
Effect of mole ratio of Y to Zn on phase constituent of Mg-Zn-Zr-Y alloys
LUO Su-qin1, TANG Ai-tao1, 2, PAN Fu-sheng1, 2, SONG Kai1, WANG Wei-qing1
1. College of Materials Science and Engineering, Chongqing University, Chongqing 400030, China;
2. National Engineering Research Center for Magnesium Alloys, Chongqing University, Chongqing 400030, China
Received 25 September 2010; accepted 25 December 2010
Abstract:
The phase constituent evolution of Mg-Zn-Y-Zr alloys with the mole ratio of Y to Zn both in the as-cast and as-annealed states at the Mg-rich corner was investigated by XRD and SEM/EDS analysis and was further explained from the ternary phase diagram calculation. The results show that the formation of the secondary phases in Mg-Zn-Y-Zr alloys firmly depends on the mole ratio of Y to Zn, and X (Mg12YZn)-phase, W (Mg3Y2Zn3)-phase and I (Mg3YZn6)-phase come out in sequence as the ratio of Y to Zn decreases. The mole ratios of Y to Zn with the corresponding phase constituent are suggested quantitatively as follows: the phase constituent is α-Mg + I when the mole ratio of Y to Zn is about 0.164; α-Mg + I +W when the mole ratio of Y to Zn is in the range of 0.164-0.33;α-Mg +W when the mole ratio of Y to Zn is about 0.33; α-Mg +W+X when the mole ratio of Y to Zn is in the range of 0.33-1.32; and α-Mg +X when the mole ratio of Y to Zn is about 1.32. The results also offer a guideline for alloy selection and alloy design in Mg-Zn-Y-Zr system.
Key words:
magnesium alloy; Mg-Zn-Zr-Y; mole ratio; phase constituent;
1 Introduction
As the lightest metal structural materials, magnesium alloys are being used to speed up the process of lightweight in automotive, motorcycle and aerospace field due to their high specific strength, good stiffness and low density[1]. The Mg-Zn system with small addition of alloying elements is one of the most popular commercial wrought magnesium alloys. It was reported that the Y addition to Mg-Zn based alloys could not only increase the eutectic temperature of Mg-Zn-Y alloy significantly but also form several secondary phases[2], and had great effects on the mechanical properties. Particularly, BAE et al[3-4] pointed out that the Mg-Zn-Y alloys containing icosahedral phase (I-phase) as a secondary solidification phase exhibited good mechanical properties at room temperature as well as at elevated temperature. To the reason why the icosahedral phase (I-phase) can promote the properties of magnesium alloys, LEE et al[5] explained that it was due to the unique intrinsic properties of I-phase, such as higher hardness and strength, the orientation relationship with α-Mg matrix and low interfacial energy with the matrix. While XU et al[6] indicated that W-phase had no grain refinement on Mg-Zn-Y-Zr alloys, and W-phase would form obviously coarsened net-like microstructure at the grain boundaries and degrade the mechanical properties greatly when the volume fraction of W-phase exceeded 17.5%. Therefore, not all the secondary phases have positive effects on the mechanical properties. So, it is essential to reveal the phase constituent for the Mg-Zn-Y-Zr system alloys.
There are three kinds of ternary equilibrium phases in Mg-Zn-Y alloys in the Mg-rich region, which are X-phase (Mg12YZn)[2], W-phase (Mg3Y2Zn3, cubic structure)[2] and I-phase (Mg3YZn6, icosahedral quasicrystal structure, quasi-periodically ordered)[7]. Since Zr addition has no effect on the phase constituent, generally, the ternary equilibrium phases in Mg-Zn-Y-Zr system are the same with the Mg-Zn-Y system. The previous investigations indicated that the phase constituent of the Mg-Zn-Y-Zr alloys closely dependent on the Zn/Y ratio of the alloys. LEE et al[8] investigated the effect of Zn/Y ratio (from 0.018 to 0.10, mass ratio) on the formation of the I and I+W phases of as-cast Mg-Zn-Y alloys with total solute content (Zn and Y) less than 10% (mass fraction). HUANG et al[9] indicated that the dominating secondary phases in the Mg-Zn-Y-Zr alloys varied with Zn/Y ratio, and X-phase was formed during solidification in a low Zn/Y ratio and W-phase came out when Zn/Y ratio grew to 0.85(mass ratio). However, there were no reports about the effect of Y/Zn ratio on the phase constituent of α-Mg +W+X and even the reported Y/Zn ratio for X-phase precipitation was not quantitative. And there was no reasonable explanation for the relationship between the Y/Zn ratio and the corresponding phase constituent. Therefore, in the present work, the phase constituent evolution of Mg-Zn-Y-Zr alloys with Y/Zn ratio variation in Mg-rich region was investigated systematically through experimental study, and the reasonable explanation for the relationship between the mole ratio of Y to Zn and the corresponding phase constituent was given through phase diagram calculation. Significantly, the mole ratio of Y to Zn or range of ratios quantitatively for all the phase regions involved the secondary phase according to the phase diagram calculation. Moreover, the thermodynamic database was constructed with little modification from the optimized thermodynamic parameters[10] for the Mg-Y-Zn system.
2 Experimental
Alloys with different Y/Zn ratio were prepared from Mg (purity 99.9%), Zn (purity 99.9%), Mg-30Y and Mg-31Zr master alloys in a carbon crucible under the protection of a mixed atmosphere of SF6 (10%, volume fraction) and CO2. After the melt was stirred equably and held for 10 min, it was poured into a permanent steel mould which was pre-heated to 300 °C. The samples were annealed at 420 °C (except for WZ012 at 350 °C) for 168 h (7 d), and then water quenched. The alloys were characterized by X-ray diffraction (XRD) (Dmax-1200VBX) with Cu Kα diffraction and 4(°) take-off angle and scanning electron microscopy (SEM) (Philips XL30) and using energy dispersive spectroscopy (EDS) (VANTAGE). The purpose of the annealing process was to eliminate the elemental segregation as much as possible to make the alloys approach the equilibrium state. Because the as-cast alloy WZ012 may contain I-phase and the invariant reaction to form I-phase happened at about 400-440 °C[9], the annealing temperature for alloy WZ012 was set at 350 °C.
3 Results
The XRD analysis for as-annealed alloys revealed that the phase constituent varied with the mole ratio of Y to Zn, as shown in Fig.1 and Table 1. For alloy WZ012, the main phases included α-Mg and I-phase. However, as the mole ratio of Y to Zn increased, no I-phase can be detected within the sensitivity limits of X-ray diffraction, and W-phase became the main second phases for alloys WZ029 and WZ039. With the further increase of mole ratio of Y to Zn, the main phases of the alloys were W-phase, X-phase and the α-Mg matrix, and the diffraction peak of X-phase can be detected (alloy WZ050) and was gradually intensified (alloy WZ076). When the mole ratio of Y to Zn increased to 1.50(alloy WZ150), the main phases were X-phase and α-Mg matrix. The SEM microstructure observations (Fig.2) and the EDS analysis showed the same result with the XRD analysis, except that the X-phase in alloy WZ050 could not be distinguished because of the few amount.
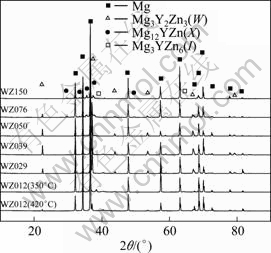
Fig.1 XRD patterns of as-annealed alloys
Table 1 Chemical composition and phase constituent of as-annealed alloys
Therefore, it can be concluded that the formation of the secondary phases in Mg-Zn-Y-Zr alloys firmly depended on the mole ratio of Y to Zn, and the X-phase, W-phase and I-phase came out in sequence as the mole ratio of Y to Zn decreased from about 1.50 to 0.12.
In order to check out whether the annealing treatment had effects on the elemental segregation, the phase constituent of alloys WZ012, WZ029 and WZ050 was compared in the as-cast state and as-annealed state. Figure 3 shows the XRD patterns for the chosen as-cast alloys. And the phase constituent for the chosen alloys in as-cast and as-annealed states was shown in Table 2. The main secondary phases in as-cast WZ012 alloy were W-phase and I-phase, which were changed to be only I-phase after the annealing treatment. And the change also happened on the alloy WZ050 (Table 2). This indicated that the annealing process indeed changed the phase constituent of the alloys, and the alloys had approached to the equilibrium state after the annealing treatment.
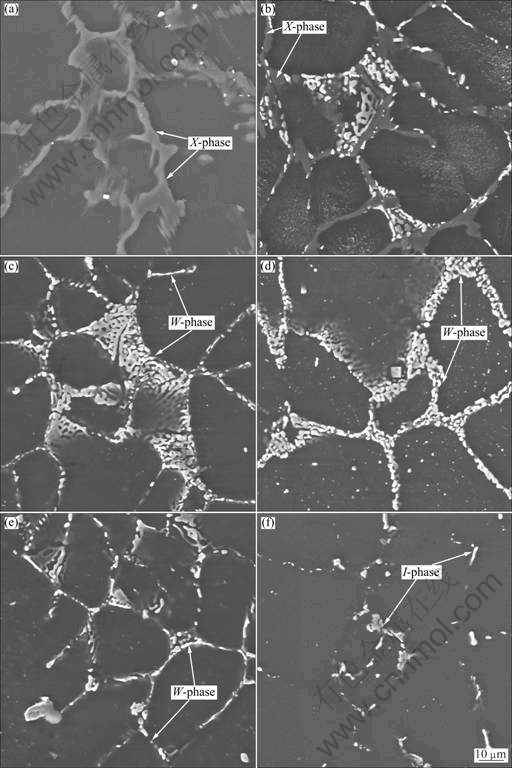
Fig.2 Microstructures of alloys WZ150 (a), WZ076 (b), WZ050 (c), WZ039 (d) and WZ039 (e) after annealing at 420 °C for 7 d, and alloy WZ012 (f) at 350 °C for 7 d
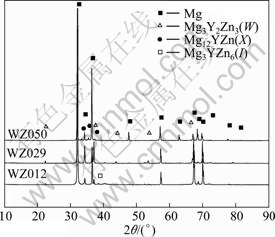
Fig.3 XRD patterns of as-cast alloys
Table 2 Phase constituent of alloys in as-cast and as-annealed states

4 Discussion
In order to understand the quantitative relationship between the determinant mole ratio of Y to Zn and the corresponding phases constituent, isothermal sections at 25 °C and 420 °C of Mg-Zn-Y ternary system were calculated using Thermo-Calc software[11] and the constructed thermodynamic database. Figure 4 shows the isothermal sections at 25 °C and 420 °C. It was noticed that the ternary phases such as X, W, I and Z (Mg28Y7Zn65)[12-13] were in equilibrium with α-Mg respectively, and the phase boundary extended to the ternary phase position from the α-Mg phase region. It was necessary to point out that the ternary phase presented a platform at high temperature (Fig.4(c)), and the platform was parallel to the Mg-Zn edge. This may be due to that there was a continuous series of solid solutions of elements Zn and Mg in these ternary phases at certain temperature. This phenomenon was a little bit general on the secondary phase in magnesium alloys [6, 14-15].
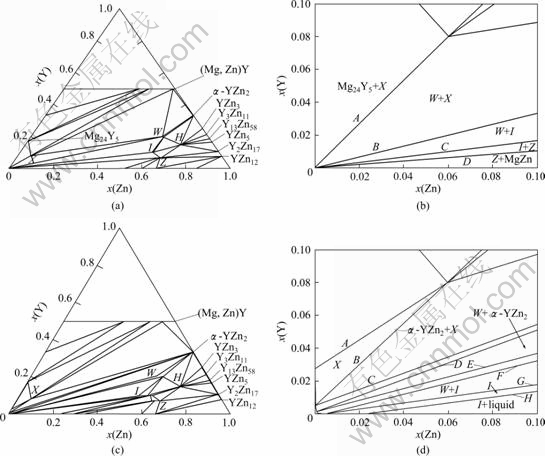
Fig.4 Calculated isothermal sections of Mg-Zn-Y system at 25 °C (a) and 420 °C (c); Enlarged part (b) and (d) for Mg-rich region of (a) and (c), respectively (The α-Mg matrix phase was not labeled and the capital letters A-H refer to the phase boundary in (b) and (d))
It can be observed from Figs.4 (b) and (d) that the mole ratio of Y to Zn was equal to the slope of the phase boundary in the Mg-rich region. The ternary phases X, W, I and Z came out in sequence from the Mg-Y edge to the Mg-Zn edge. Because the slope of the phase boundary decreased from the Mg-Y edge to the Mg-Zn edge, it can be concluded that the X-phase precipitated at high mole ratio of Y to Zn while the I-phase and Z-phase precipitated at a lower mole ratio of Y to Zn. That was consistent with our experimental study and the previous investigations[9, 16]. The slope of each phase region boundary was calculated and listed quantitatively in Table 3. It could be found that the phase constituent of the alloys was closely dependent on the Y/Zn ratio. The equilibrium phase constituent at room temperature from thermodynamic calculation was α-Mg + I(Mg3YZn6) when the mole ratio of Y to Zn was about 0.164; α-Mg + I-phase +W(Mg3Y2Zn3) when the ratio of Y to Zn was in the range of 0.164-0.33; α-Mg+W when the mole ratio of Y to Zn was about 0.33; α-Mg+W+X(Mg12YZn) when the mole ratio of Y to Zn was in the range of 0.33-1.32; and α-Mg +X when the mole ratio of Y to Zn was about 1.32. Comparing the mole ratio of Y to Zn and the corresponding phase constituent of the as-annealed alloys in this work with the results from the phase diagram calculation, it can be found that the experimental results agreed well with the results from calculation (Table 3), in other words, the information from the phase diagram calculation can explain the experimental results successfully. Additionally, the calculation results provided the information about the referenced critical mole ratio of Y to Zn and the corresponding phase constituent in the Mg-Zn-Y-Zr alloys, and also offered a guideline for alloy selection and alloy design in this system.
Table 3 Calculated mole ratio of Y to Zn and corresponding phase constituent at 25 °C
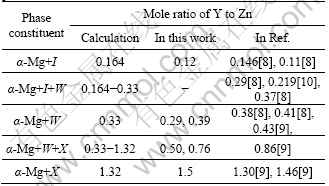
Combining the change of the phase constituent between the as-cast and as-annealed alloys (Table 2) with the phase diagram at room temperature (Fig.4(b)), it can be obviously found that the phase region for the as-cast alloys had a shift from the equilibrium phase region(Fig.4(b)) to that with a higher mole ratio of Y to Zn. For example, as for alloy WZ050, the phase constituent was α-Mg +X in the as-cast state, while was α-Mg+W+X in the as-annealed state (Table 2). According to the phase diagram calculation (Table 3), when the phase constituent was α-Mg+W+X and α-Mg+X, the mole ratios of Y to Zn are suggested to be in the range of 0.33-1.32 and about 1.32, respectively. So the phase constituent of alloy WZ050 in the as-cast state shifted to the equilibrium phase region with a higher mole ratio of Y to Zn. This was due to the un-equilibrium solidification of the as-cast alloys. When the alloys were solidified in a rapid cooling speed, the Y and Zn solute cannot be sent to the position away from the solid/liquid interface, so the enrichment of Y and Zn solute happened in front of the solid/liquid interface during un-equilibrium solidification. As that process went on, the concentration of the Y and Zn solute reached the requirement to precipitate a ternary Mg-Zn-Y phase with a higher mole ratio of Y to Zn. Therefore, the shift of the phase constituent from the ideal equilibrium state to the real as-cast state can also be easily understood from the calculated phase diagram, which also provided the general idea for the phase constituent control for Mg-Zn-Y-Zr cast alloys.
5 Conclusions
1) The formation of the secondary phases in Mg-Zn-Y-Zr alloys firmly depends on the mole ratio of Y to Zn. The X-phase, W-phase and I-phase come out in sequence as the mole ratio of Y to Zn decreases from about 1.50 to 0.12.
2) The phase constituent for the as-cast alloys has a shift from the equilibrium phase region to that of the alloys with a higher mole ratio of Y to Zn.
3) The equilibrium phase constituent at room temperature from thermodynamic calculation is α-Mg + I(Mg3YZn6) when the mole ratio of Y to Zn is about 0.164; α-Mg + I-phase +W(Mg3Y2Zn3) when the mole ratio of Y to Zn is in the range of 0.164-0.33; α-Mg +W when the mole ratio of Y to Zn is about 0.33; α-Mg +W+X(Mg12YZn) when the mole ratio of Y to Zn is in the range of 0.33-1.32; and α-Mg +X when the mole ratio of Y to Zn is about 1.32. The results from the phase diagram calculation can well explain the experimental observations.
References
[1] YANG Z, LI J P, ZHANG J X, LORIMER G W, ROBSON J. Review on research and development of magnesium alloys [J]. Acta Metallurgica Sinica (English Letters), 2008, 21(5): 313-328.
[2] PADEZHNOVA E M, MEL’NIK E V, MILIYEVSKIY R A, DOBATKINA T V, KINZHIBALO V V. Investigation of the Mg-Zn-Y system [J]. Russ Metall, 1982, 4(4): 185-188. (in Russian)
[3] BAE D H, LEE M H, KIM K T, KIM W T, KIM D H. Application of quasicrystalline particles as a strengthening phase in Mg-Zn-Y alloys [J]. J Alloys Compd, 2002, 342(1-2): 445-450.
[4] BAE D H, KIM S H, KIM D H, KIM W T. Deformation behavior of Mg-Zn-Y alloys reinforced by icosahedral quasicrystalline particles [J]. Acta Mater, 2002, 50(9): 2343-2356.
[5] LEE J Y, LIM H K, KIM D H, KIM W T, JIM D H. Effect of icosahedral phase particles on the texture evolution in Mg-Zn-Y alloys [J]. Mater Sci Eng A, 2008, 491(1-2): 349-355.
[6] XU D K, TANG W N, LIU L, XU Y B, HAN E H. Effect of W-phase on the mechanical properties of as-cast Mg-Zn-Y-Zr alloys [J]. J Alloys Compd, 2008, 461(1-2): 248-252.
[7] LUO Z P, ZHANG S Q, TANG Y L, ZHAO D S. Quasicrystals in as-cast Mg-Zn-RE alloys [J]. Scr Metall Mater, 1993, 28(12): 1513-1518.
[8] LEE J Y, KIM D H, KIM H K, LIM H K, KIM D H. Effects of Zn/Y ratio on microstructure and mechanical properties of Mg-Zn-Y alloys [J]. Materials Letters, 2005, 59(29-30): 3801-3805.
[9] HUANG Z H, LIANG S M, CHEN R S, HAN E H. Solidification pathways and constituent phases of Mg-Zn-Y-Zr alloys [J]. J Alloys Compd, 2009, 468(1-2): 170-178.
[10] SHAO G, VARSANI V, FAN Z. Thermodynamic modelling of the Y-Zn and Mg-Zn-Y systems [J]. Calphad, 2006, 30(3): 286-295.
[11] SUNDMAN B, JANSSON B, ANDERSON J O. The Thermo-Calc databank system [J]. CALPHAD, 1985, 9(2): 153-190.
[12] TSAI A P, MURAKAMI Y, NIIKURA A. The Zn-Mg-Y phase diagram involving quasicrystals [J]. Philosophical Magazine A, 2000, 80(5): 1043-1054.
[13] LI M R, DENG D W, KUO K H. Crystal structure of the hexagonal (Zn, Mg)4Ho and (Zn, Mg)4Er [J]. J Alloys Compd, 2006, 414(1-2): 66-72.
[14] LUO Su-qin, TANG Ai-tao, PAN Fu-sheng, CHEN Yi, FENG Zhong-xue. Phase diagram calculation and experimental study on the new type Mg-Al-Zn-Ca-Sr alloy in the Mg-rich region [J]. Mater Sci Forum, 2010, 650: 246-252.
[15] JANZ A, GROBNER J, SCHMID-FETZER R. Thermodynamics and constitution of Mg-Al-Ca-Sr-Mn alloys: Part II. Procedure for multicomponent key sample selection and application to the Mg-Al-Ca-Sr and Mg-Al-Ca-Sr-Mn systems [J]. Journal of Phase Equilibria and Diffusion, 2009, 30(2): 157-175.
[16] ZHANG Ya, ZENG Xiao-qin, LIU Liu-fa, LU Chen, ZHOU Han-tao, LI Qiang, ZHU Yan-ping. Effects of yttrium on microstructure and mechanical properties of hot-extruded Mg-Zn-Y-Zr alloys [J]. Mater Sci Eng A, 2004, 373(1-2): 320-327.
Y/Zn 摩尔比对 Mg-Zn-Zr-Y 合金相组成的影响
罗素琴1, 汤爱涛1, 2, 潘复生1, 2, 宋 锴1, 王维青1
1. 重庆大学 材料科学与工程学院, 重庆 400030;2. 重庆大学 国家镁合金工程技术研究中心, 重庆 400030
摘 要:采用XRD和SEM/EDS等分析方法研究铸态和退火态富镁Mg-Zn-Zr-Y合金中相组成随着Y/Zn摩尔比的变化而演变的规律,并从相图计算的角度解释这种演变规律。结果表明:Mg-Zn-Zr-Y 合金中第二相的形成严格依赖于Y/Zn摩尔比,X 相(Mg12YZn)、W相(Mg3Y2Zn3)和I相(Mg3YZn6)随着Y/Zn摩尔比的降低依次析出。与相组成对应的摩尔比或摩尔比范围定量描述如下:当Y/Zn摩尔比约为0.164时,相组成为 α-Mg + I;当Y/Zn摩尔比为0.164~0.33时,相组成为 α-Mg + I +W;当Y/Zn摩尔比约为0.33时,相组成为α-Mg +W;当Y/Zn摩尔比为0.33~1.32时,相组成为α-Mg +W+X;当Y/Zn摩尔比约为1.32时,相组成为α-Mg +X。该研究为Mg-Zn-Zr-Y合金设计和合金选用提供了指导。
关键词:镁合金;Mg–Zn–Zr–Y;摩尔比;相组成
(Edited by LI Xiang-qun)
Foundation item: Project (50725413) supported by the National Natural Science Foundation of China
Corresponding author: PAN Fu-sheng; Tel: +86-23-65112635; E-mal: fspan@cqu.edu.cn
DOI: 10.1016/S1003-6326(11)60783-8


