Trans. Nonferrous Met. Soc. China 27(2017) 1476-1482
Microstructural evolution of new type Al-Zn-Mg-Cu alloy with Er and Zr additions during homogenization
Hao WU, Sheng-ping WEN, Jun-tai LU, Zhen-peng MI, Xian-long ZENG, Hui HUANG, Zuo-ren NIE
School of Materials Science and Engineering, Beijing University of Technology, Beijing 100124, China
Received 26 April 2016; accepted 25 October 2016
Abstract:
A comprehensive study on the microstructural evolution of a new type Al-Zn-Mg-Cu-Er-Zr alloy during homogenization was conducted by optical microscope, scanning electron microscope, transmission electron microscopy and X-ray diffraction analysis. The results show that serious segregation exists in as-cast alloy, and the primary phases are T(AlZnMgCu), S(Al2CuMg) and Al8Cu4Er, which preferentially locate in the grain boundary regions. The soluble T(AlZnMgCu) and S(Al2CuMg) phases dissolve into the matrix gradually during single-stage homogenized at 465 °C with prolonging holding time, but the residual Al8Cu4Er phase cannot dissolve completely. Compared with the single-stage homogenization, both a finer particle size and a higher volume fraction of L12-structured Al3(Er,Zr) dispersoids can be obtained in the two-stage homogenization process. A suitable homogenization scheme for the present alloy is (400 °C, 10 h)+(465 °C, 24 h), which is consistent with the results of homogenization kinetic analysis.
Key words:
Al-Zn-Mg-Cu-Er-Zr alloy; homogenization; microstructural evolution; primary phases; Al3(Er,Zr) particles;
1 Introduction
The comprehensive performance improvement is an eternal pursuit in the development of aluminum alloy. High-strength 7xxx series Al-Zn-Mg-Cu alloys have been widely used for structure components in aircraft and aerospace industries due to their outstanding properties, such as high strength, fracture toughness and stress corrosion cracking resistance [1,2]. Over the past years, intensive researches have been conducted to improve the comprehensive performance of Al-Zn- Mg-Cu alloys via various methods [3]. WU et al [4,5] have carried out in-depth investigation on erbium- containing aluminium alloy and developed a new type Al-Zn-Mg-Cu alloy microalloyed with Er and Zr elements. The new type Al-Zn-Mg-Cu alloy possesses an outstanding comprehensive performance due to the formation of stable and coherent L12-structured Al3(Er,Zr) dispersoids, which can inhibit recrystallization by grain boundary pinning during subsequent high- temperature processing [6,7].
As well known, homogenization treatment, as an indispensable processing step, plays a key role in removing the microsegregation and dissolving large soluble non-equilibrium intermetallic phases formed during solidification [8-11]. In addition, a suitable homogenization scheme is crucial for the formation of fine dispersoid particles [12-14]. A large amount of work has been done on Al-Zn-Mg-Cu alloys homogenization [15,16]. LI et al [17] investigated the microstructural evolution of Al-Zn-Mg-Zr alloy with trace amount of Sc during homogenization treatment. SHI et al [18] found that the evolution of primary eutectic structure of 7085 alloy during homogenization consists of three processes: dissolution of eutectic α(Al)+T(AlZnMgCu) microstructure, phase transformation from T to S(Al2CuMg) phase and the dissolution of S phase.  et al [14] studied the distribution of Al3Zr dispersoids in commercial 7150 alloy, and concluded that two-step homogenization treatments resulted in Al3Zr dispersoids with a finer particle size and a larger number density and volume fraction. Moreover, ROBSON [19] verified that a two-step homogenization treatment can improve the dispersoid particle distribution and minimize the width of precipitation free zone in the region near the grain boundary, which results in a significant decrease in the recrystallized fraction.
et al [14] studied the distribution of Al3Zr dispersoids in commercial 7150 alloy, and concluded that two-step homogenization treatments resulted in Al3Zr dispersoids with a finer particle size and a larger number density and volume fraction. Moreover, ROBSON [19] verified that a two-step homogenization treatment can improve the dispersoid particle distribution and minimize the width of precipitation free zone in the region near the grain boundary, which results in a significant decrease in the recrystallized fraction.
In this study, in order to eliminate the large soluble non-equilibrium intermetallic phases and obtain a homogeneous distribution of L12-structured Al3(Er,Zr) dispersoids, a series homogenization schemes of the present as-cast Al-Zn-Mg-Cu-Er-Zr alloy have been conducted. Meanwhile, the microstructural evolution of the new type Al-Zn-Mg-Cu-Er-Zr alloy during homogenization treatments was investigated.
2 Experimental
The as-cast Al-Zn-Mg-Er-Zr alloy used in this study was provided by Southwest Aluminum Co., Ltd., China. The chemical composition (mass fraction, %) of the Al-Zn-Mg-Cu-Er-Zr alloy used in this study is as follows: 5.6 Zn, 2.1 Mg, 1.2 Cu, 0.1 Er, 0.1 Zr, 0.1 Mn, 0.07 Fe, 0.1 Si, and balance Al. Specimens with dimensions of 10 mm × 10 mm × 7 mm were used for homogenization treatments. Seven types of homogenization conditions were conducted as below: (465 °C, 6 h), (465 °C, 12 h), (465 °C, 24 h), (465 °C, 48 h), (465 °C, 72 h), (400 °C, 10 h) and (400 °C, 10 h)+ (465 °C, 24 h). The microstructural evolution of the as-cast and homogenized samples was characterized using Olympus BX51M optical microscope (OM), FEI Quanta 650 FEG scanning electron microscope (SEM) and JEOL 2100 transmission electron microscopy (TEM) with an operating voltage of 200 kV. The OM specimens were polished and etched with the Keller’s reagent solution at room temperature. SEM observation was carried out in the backscattered electron imaging mode, operated at 20 kV. The map scanning analyses were conducted on an energy dispersive X-ray spectrometer operated at 20 kV. X-ray diffraction (XRD) studies were performed on a D/max 2500PC diffractometer with Cu Kα1 radiation. TEM foils were prepared by mechanical polishing to less than 100 μm, subsequently punched into 3 mm discs and final twin-jet polishing with an electrolyte solution of 30% nitric acid and 70% methanol at the voltage of ~15 V DC and the temperature below -25 °C.
3 Results and discussion
3.1 Characterization of as-cast microstructure
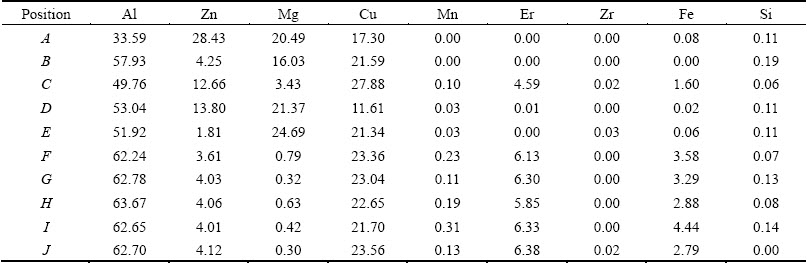
The OM and SEM microstructures of the as-cast Al-Zn-Mg-Cu-Er-Zr alloy are shown in Fig. 1. It can be seen that a large amount of coarse phases distribute along grain boundaries. Figure 1(b) shows a higher magnification image of the coarse phase, which is similar to the lamellar eutectic structure. Three zones with different composition contrasts, as shown by arrows A, B and C, were identified by energy dispersive X-ray spectrometry (EDX), and the EDX results are listed in Table 1. EDX analysis reveals that the light-grey (as indicated by A), the dark grey (B) and the bright white (C) contrast intermetallic phases can be identified as T(AlZnMgCu), S(Al2CuMg) and Al8Cu4Er type compounds, respectively. Figure 2 shows the scanning electron microstructure and the corresponding elements of Zn, Mg, Cu, Er and Zr distribution in the as-cast alloy. The main elements Zn, Mg and Cu and the microalloying element Er are enriched at grain boundaries. Therefore, a proper homogenization treatment is required to remove severe microsegregation in as-cast Al-Zn-Mg-Cu-Er-Zr alloy.
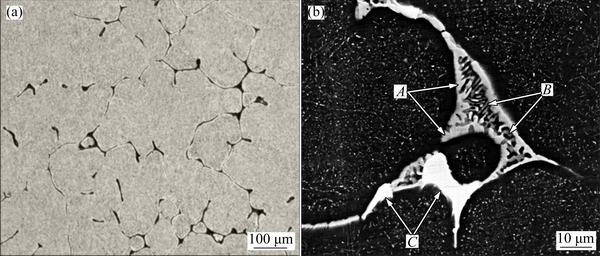
Fig. 1 OM (a) and SEM (b) images of as-cast Al-Zn-Mg-Cu-Er-Zr alloy
Table 1 EDX results of intermetallic phases in as-cast Al-Zn-Mg-Cu-Er-Zr alloy (mole fraction, %)
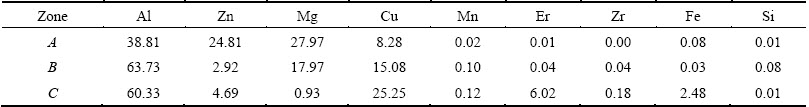
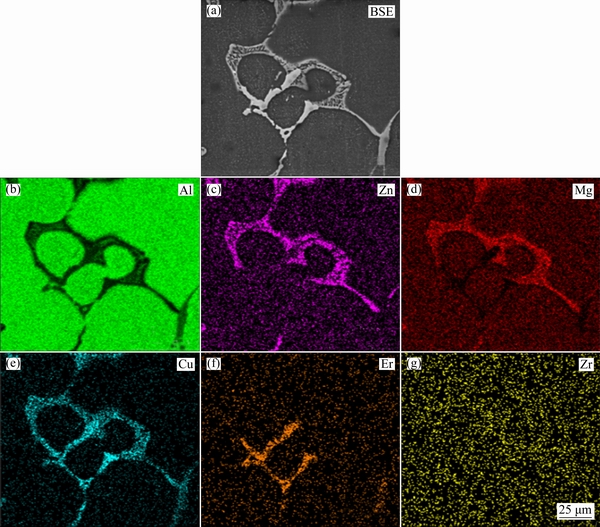
Fig. 2 SEM images and corresponding elements distribution maps of as-cast alloy
3.2 Microstructural evolution during homogenization
Figure 3 shows the evolution of statistical amount of the grain boundary phase after homogenization at 465 °C for various time. The statistics of every condition was analyzed using Image-Pro Plus software and by selecting more than 10 images at a same magnification. The grain boundary phase firstly decreased sharply within the initial 12 h, and its amount decreased slightly with further prolonging of homogenization time.
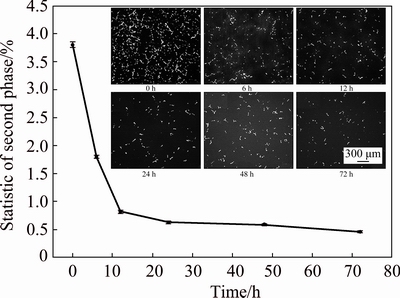
Fig. 3 Evolution of statistical amount of grain boundary phase after homogenizing at 465 °C for various time
Figure 4 displays the backscattered electron images of the residual phases under various homogenization conditions, and the corresponding EDX results of the intermetallic phases are shown in Table 2. After homogenizing at 400 °C for 10 h, three zones with different compositions contrast still exist (as arrowed A, B and C in Fig. 4(a)). According to the EDX results, the three zones A, B and C are T(AlZnMgCu), S(Al2CuMg) and Al8Cu4Er phases, respectively. The content of Cu in T(AlZnMgCu) phase in this condition is a little higher than that of T(AlZnMgCu) phase in as-cast alloy, which could be attributed to the lower diffusivity of Cu. The type of residual phase in the alloy after homogenizing at 465 °C for 6 h, as arrowed by D, E and F in Fig. 4(b), is roughly similar to that in the as-cast alloy and alloy homogenized at 400 °C for 10 h. But the continuous lamellar structures dissolved gradually and evolved to isolate particles after homogenization at 465 °C. With further prolonging of homogenization time, the T and S phases gradually dissolve into matrix and disappear finally. The residual bright white contrast phases, as arrowed in Figs. 4(c)-(f), are identified as the Al8Cu4Er phase by EDX analysis, which cannot be dissolved and eliminated completely in Al matrix during homogenization treatment at 465 °C for 48 h. This is due to the fact that its melting point is higher than the homogenization temperature.
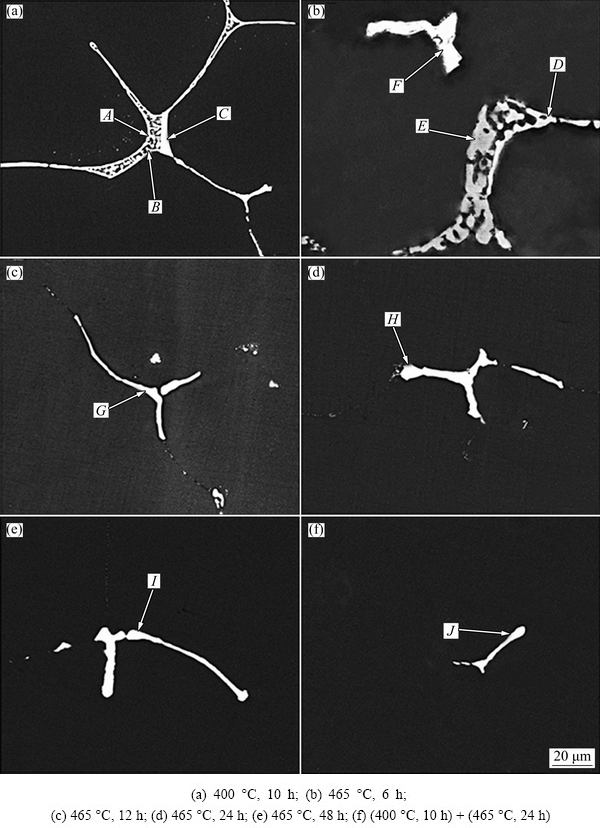
Fig. 4 Backscattered electron images of alloys under different homogenization conditions
Table 2 EDX results of intermetallic phases in Fig. 4 (mole fraction, %)
X-ray diffraction (XRD) patterns of the as-cast and homogenized alloys are shown in Fig. 5. The as-cast alloys are mainly composed of α(Al), η(MgZn2), S(Al2CuMg) phase. Compared with XRD patterns of the as-cast alloy, there is no obvious difference in the peaks of S(Al2CuMg) phase in alloy homogenized at 400 °C for 10 h. The S(Al2CuMg) phase, which formed during the non-equilibrium solidification process, has not dissolved into α(Al) matrix after 400 °C, 10 h pre-treatment. With prolonging time of homogenization treatment at 465 °C for 24 h, the peak of S(Al2CuMg) phase disappears. The Al8Cu4Er phase cannot be detected by XRD, which may be attributed to its low content in the experimental alloys. Moreover, except for α(Al), there is no obvious diffraction peak in XRD patterns after being homogenized at 465 °C for 48 h and (400 °C, 10 h) + (465 °C, 24 h).
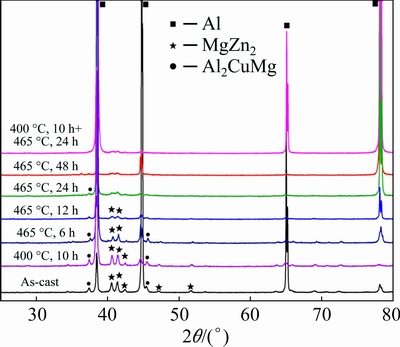
Fig. 5 XRD patterns of as-cast and homogenized alloys

Fig. 6 TEM images of as-cast and homogenized alloys
Figure 6 exhibits TEM images of the as-cast and homogenized alloys. Only rod-shape non-equilibrium T(AlZnMgCu) phase exists in the as-cast alloy, as shown in Fig. 6(a). No Al3(Er,Zr) particles can be detected in this condition. The non-equilibrium T(AlZnMgCu) phase disappears and homogeneously distributed Al3(Er,Zr) precipitates form after homogenization at 465 °C for 24 h. It can be seen from Fig. 6(c) that both finer particle size and higher number density of Al3(Er,Zr) dispersoids are obtained after homogenization in (400 °C, 10 h)+ (465 °C, 24 h) condition, because the first step held at 400 °C has an significant effect on the precipitation of Al3(Er,Zr) particles. Figure 6(d) shows the higher magnification bright field TEM image of Fig. 6(c), and the inset selected area diffraction (SAD) pattern implies that the Al3(Er,Zr) precipitates have the L12 structured lattice, as shown in the upper right inset of Fig. 6(d). The first step homogenized at 400 °C for 10 h can promote the nucleation of a large number of dispersoids out of the Al matrix due to a high Er and Zr supersaturation at low temperature. Therefore, much finer particle size and more homogenous distribution of Al3(Er,Zr) dispersoids can be obtained in (400 °C, 10 h) + (465 °C, 24 h) condition.
3.3 Homogenization kinetic analysis
Generally, homogenization time is determined by the interdendritic phase spacing. Effect of the spacing on the homogenization process could be discussed by the homogenization kinetic analysis. According to the theory of SHEWMAN [20], the homogenization kinetic equation is mathematically expressed as follows:
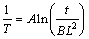 (1)
(1)
where A=R/Q, B=4.6/(4π2D0), R is the mole gas constant; Q is the diffusion activation energy; D0 is the diffusion coefficient; L is the interdendritic T(AlZnMgCu) phase spacing; T ant t are the homogenization temperature K and time s, respectively.
If the parameters of as-cast microstructure are given, the homogenization kinetic curves can be obtained. The diffusion coefficient of Cu in Al is much lower than Mg, and Zn at the same temperature [21]. So, the homogenization process is mainly controlled by the diffusion process of Cu [22-24]. SEM analysis shows that the average interdendritic phase spacings (L) in the studied alloy after the first step and the second step homogenization treatment are 60 and 32 μm, respectively. By substituting of D0(Cu)=0.084 cm2/s, Q(Cu)=136.8 kJ/mol and R=8.31 kJ/(mol·K) into Eq. (1), the homogenization kinetic curves of Al-Zn-Mg-Cu-Er-Zr alloy for a certain interdendritic phase spacing can be obtained, as shown in Fig. 7. This reveals that the holding time increases dramatically with the decrease of the homogenization temperature. According to the homogenization kinetic curve, the optimized homogenization conditions of the present as-cast Al-Zn-Mg-Cu-Zr-Er alloy are 25.2 h and 465 °C, which are well consistent with the experimental results. Therefore, taking the precipitation of Al3(Er,Zr) particles into comprehensive consideration, the most optimized homogenization scheme for the present Al-Zn-Mg-Cu- Zr-Er alloy is (400 °C, 10 h) + (465 °C, 24 h).
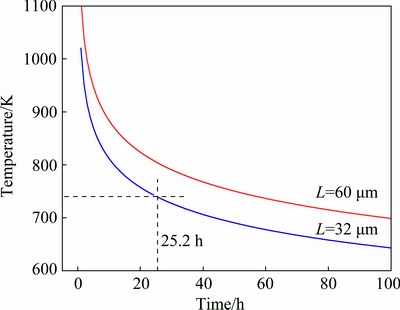
Fig. 7 Curves of homogenization kinetics at interdendritic phase spacing of 60 μm
4 Conclusions
1) Serious segregation exists in the as-cast alloy, and the primary phases preferentially locate in the grain boundary regions, which are mainly T(AlZnMgCu), S(Al2CuMg) and Al8Cu4Er phases.
2) T(AlZnMgCu) and S(Al2CuMg) phases dissolve completely into Al matrix after homogenization at 465 °C for 24 h, but the Al8Cu4Er phase cannot be dissolved and eliminated in Al matrix because its melting point is higher than the homogenization temperature.
3) Compared with the single-stage homogenization treatment, both a finer particle size and a higher number density of Al3(Er,Zr) dispersoids can be obtained with the two-stage homogenization treatment.
4) Taking the precipitation of Al3(Er,Zr) particles into consideration, the optimized homogenization scheme for the present Al-Zn-Mg-Cu-Zr-Er alloy is (400 °C, 10 h) + (465 °C, 24 h), which is consistent with the results of homogenizing kinetic analysis.
References
[1] SRIVATSAN T S, ANAND S, SRIRAM S, VASUDEVAN V K. The high-cycle fatigue and fracture behaviour of aluminium alloy 7055 [J]. Materials Science and Engineering A, 2000, 281: 292-304.
[2] WILLIAMS J C, STARKE E A. Progress in structural materials for aerospace systems [J]. Acta Materialia, 2003, 51: 5775-5799.
[3] ROMETSCH P A, ZHANG Y, KNIGHT S. Heat treatment of 7xxx series aluminium alloys—Some recent developments [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 2003-2017.
[4] WU H, WEN S P, GAO K Y, HUANG H, WANG W, NIE Z R. Effect of Er additions on the precipitation strengthening of Al–Hf alloys [J]. Scripta Materialia, 2014, 87: 5-8.
[5] WU H, WEN S P, WU X L, GAO K Y, HUANG H, WANG W, NIE Z R. A study of precipitation strengthening and recrystallization behavior in dilute Al-Er-Hf-Zr alloys [J]. Materials Science and Engineering A, 2015, 639: 307-313.
[6] WU H, WEN S P, WU X L, HUANG H, GAO K Y, WANG W, NIE Z R. Hot deformation behavior and constitutive equation of a new type Al-Zn-Mg-Er-Zr alloy during isothermal compression [J]. Materials Science and Engineering A, 2016, 651: 415-424.
[7] WU H, WEN S P, HUANG H, GAO K Y, WU X L, WANG W, NIE Z R. Hot deformation behavior and processing map of a new type Al-Zn-Mg-Er-Zr alloy [J]. Journal of Alloys and Compounds, 2016, 685: 869-880.
[8] DENG Y, YIN Z M, CONG F G. Intermetallic phase evolution of 7050 aluminum alloy during homogenization [J]. Intermetallics, 2012, 26: 114-121.
[9] HOU W R, JI W B, ZHANG Z H, XIE J X, CHENG X L. The effect of homogenization temperature on the corrosion resistance of extruded 7050 Al-alloy bars [J]. Journal of Materials Processing Technology, 2014, 214: 635-640.
[10] DENG Y L, WAN L, WU L H, ZHANG Y Y, ZHANG X M. Microstructural evolution of Al-Zn-Mg-Cu alloy during homogenization [J]. Journal of Materials Science, 2011, 46: 875-881.
[11] WESTERMANN I, HAUGSTAD A L, LANGSRUD Y, MARTHINSEN K. Effect of quenching rate on microstructure and mechanical properties of commercial AA7108 aluminium alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1872-1877.
[12] ROBSON J D. A new model for prediction of dispersoid precipitation in aluminium alloys containing zirconium and scandium [J]. Acta Materialia, 2004, 52: 1409-1421.
[13] ROBSON J D, PRANGNEL P B. Dispersoid precipitation and process modelling in zirconium containing commercial aluminium alloys [J]. Acta Materialia, 2001, 49: 599-613.
[14]  X Y, GUO E J, ROMETSCH P, WANG L J. Effect of one-step and two-step homogenisation treatments on the distribution of Al3Zr dispersoids in commercial aluminium alloy 7150 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2645-2651.
X Y, GUO E J, ROMETSCH P, WANG L J. Effect of one-step and two-step homogenisation treatments on the distribution of Al3Zr dispersoids in commercial aluminium alloy 7150 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2645-2651.
[15] HE L Z, JIA P F, ZHANG L, CUI J Z. Evolution of secondary phases and properties of 7B04 aluminum alloy during DC homogenization [J]. Transactions of Nonferrous Metals Society of China 2016, 26: 319-327.
[16] LIU Q, ZHU R H, LI J F, CHEN Y L, ZHANG X H, ZHANG L, ZHENG Z Q. Microstructural evolution of Mg, Ag and Zn micro-alloyed Al-Cu-Li alloy during homogenization [J]. Transactions of Nonferrous Metals Society of China 2016, 26: 607-619.
[17] LI B, PAN Q L, SHI Y J, LI C, YIN Z M. Microstructural evolution of Al-Zn-Mg-Zr alloy with trace amount of Sc during homogenization treatment [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3568-3574.
[18] SHI Y J, PAN Q L, LI M J, LIU Z M, HUANG Z Q. Microstructural evolution during homogenization of DC cast 7085 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3560-3568.
[19] ROBSON J D. Optimizing the homogenization of zirconium containing commercial aluminium alloys using a novel process model [J]. Materials Science and Engineering A 2002, 338: 219-229.
[20] SHEWMAN P G. Diffusion in solids [M]. New York: McGraw-Hill, 1963.
[21] LI W B, PAN Q L, XIAO Y P, HE Y B, LIU X Y. Microstructural evolution of ultra-high strength Al-Zn-Mg-Cu-Zr alloy containing Sc during homogenization [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2127-2133.
[22] Mondolfo L F. Aluminum alloys structure and properties [M]. London: Butterworths, 1976.
[23] Rokhlin L L, Dobatkina T V, Bochvar N R. Lysova E V, Mondolfo L F. Investigation of phase equilibria in alloys of the Al-Zn-Mg-Cu-Zr-Sc system [J]. Journal of Alloys and Compounds, 2004, 367:10-16.
[24] LIU X Y, PAN Q L, FAN X, HE Y B, LI W B, LIANG W J. Microstructural evolution of Al-Cu-Mg-Ag alloy during homogenization [J]. Journal of Alloys and Compounds, 2009, 484: 790-794.
新型Er、Zr微合金化Al-Zn-Mg-Cu合金在均匀化中的显微组织演变
吴 浩,文胜平,卢军太,米振鹏,曾宪龙,黄 晖,聂祚仁
北京工业大学 材料科学与工程学院,北京 100124
摘 要:通过使用光学显微镜、扫描电镜、透射电镜及X射线衍射等手段研究一种新型Er、Zr微合金化Al-Zn-Mg-Cu合金在均匀化过程中的显微组织演变。结果表明:铸态合金组织存在严重的偏析,此时合金中含有大量的T(AlZnMgCu)、S(Al2CuMg) 和 Al8Cu4Er 相,这些初生相大量偏聚于晶界。随着在465 °C单级均匀化处理的进行,第二相含量大幅度降低,可熔的T相和S相会逐步地熔入基体,但Al8Cu4Er相不能完全消除,仍有少量残留。相对于单级均匀化工艺,合金在双级均匀化处理时不仅能够消除铸态合金偏析组织,而且能够析出大量细小弥散分布的L12结构的Al3(Er,Zr)相。结合均匀化动力学分析,可以得出合金合理的均匀化热处理制度为 (400 °C, 10 h) + (465 °C, 24 h)。
关键词:Al–Zn–Mg–Cu–Er–Zr合金;均匀化;显微组织演变;初生相;Al3(Er,Zr)粒子
(Edited by Xiang-qun LI)
Foundation item: Project (2012CB619503) supported by the National Basic Research Program of China; Project (51201003) supported by the National Natural Science Foundation of China; Project (2142007) supported by Natural Science Foundation of Beijing, China
Corresponding author: Hao WU; Tel/Fax: +86-10-67391536; E-mail: wuhao2469@126.com
DOI: 10.1016/S1003-6326(17)60168-7
Abstract: A comprehensive study on the microstructural evolution of a new type Al-Zn-Mg-Cu-Er-Zr alloy during homogenization was conducted by optical microscope, scanning electron microscope, transmission electron microscopy and X-ray diffraction analysis. The results show that serious segregation exists in as-cast alloy, and the primary phases are T(AlZnMgCu), S(Al2CuMg) and Al8Cu4Er, which preferentially locate in the grain boundary regions. The soluble T(AlZnMgCu) and S(Al2CuMg) phases dissolve into the matrix gradually during single-stage homogenized at 465 °C with prolonging holding time, but the residual Al8Cu4Er phase cannot dissolve completely. Compared with the single-stage homogenization, both a finer particle size and a higher volume fraction of L12-structured Al3(Er,Zr) dispersoids can be obtained in the two-stage homogenization process. A suitable homogenization scheme for the present alloy is (400 °C, 10 h)+(465 °C, 24 h), which is consistent with the results of homogenization kinetic analysis.


