微观组织和pH对Mg-Gd-Y-Zr镁合金酸雨腐蚀的影响
张新明,易建龙,邓运来,刘杰,唐昌平,李理
(中南大学 材料科学与工程学院,湖南 长沙,410083)
摘 要:
摘 要:为探求Mg-Gd-Y -Zr合金板在酸雨环境下的腐蚀情况,采用析氢法、极化曲线、交流阻抗、扫描电镜、X线衍射仪等手段研究Mg-Gd-Y-Zr T6态合金板在不同pH酸雨中的腐蚀行为。研究结果表明:酸雨pH越低,腐蚀孕育期愈短,腐蚀电位负移,该合金板的腐蚀速率愈大;合金板在酸雨中浸泡初期腐蚀速率较快,以局部腐蚀为主;腐蚀孕育期镁合金板中α相溶解及腐蚀产物的堆积导致合金腐蚀速率减慢,表现为均匀腐蚀;当具有部分保护作用的氢氧化镁膜破裂时,腐蚀加速,出现大量点蚀坑;初期的腐蚀产物主要是氢氧化镁和氧化镁,随空气中二氧化碳在酸雨中的溶解,最终形成花瓣状的Mg5(CO3)4(OH)2?5H2O。
关键词:
中图分类号:TQ050.9 文献标志码:A 文章编号:1672-7207(2010)06-2138-05
Influence of microstructure and pH on corrosion behavior of
Mg-Gd-Y-Zr magnesium alloy plate in acid rain
ZHANG Xin-ming, YI Jian-long, DENG Yun-lai, LIU Jie, TANG Chang-ping, LI Li
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The corrosion behavior of Mg-Gd-Y-Zr T6 magnesium alloy plate in the stimulated acid rain was investigated by means of hydrogen evolution technology, polarization curve, electrochemical impedance spectroscopy, scanning electronic microscopy and X-ray diffractrometry. The shorter incubation period to the onset of corrosion, the more negative corrosion potential and the lower pH the higher corrosion rate value in stimulated acid rain is. The magnesium alloy plate prefers to local corrosion with high corrosion rate at initial stages. During corrosion incubation period, α-matrix dissolves preferentially while the second phase particles are left on the surface because they are inert to the corrosion attack. Corrosion rate slows down because the corrosion products pile up on the alloy plate surface. When the partially protective surface film is broken, corrosion rate of the plate is accelerated with a lot of pitting in the surface. The corrosion products contain magnesium hydroxide and magnesia. In the presence of carbon dioxide they are turned into Mg5(CO3)4(OH)2?5H2O.
Key words: magnesium alloy; acid rain; corrosion
镁稀土合金具有高强耐热等优异性能,在航空航天等领域有广阔的应用前景[1]。但是,镁合金耐蚀性能差,其应用受到严重制约。最近人们开始关注镁合金的腐蚀研究,Ben-Hamu等[2]发现镁稀土合金中富锆部分表现出更好的耐蚀性能。Chang等[3]认为Mg-Gd-Y-Zr合金的耐蚀性能与析出相β′的含量密切相关。随着经济发展,大气污染日趋严重,酸雨对结构材料造成极大的腐蚀破坏,造成重大经济损失和安全事故。中国已成为继欧洲和北美之后世界第三大重酸雨区,中国的酸雨区面积占国土面积的1/3,目前中国的酸雨正在发展,酸雨面积正在扩大,降雨酸度也在提高[4]。人们对于铝、铜、锌等有色金属的大气腐蚀已有大量研究[5-8],有关镁锌系镁合金大气腐蚀也有报道,Lindstrom等[9]在实验室研究了NaCl和CO2对AZ91镁合金大气腐蚀的影响;Jonsson等[10]研究了农村、城市和海洋3种大气环境对AZ91D镁合金的腐蚀。但目前镁稀土合金的酸雨腐蚀还未见报道。为此,本文作者探求镁稀土合金在酸雨环境中的腐蚀行为,以便为研究该材料在酸雨环境下的服役性能和失效提供理论指导。
1 实验
实验材料为自行制备的Mg-Gd-Y-Zr合金T6态板材,该合金的化学成分(质量分数)为:Gd 8.11%, Y 3.28%,Zr 0.51%,余量为Mg。镁合金样品分别经400号、800号、1200号金相砂纸打磨并抛光,用环氧树脂封样仅留1个工作面,用氢氧化钠溶液清洗,二次蒸馏水清洗,风干,再用丙酮浸泡5 min,二次蒸馏水清洗,风干待用。酸雨的pH采用硫酸和氢氧化钠调节。极化曲线和交流阻抗在CHI660C上测得,参比电极为饱和甘汞电极,对电极为不锈钢电极,镁稀土合金为工作电极。极化时扫描速率为1 mV/s。交流阻抗均是在开路电位稳定以后测量,扰动信号为5 mV,测量频率从100 kHz到0.01 Hz。X线衍射分析在日本D/max 2000型18 kW转靶X线衍射仪上完成。光电管功率为40 kV,300 mA;铜靶,取样步宽0.02°,合金中的组织通过Sirion 200场发射电镜观察,利用附带的Genesis 60s能谱分析仪进行微区成分分析。析氢试验的方法和原理与文献[11]中的相同。模拟酸雨的成分如表1所示。
表1 模拟酸雨离子质量浓度
Table 1 Ion concentration of solution employed as simulated acid rain mg/L

2 结果与分析
2.1 pH对镁稀土合金酸雨腐蚀的影响
容量法测定镁合金的腐蚀速率具有灵敏度高、操作简便等特点,析氢速率与腐蚀速率可以通过下式进行转换[12]:
vi = 2.279 vH (1)
式中:vi为腐蚀速率,mm/a;vH为析氢速率,mL/(cm2?d)。当pH分别为3.5和4.5时,该镁稀土合金在313 K的模拟酸雨中浸泡前4 d的平均腐蚀速率分别为 0.64和0.36 mm/a。Mg-Gd-Y-Zr T6态合金板在酸雨中析氢体积与浸泡时间的关系及极化曲线如图1所示。由图1(a)可见:在温度、离子浓度等其他条件完全相同的情况下,析氢体积随着时间延长而持续增加,说明Mg-Gd-Y-Zr合金在酸雨环境中不稳定。根据Mg-H2O体系电位-pH曲线,样品表面形成的氢氧化镁膜在溶液中pH低于10.5时不稳定。样品经1 d浸泡之后析氢平均速率由快变慢,可见该合金板在酸雨腐蚀过程中形成了一层有部分保护作用的膜;酸雨pH越低,腐蚀孕育期愈短,容易使具有部分保护作用的氢氧化镁膜破裂,该合金的腐蚀速率也更快。当酸雨的pH由4.5降低到3.5时,该合金的腐蚀电位负移约48 mV(见图1(b)),说明该合金在酸性强的溶液中更不稳定,溶液酸性越强越容易导致合金表面膜层局部破裂,表现出很明显的点蚀现象。
图2所示为Mg-Gd-Y-Zr T6态合金板在313 K,pH为3.5和4.5的酸雨中极化电阻与浸泡时间的关系,并采用文献[13]中的等效电路图拟合所得。腐蚀电流密度J可以通过Stern-Geary 方程获得[11]:
![]() (2)
(2)
其中:![]() 。K与金属和电解质的性质有关,腐蚀电流密度与极化电阻成反比。腐蚀速率通过下式进行转换:
。K与金属和电解质的性质有关,腐蚀电流密度与极化电阻成反比。腐蚀速率通过下式进行转换:
vi = 22.85 Jcorr (3)
其中:vi为腐蚀速率,mm/a;Jcorr为腐蚀电流密度,mA/cm2。从图2可见:样品在酸雨中浸泡时间越长,极化电阻越大,其腐蚀电流密度不断减少,开始反应速率比较快随后逐渐减慢;随着pH升高,极化电阻也增加,相应的腐蚀速率下降,其结果与图1所示结果完全一致。
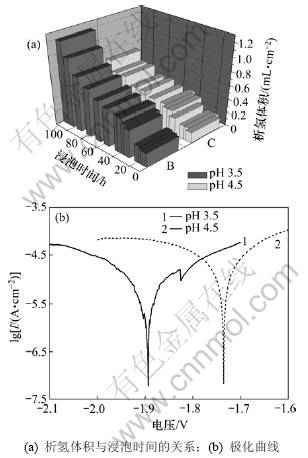
图1 Mg-Gd-Y-Zr T6态合金板在酸雨中析氢体积与浸泡时间的关系和极化曲线
Fig.1 Relationships between hydrogen evolution volume and immersion time, and polarization curve of Mg-Gd-Y-Zr magnesium alloy plate (T6) in simulated acid rain
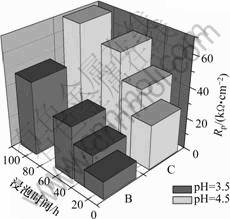
图2 Mg-Gd-Y-Zr T6态合金板在313 K的酸雨中极化电阻与浸泡时间的关系
Fig.2 Relationships between polarization resistance and immersion time in simulated acid rain for Mg-Gd-Y-Zr magnesium alloy plate (T6)
2.2 微观组织对镁稀土合金酸雨腐蚀的影响
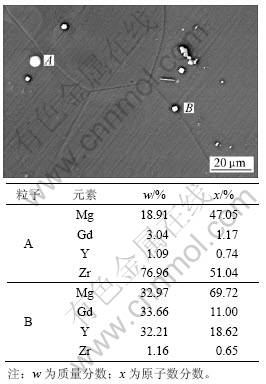
Mg-Gd-Y-Zr镁合金的微观组织如图3所示。由图3可知:合金由基体α相和第二相粒子等组成;第二相粒子的形貌为白色,主要由圆形富锆粒子和方块状富钇和钆粒子组成。在实验过程中发现第二相粒子相对基体α相更稳定,在腐蚀过程中它们作为阴极,可能是这些稀土在腐蚀过程中形成了非常稳定的稀土氧化物[14],这些阴极相在酸雨溶液中非常稳定而不被腐蚀。
Song等[15]发现在腐蚀初期AZ91D镁合金在浓度为1 mol/L的NaCl溶液中腐蚀时氢气泡主要集中在沿β相边缘的一些点处,而不是全部集中在α相或β相上。Mg-Gd-Y-Zr T6态合金板在温度为313 K、pH为4.5的酸雨中浸泡1 h的金相表明腐蚀集中发生在第二相粒子与α相相邻的区域(图4(a))。腐蚀驱动力源于α相与第二相粒子之间形成的电极电位差,在浸泡初期,镁稀土合金析氢速率很快,是第二相富稀土粒子在表面分布不连续以及数量不多所致[16]。因此,在浸泡初期,第二相粒子的作用是加速腐蚀,这与析氢法所测腐蚀速率是一致的。浸泡8~48 h的形貌显示以均匀腐蚀为主,浸泡后期腐蚀速率降低主要是由于合金表面腐蚀产物的堆积引起局部pH升高导致腐蚀速率减慢。但是,酸雨长期腐蚀将破坏合金表面,形成具有部分保护能力的膜,从而出现大量点蚀坑。
 。
。
图3 Mg-Gd-Y-Zr T6态合金板微观组织的SEM像和能谱分析结果
Fig.3 SEM image and EDS result of Mg-Gd-Y-Zr magnesium alloy plate (T6)
2.3 腐蚀产物成分分析
图5所示为Mg-Gd-Y-Zr合金板经pH=4.5、温度为313 K的酸雨腐蚀4 d后腐蚀产物的SEM像和能谱分析结果。从图5(a)可见:Mg-Gd-Y-Zr合金板在pH=4.5、温度为313 K酸雨中浸泡4 d之后,表面存在大量白色花瓣状和球状的腐蚀产物。球状腐蚀产物最先在第二相粒子与α相相邻的部分成核(图5(b)),在腐蚀过程中长大呈球状(图5(c))。根据能谱结果分 析,镁和氧的物质的量比约为1?2。X线衍射谱如图6所示。图6显示有非常明显的氧化镁及氢氧化镁的衍射峰,说明其腐蚀产物主要为氢氧化镁和氧化镁,而花瓣状腐蚀产物其成分主要是碳氧镁3种元素(图5(d)),根据物质的量比计算该腐蚀产物为Mg5(CO3)4(OH)2? 5H2O,与Lindstrom等[9]报道的AZ91镁合金大气腐蚀产物相同。Mg5(CO3)4(OH)2? 5H2O主要是空气中二氧化碳溶解在酸雨中进一步与氢氧化镁反应所致。腐蚀产物成分分析结果表明不含稀土元素,说明腐蚀过程中只是α相溶解。稀土元素的加入,对镁合金的第二相粒子及其耐蚀性能的影响过程有待进一步研究。

图4 Mg-Gd-Y-Zr T6态合金板在pH=4.5,313 K酸雨中分别浸泡1, 8, 48和240 h的显微组织
Fig.4 Microstructures of Mg-Gd-Y-Zr magnesium alloy plate (T6) immersed in simulated acid rain
with pH=4.5 at 313 K for 1, 8, 48 and 240 h
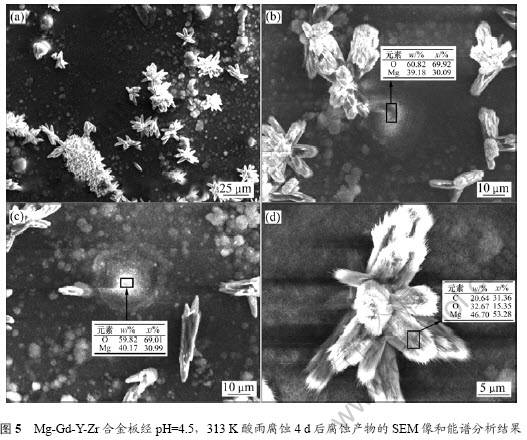
Fig.5 SEM images and EDS results of Mg-Gd-Y-Zr magnesium alloy plate microstructure in simulated acid rain
with pH=4.5 at 313 K for 4 d

图6 Mg-Gd-Y-Zr合金板经酸雨腐蚀4 d之后的X线衍射谱
Fig.6 XRD pattern of Mg-Gd-Y-Zr magnesium alloy plate in simulated acid rain with pH=4.5 at 313 K for 4 d
3 结论
(1) Mg-Gd-Y-Zr合金板在酸雨中的腐蚀不稳定,随pH从4.5降低到3.5,腐蚀电位负移约48 mV,腐蚀速率加快。浸泡前期酸雨中合金样品的极化电阻随时间延长而增大,说明其表面膜层有部分保护作用。
(2) 微观结构对于Mg-Gd-Y-Zr合金在酸雨中的腐蚀行为具有决定作用。在腐蚀过程中基体α相溶解,第二相粒子作为阴极相不溶解,腐蚀产物在表面堆积导致合金样品的腐蚀速率下降。
(3) Mg-Gd-Y-Zr合金在酸雨中最先形成氧化镁和氢氧化镁,随空气中二氧化碳在酸雨中溶解,最终形成花瓣状的Mg5(CO3)4(OH)2?5H2O。
参考文献:
[1] 肖阳, 张新明, 陈健美, 等. Mg-9Gd-4Y-0. 6Zr 合金挤压T5 态的高温组织与力学性能[J]. 中国有色金属学报, 2006, 16(4): 709-714.
Xiao Yang, Zhang Xin-ming, CHEN Jian-mei, et al. Microstructures and mechanical properties of extruded Mg-9Gd-4Y-0.6Zr-T5 at elevated temperatures[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(4): 709-714.
[2] Ben-Hamu G, Eliezer D, Shin K S, et al. The relation between microstructure and corrosion behavior of Mg-Y-RE-Zr alloys[J]. Journal of Alloys and Compounds, 2007, 431(1/2): 269-276.
[3] Chang Jian-wei, Guo Xing-wu, He Shang-ming, et al. Investigation of the corrosion for Mg-xGd-3Y-0.4Zr (x= 6, 8, 10, 12 wt%) alloys in a peak-aged condition[J]. Corrosion Science, 2008, 50(1): 166-177.
[4] 张学元, 韩恩厚, 李洪锡, 等. 中国的酸雨对材料腐蚀的经济损失估算[J]. 中国腐蚀与防护学报, 2002, 22(5): 316-319.
ZHANG Xue-yuan, HAN En-hou, LI Hong-xi, et al. Estimation of the corrosion losses by the acid rain in China[J]. Journal of Chinese Society for Corrosion and Protection, 2002, 22(5): 316-319.
[5] Antonio R. Mendoza F C. Outdoor and indoor atmospheric corrosion of non-ferrous metals[J]. Corrosion Science, 2000, 42(7): 1123-1147.
[6] Vera R, Delgado D, Rosales B M. Effect of atmospheric pollutants on the corrosion of high power electrical conductors: Part 1. Aluminium and AA6201 alloy[J]. Corrosion Science, 2006, 48(10): 2882-2900.
[7] SUN Shuang-qing, ZHENG Qi-fei, LI De-fu, et al. Long-term atmospheric corrosion behaviour of aluminium alloys 2024 and 7075 in urban, coastal and industrial environments[J]. Corrosion Science, 2009, 51(4): 719-727.
[8] El-Mahdy G A, Kim K B. Ac impedance study on the atmospheric corrosion of aluminum under periodic wet-dry conditions[J]. Electrochimica Acta, 2004, 49(12): 1937-1948.
[9] Lindstrom R, Johansson L G. The influence of NaCl and CO2 on the atmospheric corrosion of magnesium alloy AZ91[J]. Materials and Corrosion, 2003, 54(8): 587-594.
[10] Jonsson M, Persson D, Leygraf C. Atmospheric corrosion of field-exposed magnesium alloy AZ91D[J]. Corrosion Science, 2008, 50(5): 1406-1413.
[11] Yi Jian-long, Zhang Xin-ming, Chen Ming-an, et al. Effect of Na2CO3 on corrosion resistance of cerium-based conversion coatings on Mg-Gd-Y-Zr magnesium alloys[J]. Scripta Materialia, 2008, 59(9): 955-958.
[12] Zhao Ming-chun, liu Ming, Song Guang-ling, et al. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41[J]. Corrosion Science, 2008, 50(11): 3168-3178.
[13] Yi Jian-long, Zhang Xin-ming, Chen Ming-an, et al. Corrosion resistance of cerium conversion film electrodeposited on Mg-Gd-Y-Zr magnesium alloy[J]. Journal of Central South University of Technology, 2009, 16(1): 38-42.
[14] Ben-Hamu G, Eliezer D, Shin K S. The relation between microstructure and corrosion behavior of Mg-Y-RE-Zr alloys[J]. Journal of Alloys and Compounds, 2007, 431(1/2): 269-276.
[15] Song Guang-ling, Atrens A. Influence of microstructure on the corrosion of diecast AZ80D[J]. Corrosion Science, 1999, 41(1): 138-162.
[16] Song Guang-ling. Understanding magnesium corrosion—A framework for improved alloy performance[J]. Advanced Engineering Materials, 2003, 5(12): 837-857.
(编辑 陈爱华)
收稿日期:2009-10-15;修回日期:2009-12-08
基金项目:国家安全重大基础研究项目(5133001E)
通信作者:易建龙(1975-),男,湖南湘潭人,博士研究生,从事镁合金的腐蚀防护研究;电话:0731-88830265; E-mail: yijianlong@126.com


