic glass decreases with the addition of Y. In previous work, a drop in ultimate fracture strength induced by the addition of Y was also found in a Zr-based bulk metallic glass. As known, the ultimate fracture strength of BMGs is related to the binding energy of constituent elements [23]. For BMGs, the binding energy is proportional to the glass transition temperature [24]. That is, the higher Tg is, the higher the binding energy is. As shown in Fig. 2, the value of Tg decreases greatly with the addition of Y. Thus, the decrease in the fracture strength of Cu46Zr42Al7Y5 BMG can be ascribed to the decrease of the binding energy induced by Y addition.
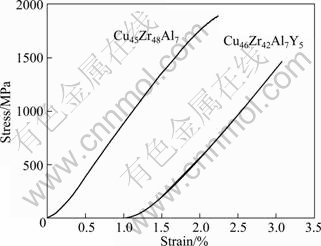
Fig. 3 Engineering stress—strain curves of Cu45Zr48Al7 and Cu46Zr42Al7Y5 bulk metallic glasses under room temperature compression
Figure 4 shows the compressive fracture surface of Cu45Zr48Al7 and Cu46Zr42Al7Y5 BMGs. It can be seen that the fracture surface is dominated by typical vein-like patterns, which indicates that local softening takes place. In addition, some intermittent smooth regions are also observed on the surface. However, as shown in Fig. 4(b), the fracture surface of Cu46Zr42Al7Y5 BMG is characterized by smooth featureless regions with larger spaced striations on the surface, which form during the fast crack propagation. Generally, the fracture surface of BMGs contains vein-like patterns, river-like patterns and smooth regions. The vein-like patterns are indicative of the ductile fracture in BMGs [25,26]. Based on the changes in the fracture morphology induced by Y, it can be concluded that the Cu46Zr42Al7Y5 BMG is more brittle than Cu45Zr48Al7 BMG, which is also supported by the fact that the samples of Cu46Zr42Al7Y5 BMG shatter into dozens of pieces when compressed.
To understand the different brittle fracture behavior of Cu45Zr48Al7 and Cu46Zr42Al7Y5 BMGs, TEM observation was carried out to reveal the difference in their microstructures. Figure 5(a) shows the HRTEM image of the Cu45Zr48Al7 BMG exhibiting the microstructure of nanometer-sized crystalline phase embedded in the amorphous matrix. Moreover, the selected-area diffraction electron pattern (inset in Fig. 5(a)) presents strong diffraction spots on the background of halo rings, indicating a composite microstructure of nanocrystalline phases dispersed in amorphous matrix. However, the TEM image of Cu46Zr42Al7Y5 BMG demonstrates a uniform amorphous structure within the resolution of the TEM adopted, as shown in Fig. 5(b). The corresponding selected-area electron diffraction inserted in Fig. 5(b) presents only halo diffraction intensity, which further supports the fully amorphous structure of Cu46Zr42Al7Y5 BMG. From the differences in microstructure of Cu45Zr48Al7 and Cu46Zr42Al7Y5 BMGs, it can be concluded that the formation of nanometer-sized clusters in CuZrAl BMGs is hindered by the addition of Y, which leads to the improvement of GFA and the formation of fully amorphous structure. The more brittle nature of Cu46Zr42Al7Y5 BMGs may be related to the fully amorphous structure.
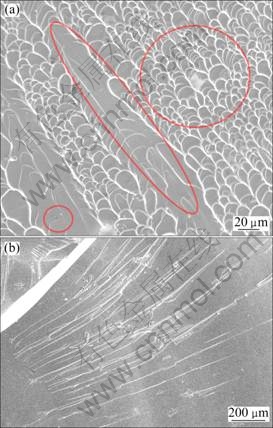
Fig. 4 SEM images of compressive fracture surface of Cu45Zr48Al7 (a) and Cu46Zr42Al7Y5 (b) BMGs
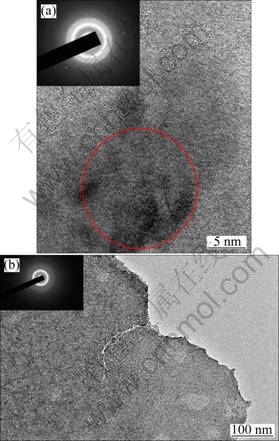
Fig. 5 HRTEM images of Cu45Zr48Al7 (a) and Cu46Zr42Al7Y5 (b) BMGs and corresponding selected-area electron diffraction patterns
4 Conclusions
1) Cu45Zr48Al7 and Cu46Zr42Al7Y5 bulk metallic glasses are prepared by copper mold melting method. Compared to Cu45Zr48Al7 bulk metallic glass, the Tg of Cu46Zr42Al7Y5 BMGs decreases and the supercooled liquid region widens, indicating the improvement of GFA due to Y addition.
2) The fracture strength decreases after Y addition due to the reduction in binding energy. The fracture surface is dominated by vein-like patterns in Cu45Zr48Al7 BMG, however, it changes to smooth regions in Cu46Zr42Al7Y5 BMGs, indicating Cu46Zr42Al7Y5 BMG is more brittle than Y-free Cu45Zr48Al7 BMG.
3) The TEM results show that Cu45Zr48Al7 BMG has a composite microstructure of nanocrystalline phases dispersed in amorphous matrix. However, the Cu46Zr42Al7Y5 BMG exhibits a fully amorphous structure. It is suggested that the improvement in GFA and decrease of mechanical properties induced by Y addition in CuZrAl-based BMGs should be carefully balanced.
References
[1] INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J]. Acta Mater, 2000, 48(1): 279-306.
[2] SCHUH C A, HUFNAGEL T C, RAMAMURTY U. Mechanical behavior of amorphous alloys [J]. Acta Mater, 2007, 55(12): 4067-4109.
[3] WU Y, XIAO Y H, CHEN G L, LIU C T, LU Z P. Bulk metallic glass composites with transformation mediated work-hardening and ductility [J]. Adv Mater, 2010, 22(25): 2770-2773.
[4] LIU L, QIU C L, CHEN Q, ZHANG S M. Corrosion behavior of Zr-based bulk metallic glasses in different artificial body fluids [J]. J Alloys Compd, 2006, 425(1-2): 268-273.
[5] CORNELISON S G, ZHAO J G, SELLMYER D J. Rare earth gallium iron glasses (II): Anomalous magnetic hysteresis in alloys based on Pr, Nd and Sm [J]. Phys Rev B, 1984, 30(5): 2857-2865.
[6] MATTERN N, VAINIO U, PARK J M, HAN J H, SHARIQ A, KIM D H, ECKERT J. Phase separation in Cu46Zr47-xAl7Gdx metallic glasses [J]. J Alloys Compd, 2010, 509(S1): s23-s26.
[7] ZHANG Y, CHEN J, CHEN G L, LIU X J. Glass formation mechanism of minor yttrium addition in CuZrAl alloys [J]. Appl Phys Lett, 2006, 89(13): 131904-1-3.
[8] LU Z P, LIU C T, THOMPSON J R, PORTER W D. Structural amorphous steels [J]. Phys Rev Lett, 2004, 92(24): 245503-1-4.
[9] LU Z P, LIU C T, PORTER W D. Role of yttrium in glass formation of Fe-based bulk metallic glasses [J]. Appl Phys Lett, 2003, 83(13): 2581-2584.
[10] HAO G J, REN F, ZHANG Y, LIN J P. Role of yttrium in glass formation of Ti-based bulk metallic glasses [J]. Rare Metals, 2009, 28(1): 68-71.
[11] LU Z P, LIU C T. Glass formation criterion for various glass-forming systems [J]. Phys Rev Lett, 2003, 91(11): 115505-1-4.
[12] XU H, DUAN G, JOHNSON W L. Unusual glass-forming ability of bulk amorphous alloys based on ordinary metal copper [J]. Phys Rev Lett, 2007, 92(24): 245504-1-4.
[13] SUN G Y, CHEN G, CHEN G L. Comparison of microstructures and properties of Zr-based bulk metallic glss composites with dendritic and spherical bcc phase precipitates [J]. Intermetallics, 2007, 15(5-6): 632-634.
[14] CHENG J L, CHEN G, XU F, DU Y L, LI Y S, LIU C T. Correlation of the microstructure and mechanical properties of Zr-based in-situ bulk metallic glass matrix composites [J]. Intermetallics, 2010, 18(12): 2425-2430.
[15] SUN G Y, CHEN G, LIU C T, CHEN G L. Innovative processing and property improvement of metallic glass based composites [J]. Scripta Materialia, 2006, 55: 375-378.
[16] INOUE A, ZHANG W, ZHANG T, KUROSAKA K. High-strength Cu-based bulk glassy alloy in Cu-Zr-Ti and Cu-Hf-Ti ternary systems [J]. Appl Phys Lett, 2001, 49(14): 2645-2652.
[17] LOUZGUINE D V, KATO H, INOUE A. High-strength Cu-based crystal-glassy composite with enhanced ductility [J]. Appl Phys Lett, 2004, 84(7): 1088.
[18] PAULY S, DAS J, BEDNARCIK J, MATTERN N, KIM K B, KIM D H, ECKERT J. Deformation induced martensitic transformation in Cu-Zr-(Al, Ti) bulk metallic glass composites [J]. Scripta Mater, 2009, 60(6): 431-434.
[19] KOVACS Z S, REVESZ A. Effect of local ordering on the decomposition of an amorphous CuZrTi alloy [J]. Appl Phys Lett, 2005, 87(25): 251909-1-3.
[20] SUN B A, YU H B, JIAO W, BAI H Y, ZHAO D Q, WANG W H. Plasticity of ductile metallic glasses: A self-organized critical state [J]. Phys Rev Lett, 2010, 105(3): 035501-1-4.
[21] YAN M, ZOU J, SHEN J. Effect of over-doped yttrium on the microstructure, mechanical properties and thermal properties of a Zr-based metallic glass [J]. Acta Mater, 2006, 54(13): 3627-3635.
[22] TAKEUCHI A, INOUE A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element [J]. Mater Trans, 2005, 46(12): 2817-2829.
[23] LI J, NGAN A H W, GUMBSCH P. Atomistic modeling of mechanical behavior [J]. Acta Mater, 2003, 51(19): 5711-5742.
[24] CHEN H S. Metallic glasses [J]. Mater Sci Eng, 1976, 25: 59-69.
[25] ZHANG Z F, ECKERT J, SCHULTZ L. Difference in compressive and tensile fracture mechanisms of Zr59Cu20Al10Ni8Ti3 bulk metallic glass [J]. Acta Mater, 2003, 51(4): 1167-1179.
[26] PAN J, LIU L, CHAN K C. Enhanced plasticity by phase separation in CuZrAl bulk metallic glass with micro-addition of Fe [J]. Scripta Mater, 2009, 60(9): 822-825.
Y元素添加对CuZrAl块体金属玻璃的结构和力学性能的影响
许宏伟1, 2,杜宇雷1, 2,邓 昱3
1. 南京理工大学 材料评价与设计教育部工程研究中心,南京 210094;
2. 江苏省(丹阳)高性能合金材料研究院,丹阳 212300;
3. 南京大学 固态微结构国家重点实验室,现代分析中心,南京 210093
摘 要:研究添加Y元素对CuZrAl块体金属玻璃的结构和力学性能的影响。结果表明,添加Y元素提高CuZrAl体系的玻璃形成能力,而且由于添加Y元素可以降低该体系的结合能,从而降低其断裂强度。Cu45Zr48Al7块体金属玻璃的断裂表面主要呈脉状,而Cu46Zr42Al7Y5块体金属玻璃的断裂表面则很平滑。TEM观察表明,Cu45Zr48Al7的微观结构为非晶基体中含有纳米相,然而Cu46Zr42Al7Y5块体金属玻璃为全非晶结构。
关键词:块体金属玻璃;Cu基合金;Y 添加
(Edited by FANG Jing-hua)
Foundation item: Project (2010ZDJH10) supported by the NUST Research Funding; Project (BK2007213) supported by the Natural Science Foundation of Jiangsu Province, China
Corresponding author: DU Yu-lei; Tel: +86-25-84315159; Fax: +86-25-84315159; E-mail: yldu_njust@mail.njust.edu.cn
DOI: 10.1016/S1003-6326(11)61254-5
Abstract: The effects of Y addition on the structural and mechanical properties of CuZrAl bulk metallic glass (BMG) were studied. The results show that the glass forming ability of CuZrAl system is improved by the addition of Y and the fracture strength decreases with Y addition due to the reduction of binding energy induced by Y. The fracture surface is dominated by vein-like patterns in Cu45Zr48Al7 bulk metallic glass, and changes to smooth regions in Cu46Zr42Al7Y5 BMG. TEM observation shows that Cu45Zr48Al7 BMG has a composite microstructure of nanocrystalline phases dispersed in amorphous matrix. However, the Cu46Zr42Al7Y5 BMG shows a fully amorphous structure.


