J. Cent. South Univ. (2012) 19: 1679-1684
DOI: 10.1007/s11771-012-1193-0![]()
Magnetic field assisted electrocatalytic oxidation of organic pollutants in electroplating wastewater
WANG Zhen(王震), YE Kuan-wei(叶宽伟), YANG Zhe(杨哲), XIA Zhe-tao(夏哲韬),
LUO Qin-qin(骆沁沁), WAN Xian-kai(万先凯), SHI Hui-xiang(史惠祥)
Institute of Environmental Engineering, Zhejiang University, Hangzhou 310058, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
To degrade the organic compounds in the electroplating wastewater, magnetic field was tentatively introduced into electrocatalytic oxidation on Ti-PbO2 anode. The magnetic field assisted electrocatalytic oxidation can promote anion movement and the generation of active species, resulting more organic compounds to be oxidized and degraded. Oxidation parameters such as treatment time, current density and initial pH of the wastewater were systematically discussed and optimized. The mineralization of organic compounds is improved by over 15% under a magnetic density of 22 mT while the current density is 50 A/m2, pH is 1.8 and the reaction time is 1.5 h. The results indicate that the magnetic field assisted electrocatalytic oxidation has considerable potential in electroplating wastewater treatment.
Key words:
magnetic field; electrocatalytic oxidation; electroplating wastewater;
1 Introduction
The rapid development of the electroplating industry in China has resulted in an increase of electroplating wastewater containing heavy metal ions, toxic and nonbiodegradable organic pollutants discharged in the environment. For the development of electroplating industry, powerful wastewater treatment processes are necessarily employed.
In recent decades, the control technique of heavy metals in electroplating wastewater [1-4] has received extensive research and application, but little attention is paid to the disposal of organic pollutants. With the implementation of new emission standards, a promising treatment technology of organic pollutants in the wastewater is imperative. Due to high concentrations of metal ions and poor biodegradability, biological treatment is not suitable for this case. Currently, coagulation sedimentation method is widely used in dealing with wastewater, but high operating cost and a large quantity of hazardous sludge are accompanied. What’s more, chemical oxidation demand (COD) value still do not satisfy the discharge standards. Therefore, a technical support for the treatment of electroplating wastewater is urgently in need.
Recently, electrocatalytic oxidation has a rapid development as one of the ways to degrade bio- refractory organic pollutants [5-8]. It has the advantages of wide adaptation, controllability, short running period. As a result of excellent electrical conductivity, electroplating wastewater is feasible to be processed with electrocatalytic oxidation. But it is still a big problem to handle with the high energy expenditure and low current efficiency of traditional electrocatalytic oxidation method. Thus, the coupling technique is developed.
Lately, technology of magnetism has received widespread concern. It was reported that the energy and efficiency of water electrolysis was considerably strengthened under a high magnetic field and a reduction in the cell voltage occurred in a magnetic field [9]. The adhibition of the magnetic field enhanced the —NO2 group electro-reduction [10]. And it was confirmed that in a Fenton reaction system, the mineralization of methyl blue was improved by over 10% with magnetic field [11]. However, the mechanism of magnetic field assisted reaction is still not well understood and there has been less reports on the magnetic field assisted electrocatalytic oxidation by now.
In this work, the objective is to provide references for the removal of the organic pollutants in electroplating wastewater using electrocatalytic oxidization technology through exploring the effect of magnetic field on electrocatalytic oxidization of the wastewater. Besides, the factors affecting the removal of organic pollutants in plating wastewater were also investigated, which consist of the treatment time, the current density and the initial pH.
Table 1 Water quality indexes of wastewater used
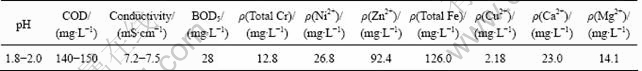
2 Experimental
2.1 Wastewater
The plating wastewater used in this work was collected from an electroplating factory located in Jiaxing City, Zhejiang Province, China. The water quality indexes are listed in Table 1. The average biological oxygen demand (BOD)/COD ratio was below 0.2 and the conductivity ranged from 7.2 to 7.5 mS/cm. The wastewater had a high concentration of metal ions and was strongly acidic.
2.2 Analytical methods
2.2.1 GC-MS analysis
Firstly, a 200 mL of the wastewater after filtration was adjusted to pH 11 with sodium hydroxide, extracted with ether, and then made acidic to pH 2 with concentrated hydrochloric acid. The aqueous layer was respectively re-extracted twice at pH 11 and pH 2. Thirdly, the extracts were combined and anhydrous sodium sulfate was added to the extract to absorb water. Lastly, the extract was concentrated to about 1 mL in a Kuderna-Danish [12].
A gas chromatography–mass spectrometry (GC/MS; Agilent 5973 GC analyzer, USA) was used for the identification of the organic compounds; GC was equipped with a HP-5 capillary column (length 30 m, i.d. 0.25 mm, liquid film 0.25 μm). The injector (splitless) was held at 250 °C. The oven temperature started at 60 °C and held for 2 min and ramped linearly to 250 °C at rate of 20 °C/min and remained constant for 5 min. Helium gas at a flow rate of 1 mL/min was used as carrier gas. The transfer line from GC to MS was set to be 250 °C. Mass spectrometer was operated in electron ionization mode (EI, 70 eV), while ion source was controlled at 250 °C. Scans were run over the range from m/z=50 to 650 at a rate of 1 s per scan.
2.2.2 Other analysis
XRD characterization was assayed by Rigaku D/MAX-RA diffractometer with Cu Kα radiation (X’Pert PRO, Holland) while atomic absorption spectro- photometer (Shimadzu AA-6300C, Japan) was used to monitor the concentrations of metal ions.
The concentration of hydrogen peroxide was analyzed by the spectrophotometry using potassium titanium(IV) oxalate [13].
Measurements of COD and BOD were based on the National Standard Method of China. The solution pH was conducted with a pH330i pH-meter (WTW, Germany). Conductivity was determined by a Cond303i conductivity measurer (WTW, Germany).
2.3 Equipment and procedures
Figure 1 illustrates the systematic process of the experiment. The experiment was carried out in intermittent conditions. The reactor (h=11.5 cm, φ= 6.5 cm) was a self-made columnar plexiglass. A Ti-PbO2 sheet obtained from Hangzhou Dakang Environmental Engineering Company Limited, China, acted as anode (6.0 cm×4.5 cm) and a stainless steel acted as cathode (6.0 cm×4.5 cm), both fixed in the cover of reactor. The magnetic field was performed with two magnets with dimension of 15.5 cm×10.0 cm. During experiment, the two magnets were placed in the reactor on both sides where the magnets were apart from 11 cm. Meanwhile, the magnetic density was measured as 22 mT. The reactor kept a volume of 300 mL, and 250 mL electroplating wastewater was treated every time. If required, the initial pH of wastewater was adjusted with 10% sodium hydroxide solution.
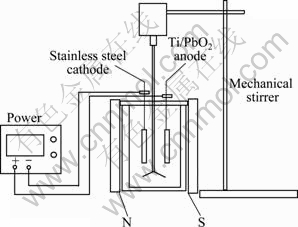
Fig. 1 Schematic diagram of experiment
Experiments were prepared as follows: First, the wastewater was filtered through the qualitative filter paper, then the pH and conductivity of the filtrate were determined. Thereafter, 250 mL reaction liquid was fed into the reactor, and magnetic field was joined based on the experiment. Second, the mechanical agitator was run at the rate of 100 r/min, and constant-current power was started. Last, after reacting for 2 h, samples were taken from reaction product to measure the concentration of H2O2 and COD.
3 Results and discussion
3.1 Electrode characterization
The phase composition and structure of the anode were investigated by XRD analysis as illustrated in Fig. 2. Strong diffraction peaks appear at 2θ=25.6°, 32.1°, 36.6°, 49.7°, indicating that the crystal structure of the anode is β-PbO2. The individual grains with an average dimension of 41 nm are quadrangular. In Ref. [14], it is pointed out that the anode covered with β-PbO2 has good electrocatalytic activity.

Fig. 2 XRD pattern of anode
3.2 Influence of experimental conditions
3.2.1 Treatment time
The influence of reaction time on COD removal rate and H2O2 concentration is reported in Fig. 3. As expected, the introduction of magnetic field enhances the degradation and mineralization of organic compounds in the wastewater. Compared with the condition without magnetic field, the removal rate of COD in a magnetic field of 22 mT increases by 7.3%-15.2%. At the same time, H2O2 concentration is higher than that without magnetic field, which is consistent with the trend of COD removal rate. A high H2O2 concentration can promote the degradation of organic compounds, mainly due to Fenton reaction as there is a high concentration of Fe in the wastewater.
3.2.2 Current density
Figure 4 presents the effect of current density on COD removal rate and H2O2 concentration. As the current density increases, the removal rate of COD gradually increases. In addition, the removal efficiency of COD in a magnetic field of 22 mT is obviously higher than that without magnetic field. Also, as can be seen from Fig. 4, the magnetic field significantly promotes the generation of H2O2, and H2O2 concentration and current density are linearly related. The cause needs further research.
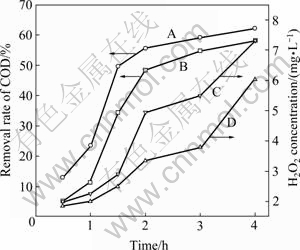
Fig. 3 Effect of reaction time on COD removal rate and H2O2 concentration (pH, 1.8; current density, 50 A/m2; room temperature): A—Removal of COD in magnetic field of 22 mT; B—Removal of COD without magnetic field; C—Concentration of H2O2 in magnetic field of 22 mT; D—Concentration of H2O2 without magnetic field

Fig. 4 Effect of current density on COD removal rate and H2O2 concentration (pH, 1.8; room temperature; reaction time, 2 h): A—Removal of COD in magnetic field of 22 mT; B—Removal of COD without magnetic field; C—Concentration of H2O2 in magnetic field of 22 mT; D—Concentration of H2O2 without magnetic field
3.2.3 pH
The effect of the initial pH of wastewater on COD removal rate and H2O2 concentration (Fig. 5) was studied under the condition of current density of 50 A/m2. From Fig. 5, we can see that the degradation of organic compounds is accelerated by the magnetic field.
It is interesting that the COD removal efficiency increases while initial pH is from to 7, but it decreases with pH from 7 to 11. To find out the possible reason, the conductivity and metal ions concentrations were measured, and the results are shown in Table 2 (The concentration value of metal ions in solution is an average of measurements obtained in three replicates). According to precipitation-dissolution equilibrium, within a certain range, the higher the pH value is, the lower the metal ion density of the solution is. Moreover, when wastewater contains several types of metal ions, there will be coprecipitation, and the pH that the coprecipitation needs is lower than that a single metal ion requires. Under acid conditions, there is a high iron ion concentration in the solution, and the Fenton reaction is prominent.
There is a hydrolysis equilibrium in the reaction:
H2O2![]() H++HO2-
H++HO2-
So with the increase of pH, the equilibrium shifts right. The increase of pH accelerates H2O2 breaking up to H+ and HO2- and speeds up the reaction. But under alkaline conditions, the metal ion concentrations are so low that the Fenton reaction can be ignored. And the increase of pH decreases the potential oxygen generation and consequently boosts the flow rate of oxygen at the electrode surface [15]. As a result, the diffusion flow of organic compounds towards the electrode decreases, leading to the decrease of degradation of organic compounds.
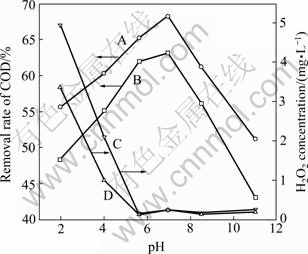
Fig. 5 Effect of pH on COD removal rate and H2O2 concentration (current density, 50 A/m2; room temperature; reaction time, 2 h): A—Removal of COD in magnetic field of 22 mT; B—Removal of COD without magnetic field; C—Concentration of H2O2 in magnetic field of 22 mT; D—Concentration of H2O2 without magnetic field
3.3 Mechanism
3.3.1 H2O2 concentration
As known to all, there would be active species generated in the electrochemical oxidation, such as hydroxyl, ozone and hydrogen peroxide [16]. This work selects H2O2 as representative for research. O2 adsorbed on the surface of the catalyst forms oxygen radicals through capturing electronics, then after a series of reactions, it transforms into hydrogen peroxide. The generation of H2O2 is illustrated in the following equations:
O2+e→O2-
or O2+H+→HO2
2H2O→H2O2+O2
O2-+HO2?→O2+HO2-
HO2-+H+→H2O2
The H2O2 concentration in the wastewater is presented in this work (Figs. 2-4). In the acid solution, the magnetic field enhances the generation of H2O2 in the electrochemical oxidation. The existence of H2O2 strengthens Fenton reaction, arousing higher formation rate of hydroxyl radical or prolonging longevity of hydroxyl radical [17], so that more organic compounds are degraded.
3.3.2 Cell voltage
Figure 6 shows the cell voltage measured when the current density is increased. Noted that a magnetic field could make voltage drop. As reported in Refs. [18-19], due to the magnetic hydrodynamic (MHD) effect caused by the Lorentz force acting on moving charged particles, the magnetic field could enhance the movement of anion. Therefore, the applied magnetic field would result in a decrease in cell voltage and energy expenditure.
Table 2 Conductivity and concentrations of metal ions in wastewater at different pH
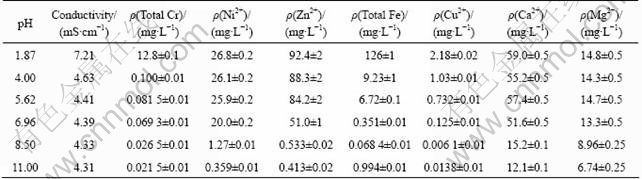
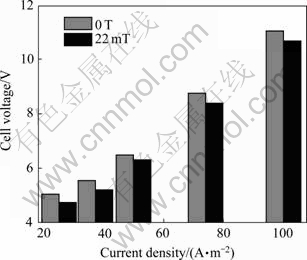
Fig. 6 Cell voltage vs current density curves (pH, 1.80; room temperature; reaction time, 2 h)
3.3.3 Analysis of wastewater
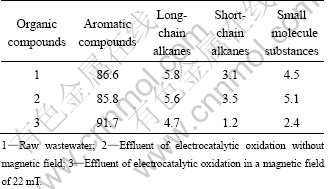
The organic components of the wastewater (the raw wastewater, effluent of electrocatalytic oxidation without magnetic field and in a magnetic field of 22 mT) were analyzed by GC-MS. Electrochemical oxidations were carried on at room temperature, initial pH 1.80 and 50 A/m2. The results are shown in Fig. 7 and Table 3. Comparing Figs. 7(a), (b) and (c), the magnetic field improves the degradation of organic compounds at different degrees, especially the alkanes and small molecule substances. As stated above, a proposed reaction mechanism is as follows: Owing to MHD, the magnetic field enhances the anion movement as well as the reaction rate, giving rise to a decrease in cell voltage. Besides, the magnetic field accelerates the generation of active species (the research focused on H2O2 concentration), which would result in more organic compounds to be oxidized and degraded. However, the clearer mechanism should be further studied by analysis of the formation of active species and intermediates.
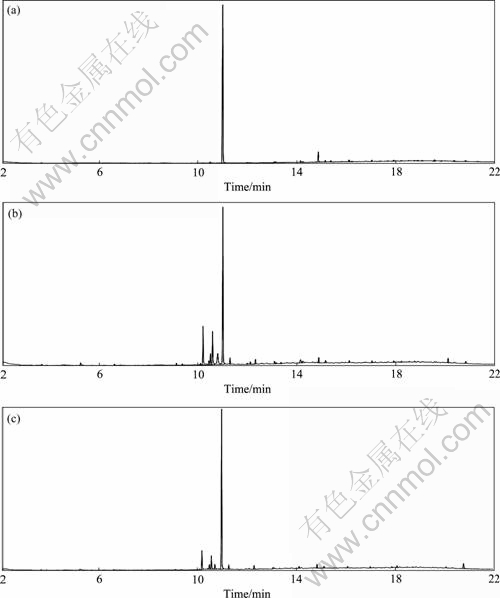
Fig. 7 Gas chromatograms of wastewater with ether as extract: (a) Raw wastewater; (b) Effluent of electrocatalytic oxidation without magnetic field; (c) Effluent of electrocatalytic oxidation in magnetic field of 22 mT
Table 3 Changes in relative amount of organic compounds in wastewater (mass fraction, %)
4 Conclusions
1) The introduction of magnetic field enhances the degradation and mineralization of organic compounds in the wastewater. Compared with no magnetic field, removal rate of COD in a magnetic field of 22 mT increases by 7.3%-15.2% at different reaction time as current density is 50 A/m2 and pH is 1.80 at room temperature.
2) The H2O2 concentration with magnetic field is higher than that of no magnetic field, which is consistent with the trend of COD removal rate. A high H2O2 concentration could promote the degradation of organics, mainly due to Fenton reaction as there is a high concentration of Fe in the wastewater.
3) The magnetic field enhances the anion movement and the generation of active species, which results in more organic compounds to be oxidized and degraded.
References
[1] CAVACO S A, FERNANDES S, QUINA M M, FERREIRA L M. Removal of chromium from electroplating industry effluents by ion exchange resins [J]. Journal of Hazardous Materials, 2007, 144(3): 634-638.
[2] KUMAR R, BISHNOI N R, GARIMA, BISHNOI K. Biosorption of chromium(VI) from aqueous solution and electroplating wastewater using fungal biomass [J]. Chemical Engineering Journal, 2008, 135(3): 202-208.
[3] SOUSA F W, SOUSA M J, OLIVEIRA I R N, OLIVEIRA A G, CAVALCANTE R M, FECHINE P B A, NETO V O S, KEUKELEIRE D D, NASCIMENTO R F. Evaluation of a low-cost adsorbent for removal of toxic metal ions from wastewater of an electroplating factory [J]. Journal of Environmental Management, 2009, 90(11): 3340-3344.
[4] CHEN S S, LI C W, HSU H D, LEE P C, CHANG Y M, YANG C H. Concentration and purification of chromate from electroplating wastewater by two-stage electrodialysis processes [J]. Journal of Hazardous Materials, 2009, 161(2/3): 1075-1080.
[5] SCIBAN M, RADETIC B, KEVRESAN D, KLASNJA M. Adsorption of heavy metals from electroplating wastewater by wood sawdust [J]. Bioresource Technology, 2007, 98(2): 402-409.
[6] CHIANG L C, CHANG J E, TSENG S C. Electrochemical oxidation pretreatment of refractory organic pollutants [J]. Water Science and Technology, 1997, 36(2/3): 123-130.
[7] MARTINEZ-HUTITLE C A, BATTISTI A D, FERRO S, REYNA S, CERRO-LOPEZ M, QUIRO M A. Removal of the pesticide methamidophos from aqueous solutions by electrooxidation using Pb/PbO2, Ti/SnO2 and Si/BDD electrodes [J]. Environmental Science & Technology, 2008, 42(18): 6929-6935.
[8] UN U T, ALTAY U, KOPARAL A S, OGUTVEREN U B. Complete treatment of olive mill wastewaters by electrooxidation [J]. Chemical Engineering Journal, 2008, 139(3): 445-452.
[9] CHEN X M, GAO F R, CHEN G H. Comparison of Ti/BDD and Ti/SnO2-Sb2O5 electrodes for pollutant oxidation [J]. Journal of Applied Electrochemistry, 2005, 35(2): 185-191.
[10] IIDA T, MATSUSHIMA H, FUKUNAKA Y. Water electrolysis under a magnetic field [J]. Journal of the Electrochemical Society, 2007, 154(8): 112-115.
[11] CHATELUT M, VITTORI O. Influence of an external magnetic field on the electrochemical behaviour of ortho, meta and para nitrobenzaldehydes and nitrobenzoic acids in water dioxane solvent [J]. Elecrrochimica Acta, 1997, 42(15): 2355-2359.
[12] HAO X L, ZOU L Y, ZHANG G S, ZHANG Y B. Magnetic field assisted Fenton reactions for the enhanced degradation of methyl blue [J]. Chinese Chemical Letters, 2009, 20(1): 99-101.
[13] KEITH L H. Identification of organic-compounds in unbleached treated kraft paper-mill wastewaters [J]. Environmental Science & Technology, 1976, 10(6): 555-564.
[14] ROBIN M, SELLERS. Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) Oxalate [J]. Analyst, 1980, 105: 950-954.
[15] CASELLATO U, CATTARIN S, MUSIANI M. Preparation of porous PbO2 electrodes by electrochemical deposition of composites [J]. Electrochimica Acta, 2003, 48(27): 3991-3998.
[16] SAMET Y, ELAOUD S C, AMMAR S, ABDELHEDI R. Electrochemical degradation of 4-chloroguaiacol for waster water treatment using PbO2 anodes [J]. Journal of Hazardous Materials, 2006, B138(3): 614-619.
[17] ZHOU M H, DAI Q Z, LEI L C, MA C A, WANG D H. Long life modified lead dioxide anode for organic wastewater treatment: electrochemical characteristics and degradation mechanism [J]. Environmental Science & Technology, 2005, 39(1): 363-370.
[18] BUND A, KOEHLER S, KUEHNLEIN H H, PLIETH W. Magnetic field effects in electrochemical reactions [J]. Electrochimica Acta, 2003, 49(1): 147-152.
[19] CHO M S, YUN Y Y, NAM J D, SON Y, LEE Y. Effect of magnetic field on electrochemical polymerization of EDOT [J]. Synthetic Metals, 2008, 158(21/22/23/24): 1043-1046.
(Edited by HE Yun-bin)
Foundation item: Project(2008ZX07101-006-09) supported by the Major Science and Technology Program for Water Pollution Control and Treatment of China
Received date: 2011-03-22; Accepted date: 2011-06-15
Corresponding author: SHI Hui-xiang, Professor, PhD; Tel: +86-571-81021986; E-mail: shhx188@163.com
Abstract: To degrade the organic compounds in the electroplating wastewater, magnetic field was tentatively introduced into electrocatalytic oxidation on Ti-PbO2 anode. The magnetic field assisted electrocatalytic oxidation can promote anion movement and the generation of active species, resulting more organic compounds to be oxidized and degraded. Oxidation parameters such as treatment time, current density and initial pH of the wastewater were systematically discussed and optimized. The mineralization of organic compounds is improved by over 15% under a magnetic density of 22 mT while the current density is 50 A/m2, pH is 1.8 and the reaction time is 1.5 h. The results indicate that the magnetic field assisted electrocatalytic oxidation has considerable potential in electroplating wastewater treatment.


