
J. Cent. South Univ. (2020) 27: 1691-1702
DOI: https://doi.org/10.1007/s11771-020-4400-4
Effect of sodium sulfate on strength and microstructure of alkali-activated fly ash based geopolymer
LV Qing-feng(吕擎峰), WANG Zi-shuai(王子帅), GU Liu-yang(谷留杨),CHEN Yi(陈臆), SHAN Xiao-kang(单小康)
Key Laboratory of Mechanics on Disaster and Environment in Western China, Ministry of Education,Lanzhou University, Lanzhou 730000, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
The main objective of this paper focuses on the changes that occur in the strength and microstructural properties of sodium silicate activated fly ash based geopolymer due to varying the sulfate salt and water content. A series of tests including X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, physical adsorption and unconfined compressive strength were used to investigate this effect. The results indicate that the higher water content has an adverse effect on the alkali activation and microstructural properties of geopolymer, so the optimum mass ratio of sodium sulfate in alkali-activated geopolymer under different water-to-binder ratios shows a “peak shifting” phenomenon, i.e., the higher the water-to-binder ratio, the higher the optimum mass ratio.Lower presence of sodium sulfate has no significant effect on the alkali-activated geopolymer systems; higher addition of sodium sulfate, however, could cause the symmetrical stretching vibration of Si—O and the symmetrical stretching vibration of Si—O—Si and Al—O—Si, and promote the formation of N-A-S-H gels. Furthermore, the cement effect of the gel and sodium sulfate aggregate could improve the integrity of pore structure obviously. The maximum strength of geopolymer curing at ambient temperature was 52 MPa. This study obtains the rule that the strength properties of alkali-activated geopolymers vary with the water-to-binder ratio and sodium sulfate content. The feasibility of geopolymer co-activated by sodium sulfate and sodium silicate was investigated, and reference for engineering application of alkali-activated geopolymer in salt-bearing areas was provided.
Key words:
geopolymer; microstructure; fly ash; sodium sulfate; water-to-binder ratio;
Cite this article as:
LV Qing-feng, WANG Zi-shuai, GU Liu-yang, CHEN Yi, SHAN Xiao-kang. Effect of sodium sulfate on strength and microstructure of alkali-activated fly ash based geopolymer [J]. Journal of Central South University, 2020, 27(6): 1691-1702.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4400-41 Introduction
Geopolymer is a new type of inorganic gelling material with a three-dimensional structure. Generally, it is made by mixing aluminosilicate raw materials with alkali-activator [1]. In an alkaline environment, aluminosilicate solid materials are dissolved to produce the tetrahedron elements of [SiO4] and [AlO4], which are preserved in water and form supersaturated aluminosilicate solution. An amorphous gel with spatial structure is formed through condensation and rearrangement, and its empirical formula is Mn[—(SiO2)z—AlO2]n·wH2O, where Z is the Si/Al molar ratio, M is the alkali cation, such as Na or K, n is the polymerization degree, and w is the water content [2, 3]. Geopolymers can provide a desirable alternative to Portland cement (PC) binders, avoiding the production of large amounts of CO2 in cement manufacturing, and its raw materials come from natural products (kaolinite, clay, metakaolin, etc.) and industrial byproducts (fly ash, slag, rice husk ash, etc.) [4-7], therefore becoming the current research focuses.
Activators and curing methods are two crucial factors influencing the polymerization of geopolymers [8, 9]. It is commonly accepted that high temperature can improve the content of zeolite and the strength of specimens in a geopolymer system. However, it is difficult to achieve in engineering practice. Activators are usually a strong base or alkaline, such as NaOH, KOH and Na2SiO3. Weak alkaline salt and neutral salt can also promote the polymerization, among which Na2SO4 and Na2CO3 have a better activated effect. A strong base can provide an abundance of OH- ions, which is conducive to the release of active Al3+ and Si4+ by the breaking of Si—O—Si and Si—O—Al bonds in silicate raw materials. Subsequently, active Al3+ and Si4+ react to form crystalline nuclei and aluminosilicate oligomers with a [SiO4] and [AlO4] tetrahedron [10, 11]. However, excessive OH- ions or acceleration of the hydration reactions result in a significant increase in the formation of geological polymers and an increase in internal pressure [2]. Subsequently, microcracks are produced and the strength eventually decreases; strong base activator needs to be prepared in industry, which has the disadvantages including high cost, limited circulation and great harmfulness; the mechanism of salt activators is usually based on increasing the content of Ca(OH)2 and Tricalcium aluminate trisulphate hydrates (AFt) in the geopolymer system, filling the matrix voids and enhancing the strength [12-14]. Inorganic salts can be obtained naturally with low cost, however, the activated effect is not as good as that obtained by the use of strong base activators. Therefore, accelerating the dissolution of geopolymer raw materials by the combination of a salt and alkali is an effective way to improve the properties of a geopolymer.
CHEQUER et al [15] investigated the impact of sulfate on sodium-based geopolymers and proved that sulfates can modify the properties of geopolymers. KOMNITSAS et al [16] found that the compressive strength of geopolymers is negatively affected by the presence of SO42- ions in the starting mixture. CRIADO et al [17] discovered that the presence of sulfates in alkaline activation of fly ash retards N-A-S-H gel formation and shortens the time needed for zeolites to precipitate. ISMAIL et al [18] believed that sodium sulfate solution appears to have little or no effect on the structure of the material. ZHANG et al [19] used different contents of Na2SO4+Na2SiO3 composite activated slag to produce geopolymer strength that was increased by 15.8% compared to a single Na2SiO3 activator.
Based on previous literatures, there are relatively few studies devoted to the effect of external or non-stationary sodium sulfate. Further effort is required to understand the impact of internal sodium sulfate content on the alkali- activated geopolymer. Hence, the major objective of this study was to investigate the effect of the sodium sulfate content on the strength of alkali-activated geopolymer cementitious materials. Sodium sulfate with varying contents was added to fly ash geopolymer under different water-to-binder ratios. Mechanical properties, phase characteristics, microscopic characterization and microstructure of the products were identified respectively by unconfined compressive strength (UCS), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), and physical adsorption tests. The mechanism underlying the influence of sulfate on the water-to-binder ratio and alkali-activated geopolymer strength was discussed.
2 Experimental program
2.1 Materials
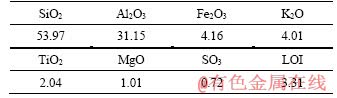
The class F fly ash (FA, ASTM C 618-03) was obtained from an electric power plant in Zhengzhou city, China, and its fineness (revised residual percentage of screening) Fc=11.2%. According to the code of fly ash used for cement and concrete (GB/T 1596-2005, PRC National Standard), the used FA meets the standard of first-class pulverized powder. Table 1 shows the chemical components of the FA. Commercially available sodium silicate consisting of 8.35 wt% Na2O and 26.54 wt% SiO2 was collected from Litian Chemical Co., Ltd., Neiqiu, China. Granular sodium hydroxide (AR, i.e. analytical regent) was mixed into sodium silicate solution to prepare the alkali activator (AA, with w(Na2O)=16.08%, w(SiO2)=23.42%). In order to reduce the effect of heat released when sodium hydroxide meets water, AA was rested for 24 h before use. Sodium sulfate (AR) used in this experiment was supplied from Lanzhou Fuming Chemical Co., Ltd.
Table 1 Composition of fly ash (wt%)
2.2 Sample preparation
Considering the workability of geopolymer and our previous experiments results [8, 9], three water-to-binder ratios (water/fly ash, W/F=0.25, 0.30, 0.35) were used in the experiment. It should be mentioned that the water used in W/F includes water in the AA solution and additional water. Since AA solution contains water, the mass ratio of NaO (from AA solution) to fly ash was selected 6% to satisfy lower W/F, and the amounts of AA and additional water were calculated accordingly. Moreover, according to the mass ratio of salt to fly ash (0, 1%, 3%, 5%, 7%, 9%, 11%, 13%, 15%, 17%, 19%, 21%, 23%, 25% and 27%), the mass of sodium sulfate added to the geopolymer system was calculated.
Sodium sulfate was added to the fly ash that was subsequently mixed with the AA solutions and additional water in a cement blender mixer for 120 s slow stirring ((140±5) r/min), 30 s break and then 120 s rapid stirring ((285±10) r/min). After the stirring process, the fresh pastes were poured in a prismatic mold with sizes of 40 mm×40 mm×40 mm and placed in the standard curing chamber (20±2)°C and a humidity of more than 90%. After 24 h, the mold was removed, and the samples were still maintained in the standard curing chamber to 28 d for the experiments of unconfined compressive strength and microstructure. The average compressive strength values obtained by two or three samples of geopolymer with different conditions were studied.
2.3 Experimental methods
A TDW-10-300 automatic cement pressure tester was used to test the unconfined compressive strength, with a loading rate of 0.8 kN/s. A PANalytical X’Pert Pro X-ray diffraction analyzer was used for phase qualitative analysis, with a powder specimen and Cu target, λ=1.5406  . The structure of the specimen material was identified by a Nicolet NEXUS-670 Fourier transform infrared light meter, and KBr tableting method over a test range of 4000-400 cm-1. The micromorphology was characterized by a Thermoscientific ApreoS scanning electron microscope, and the chemical element analysis was performed using Oxford Instruments INCA energy dispersive X-ray spectroscopy (EDS) system; the surface area, pore volume and pore size distribution were determined using an ASAP 2020M instrument.
. The structure of the specimen material was identified by a Nicolet NEXUS-670 Fourier transform infrared light meter, and KBr tableting method over a test range of 4000-400 cm-1. The micromorphology was characterized by a Thermoscientific ApreoS scanning electron microscope, and the chemical element analysis was performed using Oxford Instruments INCA energy dispersive X-ray spectroscopy (EDS) system; the surface area, pore volume and pore size distribution were determined using an ASAP 2020M instrument.
3 Results and discussion
3.1 Mechanical properties
Figure 1 shows the variation in unconfined compressive strength of alkali activated fly ash based geopolymer with salt content for the three water-to-binder ratio systems studied. The findings show that the mechanical strength of alkali- activated fly ash based geopolymer rose in all the systems with salt contents both in the lower and the higher water-to-binder ratio. With regard to alkali activated fly ash based geopolymer with sodium sulfate, the unconfined compressive strength (UCS) of reaches a maximum at different salt contents in the three water-to-binder ratios, and then drops down when the content still increases. Moreover, significant improvement in UCS occurs with a decrease in ratio of water-to-binder. The UCS of specimens with a water-to-binder ratio of 0.25 is promoted from 30.7 to 52.1 MPa as the mass fraction of salt content increases up to 13%; the salt content corresponding to the maximum strength of geopolymer system shifts as the ratio of water-to- binder increases, so we provide words with “peak shifting” to describe this interesting phenomenon. Finally, Figure 1 indicates that the strength increases or decreases disorderly in geopolymer systems with mass ratio of sodium sulfate between 0-9%. The mechanical behaviours of geopolymer are not exceptional enough in any water-to-binder ratio system, although the sodium sulfate was proved as an effective activator for fly ash based geopolymer. In fact, sodium sulfate is a neutral salt the aqueous solution of which exhibits a pH of ~7, so the lower salt content cannot have a significant effect on the geopolymerization degree when sodium silicate was the alkali activator in geopolymer systems [17]. On the other hand, the mass fraction of Ca, which can produce calcium aluminosilicate hydrate (C-S-H) in the gepolymer system and further react with sulfates to generate ettringite (AFt) [12, 14], in the raw material is lower (see Table 1), hence it is not enough to improve the strength of geopolymer.

Figure 1 Effect of water/fly ash ratio and Na2SO4 content on compressive strength of samples
The UCS test results show that the mechanical strength of specimens is closely associated with the mass ratio of water and sodium sulfate. We will discuss other test results from two aspects including the effect of water-to-binder ratio and the effect of salt content, so as to explore the relationship between mechanical strength and salt better.
3.2 Phase analysis
The crystalline and amorphous phases detected by X-ray diffraction technique are presented in Figure 2 for geopolymer with different water-to- binder ratios and salt contents (S). The main reaction product in both systems was N-A-S-H gel (see hole at 25°-40°) [17], although their peaks are rather insignificant owing to their amorphous nature. The mineral components (quartz, mullite and magnetite) existing in the raw material were distinctly detected in all formulations of the geopolymer. There is no significant difference between the XRD patterns of the geopolymer in three water-to-binder ratios with a low and dense non-crystalline phase material peak group, revealing that the water has little effect on the mineralogical nature of geopolymer. Moreover, the similar property of XRD patterns shows that water content cannot affect the long-range order of the gel [20].
For geopolymer mixed with sodium sulfate, the peak of sodium sulfate was found on the diffractogram, indicating that sodium sulfate did not react completely and unreacting parts existed in geopolymer system. As shown in Figure 2(b), with increasing salt content, the peak intensity of sodium sulfate increases, indicating the presence of sodium sulfate in geopolymer systems. Due to the salt solution was highly diluted [17], the peak of sodium sulfate was not found in the geopolymer with lower salt content. However, it cannot be proved that sodium sulfate unreacts when salt content is over a value, and their relationship needs a further explanation by other tests. In addition, the reaction products of alkali activated geopolymer are mostly amorphous, and the samples cured at room temperature are difficult to form crystalline materials such as zeolites [11, 21]. Therefore, no new peaks appeared in the XRD spectra of all samples.

Figure 2 XRD pattern of geopolymers:
3.3 Result of FTIR
Figure 3 presents the FTIR spectra for geopolymers with different water-to-binder ratios (Figure 3(a)) and different salt contents (Figure 3(b)). What can be seen from the data is that the characteristic absorption bands for fly ash and the geopolymer are in the range of 1000-1100 cm-1. As seen in Figure 3(a), the spectral bands for the different water ratios do not change significantly. The maximum difference was found in the low wavenumber region (400-800 cm-1) and the medium wavenumber region (800-1300 cm-1) [22, 23]. The absorption peak at approximately 450 cm-1 in the infrared spectrum of the specimen is related to the bending vibration of the T—O bond in the TO4 (T=Si/Al) tetrahedron. The absorption peak at approximately 790 cm-1 belongs to the Al—O bending vibration of the AlO6 octahedron, and at approximately 1010 cm-1, one can observe the asymmetric tensile vibration peak of Si—O—T, which is also the characteristic peak of a geopolymer [24, 25]. The absorption peaks at approximately 1460 and 1640 cm-1 belongs to the tensile vibration of the O—C—O bond of the CO32- group and is attributed to atmospheric carbonation on the surface of powdered geopolymeric products [20]; the weak absorption band at 1640 cm-1 represents an isolated noninteracting surface silanol group [26].

Figure 3 FTIR pattern of geopolymers:
For geopolymer with different water ratios, it can be seen that the tensile asymmetric vibration peak of Si—O—T was changed from 1076 to 1015 cm-1 in specimens compared with fly ash, which is generally considered to be the basis for geopolymer formation [27, 28]. As the water- to-binder ratio increases, the O—H—O bending vibration representing the inmobilized water moves from 3455 cm-1 to a low wavenumber of 3438 cm-1. When the wave crest moves towards a low wave number, the energy required for vibration decreases [29], which indicates that the increase in water content causes a change in the bond length of the OH- of structure water [22]. In addition, the infrared spectra change little with the change in water-to-binder ratio, revealing that no new substances appear in the samples. This is consistent with the observation from XRD.
An FTIR plot with different salt contents for a water-to-binder ratio of 0.30 is shown in Figure 3(b). It can be seen from the graph that with the increase in salt content, new absorption peaks appear near 615, 637 and 1120 cm-1, and that the peaks show a gradual increasing in intensity.The absorption peak at 615 cm-1 is owing to the Si—O—Si and Al—O—Si symmetrical stretching vibration, and the weak absorption peak at 637 cm-1 is attributed to the Si—O symmetrical stretching vibration [30], indicating the new bonds were generated in geopolymer system. It must also be mentioned that the peak near 1120 cm-1 is not related to the migration of the tensile asymmetric vibration peak of Si—O—T, but to the new anti-symmetric stretching vibration of SO42-, which can be clearly seen from a comparison of the infrared spectra for the change in salt content, [SO4] can replace [SiO4] in N-A-S-H and [SiO4] in a free state accelerates the dissolution of active alumina [31]. Therefore, the increase of salt content is likely to inspire the generation of Si—O—Si and Al—O—Si bonds in the system. A new amorphous Si—O—Si (Al) network structure was formed to improve the geopolymerization degree of the geopolymer [32]. However, when the salt content is more than a value,the Na2SO4 increase rate in the system is much faster than the dissolution rate of aluminosilicate, which is shown by the sharp 1120 cm-1 absorption peak, while the absorption peaks at 615 and 637 cm-1 do not change much.
3.4 Micromorphology and chemical element analysis
SEM study was made to investigate the cross section morphology of geopolymer. Figure 4(a) shows that the raw material (i.e. fly ash) has a great quality of vitreous beads. EDS result reflects that these microspheres are rich in silicate and aluminate, which are activated and dissolved by alkali to form an amorphous phase geopolymer matrix, and then the polymer matrix wraps partially reacted or unreacted FA particles to form a continuous, nonuniform geopolymer. As seen in Figures 4(b)- (d), an increase in mass ratio of water to binder from 0.25 to 0.30 appears to have a detrimental effect on the alkali activation, and less unreacted fly ash particles wrapped by white acicular substances can be seen in image obviously. These substances are considered to be sodium silicate, which erodes the unsuccessful solidification of the vitreous surface [2]. Water plays the role of a reaction medium, and the process of oligomers condensation releases the water that was nominally consumed during dissolution as depicted in Eqs. (1) and (2) [33]. So increasing water content in the geopolymer system would break the equilibrium of oligomer polymerization reaction, and therefore leads to restricting of the formation of the more gel. Sodium silicate is actually an inorganic binder that provides a higher bonding force between the unreacted fly ash particles and the geopolymer matrix, avoiding the formation of microcracks between the smooth fly ash and the matrix due to loss of cementation. Therefore, the specimen with a low water-to-binder ratio has the highest compressive strength. The microcracks in the geopolymer matrix are caused by pressure, and the pits on the surface of the geopolymer are formed by the shedding of fly ash microspheres [2].
(Al2O3·2SiO2)n+3nH2O+OH-→
n(OH)3—Si—O—Al-—(OH)3→
(—Si—O—Al-—O—)n+3nH2O (1)
(Al2O3·2SiO2)n+2nSiO2+4nH2O+OH-→
n(OH)3—Si—O—Al-—O—Si—(OH)3→
(—Si—O—Al-—O—Si—O—)n+4nH2O (2)
The atomic percentages of EDS elements of each feature point in Figure 4 are shown in Table 2. The results demonstrate that the composition and proportion of the products of the geopolymers with different water-to-binder ratios are approximately the same, which is also consistent with the XRD and FTIR results. The Si/Al molar ratios for the calcium (sodium) aluminosilicate hydrate (C-(N)- A-S-H) gel is basically between 3 and 4, indicating that an increase in water content will not significantly affect the Si/Al ratio of the geopolymer gel. It can be seen that the increase in the water-to-binder ratio in the geopolymer system results in a decrease in the degree of geopolymerization reaction, an increase in unreacted fly ash particles and an increase in the number of microcracks in the samples, which destroys the microstructures in the specimens and has a negative impact on the strength.

Figure 4 SEM image of FA and different water/fly ash ratio geopolymers with S=0:
Table 2 Atomic fraction of elements of each feature point in Figure 4
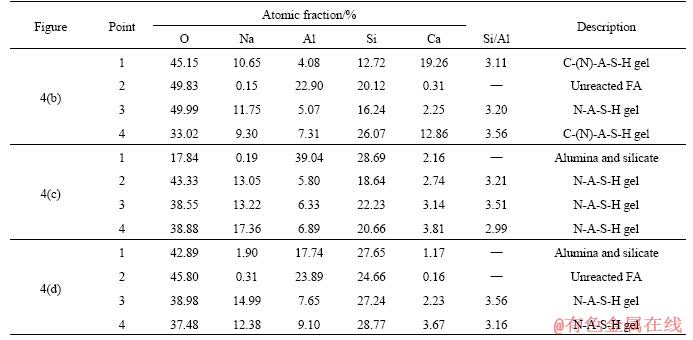
Figure 5 shows the SEM images of geopolymers with different salt contents. Combining with the EDS atomic percentage for the characteristic points in Table 3, it can be found that compared with the geopolymer without salt, the structure integrity of the geopolymer with salt has significantly improved the results from the formation of N-A-S-H gel; in addition, the cementation state between the components grows denser, and the glass microspheres with a smooth surface of fly ash basically disappear, which indicates that salt would benefit to the dissolution of aluminosilicate raw materials and improve the degree of geopolymerization reaction. The S element appeared in the geopolymer gel was attributed to the increase in the salt content, with the content increased first and then decreasd with increasing salt content; S atoms replaced part of the Si atoms in the geopolymer reaction, which also resulted in a decrease in the Si/Al ratio between 1 and 2 in the geopolymer system after salt doping. The Si/Al ratio has shown the decreasing trend with increase in salt content indicating more dissolution of active alumina and subsequently networking with SiO4 tetrahedron through geopolymerization [21], so the formation of N-A-S-H gel was promoted. This finding was consistent with the results of FTIR test.
Scientists [34, 35] have found that when Si/Al≤3, the geopolymer material will produce a three-dimensional crosslinked network structure with rigid and brittle characteristics; when the molar ratio of Si/Al>3, the geopolymer will produce a rubber-like two- dimensional network or linear connection structure. Because sodium sulfate has a smaller particle size and cannot react with alkali activator, so the remaining sodium sulfate crystal phase presenting in the system was wrapped by the geopolymer gel to serve as an aggregate (act like fine sand) [24], resulting in a denser microstructure of geopolymer. Once the salt content surpasses a certain value, the sodium sulfate in the system was excessive, resulting in the weak cement effect and intergranular force, hence the decline of the compressive strength after the peak is explained.
3.5 Pore structure analysis
Table 4 reports the BET (Brunauer, Emmett and Teller) specific surface area for different geopolymers and the pore volume and average pore diameter of BJH (Barrett, Joyner and Halenda) in the diameter of 1.7-300.0 nm. It can be seen that with increasing water-to- binder ratio, the BET specific surface area gradually decreases, the BJH pore volume gradually increases, and the nanometer pore size gradually decreases. A theory that water resides within pores in the gel could explain this phenomenon [21]. When the water of the gel and free water were evaporated, many pores would appear in the geopolymer system and make an adverse effect to the microstructure. At the same time, the phenomenon of weak cementation of the geopolymer gel and glass bead surface occurs, so that the pores and volume of the intergrains increase, the specific surface area decreases, and a loose space grid structure is formed, resulting that in the strength of the specimen is greatly reduced.

Figure 5 SEM images of different salt content geopolymers with W/F=0.30:
Table 3 Atomic fraction of EDS elements of each feature point in Figure 5

Table 4 Microstructure of samples at different geopolymer systems
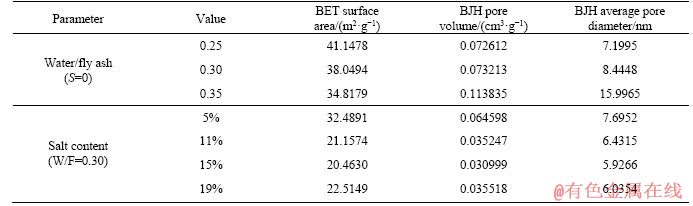
The BET specific surface area, BJH pore volume and BJH average pore size of the specimen increase at first, then decrease with increasing salt content. A possible explanation is that when the amount of salt is increased, a portion sulfate promotes the polymerization reaction, resulting in the amount of the gel increases and the unreacted fly ash decreases. Another part of sulfate is wrapped by the geopolymer gel as an aggregate, so that the specific surface area and pore volume decrease while avoiding the presence of macropores and increasing the strength of the sample. However, with sulfate increasing to a certain amount, the effect of gelling of the sulfate is gradually decreased, and the excess sulfate deposition causes an increase in pore volume and average pore diameter in the geopolymer system, resulting in a decrease in the strength of the sample.
Figure 6 shows the pore size distribution of geopolymers for different water-to-binder ratios and different salt contents. With increasing water content in the geopolymer system, the number of large pore sizes gradually increases, and the pore size distribution becomes wider. This indicates that the nanopore development is poor and has a negative effect on the strength of the sample. Figure 6(b) demonstrates that the increase in mass ratio of salt (from 5% to 15%) induces a lowering fraction of micropores, and narrows the distribution range of pores in geopolymer, which indicates that the microstructure of the specimen is significantly improved. However, when the salt content increases again, the macropores of the specimen is no longer reduced, while the number of micropores is is gradually increased, resulting in the increase of the pore volume and the destroy on the microstructure of the specimen, thus explaining the change in strength and microstructure.
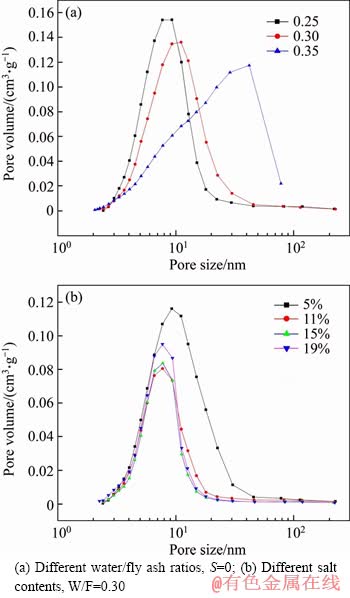
Figure 6 Pore diameter distribution of geopolymers:
4 Conclusions
1) The water-to-binder ratios have a significant effect on the mechanical strength of alkali-activated geopolymer mixed with sodium sulfate. With increasing the mass ratio of water, the bonding ability of N-A-S-H gels becomes weaker and the microstructure integrity of geopolymer specimens grow worse. Therefore, geopolymer systems need more salt to improve the strength, and the interesting phenomenon “peak shifting” is present, i,e. the salt content corresponding to the maximum strength of geopolymer system shifts as the ratio of water-to-binder increases.
2) The effect of the lower salt content on the strength property of alkali-activated geopolymer is slight. Comparing to sodium sulfate, the alkali activator (sodium silicate and sodium hydroxide) has a more significant effect in the dissolution of active ingredients from raw materials and polymerization. So the lower content of sodium sulfate has no significant effect on the phase characteristics, network structure and cross section morphology of the reaction products, and the strength of geopolymer cannot be improved obviously.
3) The presence of higher sodium sulfate in alkali-activated geopolymer systems is beneficial for the development of strength and microstructure characteristic. Sodium sulfate would cause the absorption peaks of Si—O symmetrical stretching vibration and Si—O—Si, Al—O—Si symmetrical stretching vibration to appear and gradually increase. It could accelerate the dissolution of active alumina, and thus the formation of N—A—S—H gel is promoted. The unreacted sodium sulfate acts as an aggregate to increase the integrity of pore structure, and the strength of specimens is improved obviously.
4) Sodium sulfate plays a crucial role in promoting the polymerization reaction and acts as an aggregate for the alkali-activated fly ash base polymer, so it can significantly promote the strength.
References
[1] KOLEZYNSKI A, KROL M, ZYCHOWICZ M. The structure of geopolymers-Theoretical studies [J]. Journal of Molecular Structure, 2018, 1163: 465-471. DOI: 10.1016/ j.molstruc.2018.03.0 33.
[2] FAN Feng-hong, LIU Zhen, XU Guo-ji, PENG Hui, CAI C S. Mechanical and thermal properties of fly ash based geopolymers [J]. Construction and Building Materials, 2018, 160: 66-81. DOI: 10.1016/j.conbuildmat.2017.11.023.
[3] NATH S K, MAITRA S, MUKHERJEE S. Microstructural and morphological evolution of fly ash based geopolymers [J]. Construction and Building Materials, 2016, 111: 758-765. DOI:10.1016/j.conbuildmat.2016.02.106.
[4] PEYNE J, JOUSSEIN E, GAUTRON J, DOUDEAU J, ROSSIGNOL S. Feasibility of producing geopolymer binder based on a brick clay mixture [J]. Ceramics International, 2017, 43: 9860-9871. DOI: 10.1016/j.ceramint.2017.04.169.
[5] NMIRI A, DUC M, HAMDI N, MARZOUK O Y, SRASRA E. Replacement of alkali silicate solution with silica fume in metakaolin-based geopolymers [J]. International Journal of Minerals Metallurgy and Materials, 2019, 26(5): 555-564. DOI: 10.1007/s12613-019-1764-2.
[6] GHOSH P, KUMAR H, BISWAS K. Fly ash and kaolinite-based geopolymers: Processing and assessment of some geotechnical properties [J]. International Journal of Geotechnical Engineering, 2016, 10(4): 1-10. DOI: 10.1080/19386362.2016.1151621.
[7] CHEN Xiao, ZHU Guo-rui, ZHOU Ming-kai, WANG Jie, CHEN Qian. Effect of organic polymers on the properties of slag-based geopolymers [J]. Construction and Building Materials, 2018, 167: 216-224. DOI: 10.1016/j.conbuildmat. 2018.02.031.
[8] PROVIS J L. Alkali-activated materials [J]. Cement and Concrete Research, 2018, 114: 40-48. DOI: 10.1016/ j.cemconres.2017.02.009.
[9] PROVIS J L, BERNAL S A. Geopolymers and related alkali-activated materials [J]. Annual Review of Materials Research, 2014, 44(1): 299-327. DOI: 10.1146/annurev- matsci-070813-113515.
[10] ABABNEH F A, ALAKHRAS A I, HEIKAL M, IBRAHIM S M. Stabilization of lead bearing sludge by utilization in fly ash-slag based geopolymer [J]. Construction and Building Materials, 2019, 227: 116694. DOI: 10.1016/j.conbuildmat. 2019.116694.
[11] HEIKAL M, NASSAR M Y, EL-SAYED G, IBRAHIM S M. Physico-chemical, mechanical, microstructure and durability characteristics of alkali activated Egyptian slag [J]. Construction and Building Materials, 2014, 69: 60-72. DOI: 10.1016/j.conbuildmat.2014.07.026.
[12] ODERJI S Y, CHEN B, AHMAD M R, SHAH S F A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators [J]. Journal of Cleaner Production, 2019, 225: 1-10. DOI: 10.1016/j.jclepro.2019.03.290.
[13] AHN J, KIM W S, UM W. Development of metakaolin- based geopolymer for solidification of sulfate-rich HyBRID sludge waste [J]. Journal of Nuclear Materials, 2019, 518: 247-255. DOI: 10.1016/j.jnucmat.2019.03.008.
[14] PRASITTISOPIN L, SEREEWATTHANAWUT I. Effects of seeding nucleation agent on geopolymerization process of fly-ash geopolymer [J]. Frontiers of Structural and Civil Engineering, 2018, 12: 16-25. DOI: 10.1007/s11709-016- 0373-7.
[15] CHEQUER D L, FRIZON F. Impact of sulfate and nitrate incorporation on potassium- and sodium-based geopolymers: Geopolymerization and materials properties [J]. Journal of Materials Science, 2011, 46(17): 5657-5664. DOI: 10.1007/ s10853- 011-5516-6.
[16] KOMNITSAS K, ZAHARAKI D, BARTZAS G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers [J]. Applied Clay Science, 2013, 73: 103-109. DOI: 10.1016/j.clay.2012.09.018.
[17] CRIADO M, FERNANDEZ J A, PALOMO A. Effect of sodium sulfate on the alkali activation of fly ash [J]. Cement and Concrete Composites, 2010, 32: 589-594. DOI: 10.1016/j.cemconcomp.2010.05.002.
[18] ISMAIL I, BERNAL S A, PROVIS J L, HANDAN S, van DEVENTER J S J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure [J]. Materials and Structures, 2013, 46: 361-373. DOI: 10.1617/ s11527-012-9906-2.
[19] ZHANG Yao-jun, CHAI Qian, YANG Meng-yang, FAN Bo-wen, LI Xin. Synthesis of bottom ash-based geopolymer co-activated by double salts [J]. Journal of Functional Materials, 2016, 47(9): 9176-9181. DOI: 10.3969/j.issn.100 1-9731.2016.09.034. (in Chinese)
[20] CUI Yong, WANG Dong-ming, WANG Yi-ren, SUN Rui, RUI Ya-feng. Effects of the n(H2O: Na2Oeq) ratio on the geopolymerization process and microstructures of fly ash-based geopolymers [J]. Journal of Non-Crystalline Solids, 2019, 511: 19-28. DOI: 10.1016/j.jnoncrysol.2018. 12.033.
[21] DUXSON P, A FERNANDEZ-JIMENEZ, PROVIS J L, LUKEY G C, PALOMO A, van DEVENTER J S J. Geopolymer technology: The current state of the art [J]. Journal of Materials Science, 2007, 42(9): 2917-2933. DOI: 10.1007/ s10853-006-0637-z.
[22] KUMAR S, KRISTALY F, MUCSI G. Geopolymerisation behaviour of size fractioned fly ash [J]. Advanced Powder Technology, 2015, 26(1): 24-30. DOI: 10.1016/j.apt.2014. 05.002.
[23] LI Xian-bo, YE Jun-jian, LIU Zhi-hong, QIU Yue-qin, LI Long-jiang, MAO Song, WANG Xiao-chen, ZHANG Qin. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(Ⅱ) in aqueous solution [J]. Journal of Central South University, 2018, 25(1): 9-20. DOI: 10.1007/s11771-018-3712-0.
[24] LV Qing-feng, JIANG Lu-sha, MA Bo, ZHAO Ben-hai, HUO Zhen-sheng. A study on the effect of the salt content on the solidification of sulfate saline soil solidified with an alkali-activated geopolymer [J]. Construction and Building Materials, 2018, 176: 68-74. DOI: 10.1016/j.conbuildmat. 2018.05.013.
[25] PEYNE J, JOUSSEIN E, GAUTRON J, DOUDEAU J, ROSSIGNOL S. Feasibility of producing geopolymer binder based on a brick clay mixture [J]. Ceramics International, 2017, 43(13): 9860-9871. DOI: 10.1016/j.ceramint.2017. 04.169.
[26] KROL M, ROZEK P, CHLEBDA D, MOZGAWA W. Influence of alkali metal cations/type of activator on the structure of alkali-activated fly ash–ATR-FTIR studies [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2018, 198: 33-37. DOI: 10.1016/j.saa.2018. 02.067.
[27] RAKHIMOVA N R, RAKHIMOV R Z, MOROZOV V P, GAIFULLIN A R, POTAPOVA L I, GUBAIDULLINA A M, OSIN Y N. Marl-based geopolymers incorporated with limestone: A feasibility study [J]. Journal of Non-Crystalline Solids, 2018, 492: 1-10. DOI: 10.1016/j.jnoncrysol. 2018.04.015.
[28] KOTTA A B, KARAK S K, KUMAR M. Chemical, physical, thermal, textural and mineralogical studies of natural iron ores from Odisha and Chhattisgarh regions, India [J]. Journal of Central South University, 2018, 25(12): 2857-2870. DOI: 10.1007/s11771-0 18-3958-6
[29] HAJIMOHAMMADI A, NGO T, KASHANI A. Glass waste versus sand as aggregates: The characteristics of the evolving geopolymer binders [J]. Journal of Cleaner Production, 2018, 193: 593-603. DOI: 10.1016/j.jclepro.2018.05.086.
[30] LEE W K W, van DEVENTER J S J. The effects of inorganic salt contamination on the strength and durability of Geopolymers [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 211: 115-126. DOI: 10.1016/S0927-7757(02)00239 -X.
[31] LV Qing-feng, JIA Meng-xue, WANG Sheng-xin, ZHOU Gang, WANG Qing-dong. Effect of salt content on compressive strength of solidified sulphate saline soil [J]. Journal of Central South University: Science and Technology, 2018, 49(3): 718-724. DOI: 10.11817/j.issn. 1672-7207.2018.03.027. (in Chinese)
[32] LI Qin, XU Hui, LI Fei-hu, SHEN Li-feng, ZHAI Jian-ping. Synthesis of geopolymer composites from blends of CFBC fly and bottom ashes [J]. Fuel, 2012, 97: 366-372. DOI: 10.1016/j.fuel.201 2.02.059.
[33] MAJIDI B. Geopolymer technology, from fundamentals to advanced applications: A review [J]. Materials Technology, 2009, 24(2): 79-87. DOI: 10.1179/175355509X449355.
[34] HAJIMOHAMMADI A, van DEVENTER J S J. Characterisation of one-part geopolymer binders made from fly ash [J]. Waste and Biomass Valorization, 2017, 8(1): 225-233. DOI: 10.1007/s12649-016-9582-5.
[35] LEONG H Y, ONG D E L, SANJAYAN J G, NAZARI A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based- geopolymer [J]. Construction and Building Materials, 2016, 106: 500-511. DOI: 10.1016/j.conbuildmat.2015.12.141.
(Edited by FANG Jing-hua)
中文导读
硫酸钠对碱激发粉煤灰基地质聚物的强度及微观结构的影响
摘要:本文主要研究了水玻璃激发粉煤灰基地质聚物的强度和微观结构随硫酸盐和水胶比的变化。通过X射线衍射、傅立叶红外光谱、扫描电镜、物理吸附和无侧限抗压强度等一系列试验研究了该变化。结果表明,高水胶比对地聚物的强度及微观结构有不利影响;在不同水胶比条件下,碱激发粉煤灰基地聚物中硫酸钠的最佳质量比表现出“峰移”现象,即水胶比越高,最佳质量比越高;硫酸钠的加入量越低,对碱激发粉煤灰基地聚合物体系的影响越小;硫酸钠的加入量增加,会引起Si—O的对称拉伸振动及Si—O—Si和Al—O—Si的对称拉伸振动,促进N-A-S-H凝胶的形成。凝胶和硫酸钠集料的胶结作用能明显改善孔隙结构的完整性。常温养护地聚物的最大强度为52 MPa。本文得出了碱激发粉煤灰基地聚物的强度特性随水胶比和硫酸钠含量的变化规律,研究了硫酸钠和硅酸钠共同激发地质聚合物的可行性,能够为碱激发粉煤灰基地聚物在含盐地区的工程应用提供参考。
关键词:地质聚合物;微观结构;粉煤灰;硫酸钠;水胶比
Foundation item: Project(51878322) supported by the National Natural Science Foundation of China; Project(18YF1FA112) supported by Key Research and Development Program of Gansu Province, China
Received date: 2019-08-28; Accepted date: 2020-02-04
Corresponding author: LV Qing-feng, PhD, Professor; Tel: +86-18919066282; E-mail: lvqf@lzu.edu.cn; ORCID: 0000-0003-1570-6308
Abstract: The main objective of this paper focuses on the changes that occur in the strength and microstructural properties of sodium silicate activated fly ash based geopolymer due to varying the sulfate salt and water content. A series of tests including X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, physical adsorption and unconfined compressive strength were used to investigate this effect. The results indicate that the higher water content has an adverse effect on the alkali activation and microstructural properties of geopolymer, so the optimum mass ratio of sodium sulfate in alkali-activated geopolymer under different water-to-binder ratios shows a “peak shifting” phenomenon, i.e., the higher the water-to-binder ratio, the higher the optimum mass ratio.Lower presence of sodium sulfate has no significant effect on the alkali-activated geopolymer systems; higher addition of sodium sulfate, however, could cause the symmetrical stretching vibration of Si—O and the symmetrical stretching vibration of Si—O—Si and Al—O—Si, and promote the formation of N-A-S-H gels. Furthermore, the cement effect of the gel and sodium sulfate aggregate could improve the integrity of pore structure obviously. The maximum strength of geopolymer curing at ambient temperature was 52 MPa. This study obtains the rule that the strength properties of alkali-activated geopolymers vary with the water-to-binder ratio and sodium sulfate content. The feasibility of geopolymer co-activated by sodium sulfate and sodium silicate was investigated, and reference for engineering application of alkali-activated geopolymer in salt-bearing areas was provided.


