文章编号:1004-0609(2011)01-0072-08
Nb-Al系金属间化合物及其复合材料研究进展
罗 民1, 陈焕铭2,王怀昌1, 何力军2,李 星1
(1. 宁夏大学 化学化工学院, 银川 750021; 2. 宁夏大学 物理电气信息学院, 银川 750021)
摘 要:
综述了Nb-Al系金属间化合物作为高温结构材料的最新研究进展和发展趋势。对目前国内外Nb-Al系金属间化合物及其复合材料的制备工艺、组织结构控制和力学性能的研究现状进行评述。结果表明:通过延性相增韧、合金化、层状结构设计、复合材料设计等方法,可以显著改善Nb-Al金属间化合物的室温脆性、抗氧化能力、高温强度及抗蠕变性能。Nb-Al系金属间化合物的研究方向应集中发展以Nb3Al及NbAl3金属间化合物为基体,以SiC、Al2O3及TiC等陶瓷相为增强相强化的陶瓷-铌基合金复合材料。
关键词:
中图分类号:TF841.6 文献标志码:A
Research progress of Nb-Al system intermetallics and composite
LUO Min1, CHEN Huan-ming2, WANG Huai-chang1, HE Li-jun2, LI Xing1
1. School of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China;
2. School of Physics and Electrical Information Engineering, Ningxia University, Yinchuan 750021, China)
Abstract: The recent research progress and prospects of Nb-Al intermetallics compounds for structural applications were discussed. The processing and mechanical properties of the Nb-Al intermetallics and their composites were reviewed. The results show that the ductile phase toughening, alloying, microlamination and composite design can improve the brittle fracture at room temperature, oxidation resistance, high-temperature strength and creep resistance. The research on Nb-Al system materials should be focused on the ceramics phase (SiC, Al2O3 and TiC) reinforced Nb3Al and NbAl3 matrix composites.
Key words: Nb-Al system; intermetallics; composite; high-temperature structure materials
金属间化合物由于具有密度小、熔点高、高温性能优越、化学稳定性良好等特点,在航空、舰艇和工业用燃气轮机的高温部件、航天器及火箭发动机、核反应堆、石油化工设备等领域应用具有独特的优势。在近十多年来,工业发达国家,如美国、日本、欧洲都制定了全国性的研究计划来大力发展金属间化合物结构材料,其长远目标是发展具有比Ni基高温合金性能更好的高温结构材料,特别注重发展一种使用温度和力学性能介于Ni基高温合金和高温陶瓷材料之间的高温材料、使用温度更高而高温力学性能特别是强韧性良好的材料[1]。
金属铌熔点较高、延展性和导热性优良,是密度最低的难熔金属(密度为8.6 g/cm3)。铌与铝形成的金属间化合物具有优良的高温强度、较高的熔点和较低的密度。这种材料与目前广泛研究和应用的Fe-Al系、Ni-Al系、Ti-Al系相比,具有更高的熔点和适中的密度(见表1),是一种潜在的高温结构材料。同时,Nb-Al也可作为一种高电流、高磁场下的超导材料来使 用[2-3]。由于具有复杂的晶体结构和有限的滑移带,铌铝金属间化合物的室温塑性和韧性差,因此,提高延性和增韧是其获得工业应用的必备条件。制约Nb-Al应用的另一个主要原因是其抗氧化性能较弱,铌及其合金材料在空气气氛中600 ℃以上会发生严重氧化,导致脆性断裂。通常采用合金化或延性相增韧来避免其室温脆性,通过合金化和金属陶瓷复合工艺改善其抗氧化性能。
目前研究较多的Nb-Al系金属间化合物主要包括Nb3Al和NbAl3。Nb3Al金属间化合物的高温屈服强度非常高,在1 200 ℃为800 MPa,1 300 ℃为500 MPa。Nb2Al合金很少作为结构材料来使用,单相Nb2Al非常脆, 且韧脆转折温度(BDTT)高于1 150 ℃。通过元素粉末热压反应制备单相Nb2Al的压缩实验表明,在 1 200 ℃以下发生脆性断裂, 但屈服强度比单相Nb3Al合金的屈服强度高(当应变速率为1×10-4 s-1时, 温度为1 300 ℃时为870 MPa, 温度为1 500 ℃时为290 MPa)[4]。NbAl3合金的熔点高、密度低和高温氧化性能好,在Nb-Al系3种金属间化合物中具有最低的氧化速率[5]。近年来,NbAl3合金的高温抗氧化性能可以通过添加Cr、Y和Si的微合金化过程来改善[6] 。这些元素的添加导致铝的选择性氧化,在NbAl3合金表面形成一层连续致密的氧化铝保护层。Nb-40%Al- 8%Cr-l%Y-l%Si 合金的抗氧化性能与Ni-30%Al-l%Zr的抗氧化性能相当。本文作者综述目前Nb-Al系化合物的最新研究进展,介绍Nb-Al系金属间化合物及其复合材料的制备、性能和研究现状,并分析其发展 趋势。
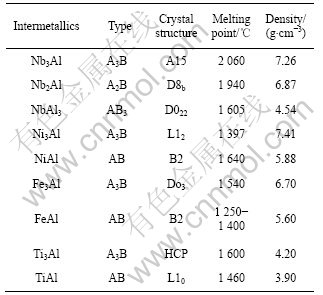
表1 金属间铝化合物的晶体结构和基本物理性能 [6-7]
Table 1 Crystal structure and basic physical properties of aluminides[6-7]
1 Nb-Al金属间化合物的制备方法
Nb-Al合金在熔炼过程中,由于铌和铝的密度和熔点存在很大的差异,容易导致低熔点元素的挥发,Nb和气氛气体、坩锅或模具之间的反应, 从而影响合金收率。电子束熔炼、真空或氩电弧熔炼、等离子电弧熔炼是熔铸Nb-Al合金的常用方法。电子束熔炼时,由于其能量高导致Al的挥发很严重,难以获得所需要的合金成分;电弧熔炼是目前广泛采用的制备难熔金属间化合物的方法,此方法可用于制备具有超高熔点的材料。OKABE等[8]用铝和钙还原氧化铌制备铌铝化合物,但必须通过酸溶液来沥滤其产物中的Ca-Al合金及CaAl2和CaO等杂质。目前,工业上采用铝热还原方法制得NbAl中间合金,在中间合金的基础上添加Nb或Al制得不同Al含量的Nb-Al合金。
不同方法熔炼的铸锭的凝固组织存在明显差别。定向凝固的Nb-Al合金铸锭的共晶组织中不产生“原位”排列的复合组织。为了改善这种共晶形貌, 在合金中添加Ti和Ni可以改变二元系的球状形貌。电弧熔炼Nb-Al合金时,加入2% Ti(摩尔分数)后, 合金铸锭的共晶形貌不变, 只改变NbAl3的枝晶形貌, 所产生的枝晶是很细小的鱼骨形组织, 而枝晶间组织形貌呈球形;当加入2%Ni(摩尔分数)时, 产生的枝晶形貌呈细小棒状。KAMATA等[9]采用高频感应凝壳炉熔炼Nb-Al合金,研究熔体与各种氧化物(Y2O3、MgO、ZrO2、HfO2、CaO)模具的反应性以实现Nb3Al合金的精密铸造, 其中,在Y2O3铸模中铸出的精密铸件无裂纹且外观良好,表层组织也无反应层,相组成与化学成分都很均匀,各组分的偏差可控制在l%(摩尔分数)以下,杂质元素氧和氮的含量在0.03%(摩尔分数) 以下。
机械合金化方法是制备Nb-Al合金的方法之一,可有效控制微观组织, 细化晶粒, 从而提高材料的力学性能。用机械合金化方法制备的Nb-Al合金粉末为晶粒尺寸可达纳米级的NbAl3金属间化合物,而富Nb的Nb3Al相只能通过后续热处理工艺才能得到。DOLLAR等[10]和DYMEK等[11] 用机械合金化-热压工艺制备了致密无裂纹的Nb3Al基合金。TRACY和GROZA[12]用Nb粉和Al粉在872~877 ℃加热后球磨形成Nb3Al粉末,粉体在67 Pa真空度,30 MPa压力,1 200 ℃进行等离子体烧结4 min后达到理论密度,样品的维氏硬度为10 GPa,如此高的硬度是由于晶粒细化和在球磨过程中用己烷作为球磨介质形成了部分碳化铌。
自蔓延高温合成(SHS)是利用一定配比原料自身的燃烧反应放热使化学反应过程自发持续进行,从而获得具有指定成分和结构产物的一种新型材料合成手段,具有能耗低、反应时间短、设备简单、产物纯度高等优点。当使用元素粉末高温自蔓延或燃烧合成来制备Nb-Al合金时, 由于Nb-Al体系化学反应放热量少,需要混合配比粉体在较高温度预热、保温或采用机械活化法活化粉体,降低活化能,还可以添加少量的B来增加反应的放热量[13-17]。MILANESE等[18]研究了NbAl金属间化合物的高温自蔓延机理。根据原料的计量比不同产生两种燃烧反应机制,即溶解-沉淀机理、固态反应机理。BHATTACHARYA等[14, 17]使用Nb + 0.33A1 + 3%B 燃烧合成了以Nb2Al为基体、Nb和Nb3Al为第二相、NbB颗粒沿晶界分布的复合材料,该材料的强度和韧性都远远高于单相Nb2Al合金的强度和韧性。GAUTHIER等[19-20]利用机械活化自蔓延高温合成(MASHS)技术制备纳米结构的NbAl3粉体:首先将Nb+3Al混合粉末预先进行机械活化,压制成块状样品,在500~600 ℃下进行自蔓延反应生成50%~55%理论密度的Nb3Al纳米粉。机械合金化过程破坏了元素粉体表面的氧化层,提高了反应物的接触面积,缩短了反应扩散距离。Nb3Al的成核过程是分散在Al(Nb)熔液中的铌表面的异质成核过程。
通过铝热反应自蔓延烧结制备Nb-Al是一种更为有效的方法。Nb3Al、Nb2Al和NbAl3 的反应焓变分别为-77.0、-74.9和-130.5 kJ/mol,铝热反应的放热量更多,有利于自蔓延合成;同时,以廉价的氧化铌作为铌源,降低了生产成本。合成反应分为两部分:首先氧化铌被铝还原得到金属铌和氧化铝副产物,然后铌和铝反应形成铌铝金属间化合物。YEH和WANG[21]研究了用铝热自蔓延高温烧结反应制备铌铝金属间化合物。结果表明:调节起始原料Al与Nb2O5的配比,可有效地控制自蔓延反应过程。当起始Al与Nb2O5的摩尔比为4.0~6.67时,形成持续的燃烧波,扩散速率随铝含量的增加而逐渐降低(34.3~10.5 mm/s),当Al与Nb2O5的的摩尔比大于7时,燃烧过程停止,这是由于随铝含量增加,反应放热量逐渐减少。
STAFFORD和HAARBERG[22]研究了铌在熔盐电解质AlCl3和NaCl (AlCl3与NaCl的摩尔比为52?48)中的溶解和Nb-Al合金的电沉积过程,发现铌在电压为1.1 V 开始氧化,铝的活性溶解在电压为1.5 V时开始。在所得到的Nb-Al合金中,Nb原子的摩尔分数为3.0%~13.4 %。虽然铌可以不断溶解,但是由于它在熔体中的溶解度太低且化学稳定性差,真正能用于电沉积的电化学活性离子的有效浓度很低。铌的电沉积反应由电流密度决定,而铝的电沉积反应则由动力学或综合因素决定。
YAN和FRAY[23]采用直接电化学还原氧化铌和氧化铝的混合物的方法制备Nb-Al合金。将混合氧化物压成阴极电极,以熔融的CaCl2或CaCl2-XCl(X=Na、Li)为电解质在900~950 ℃进行电解,阴极电极中的氧离子溶解在熔体中,在阳极放电,阴极还原成粒径为10 μm左右的Nb3Al和Nb2Al复合粉体。
2 Nb-Al金属间化合物基复合材料的研究现状
最初,Nb3Al被认为是具有高超导转变温度的超导材料而广为重视和研究。近来,铌合金又成为具有潜在应用价值的新一代高温结构材料,人们进一步研究了Nbss/Nbss+Nb3Al和Nbss+Nb3Al/Nb3Al的相线和 1 600 ℃以下的部分相图(见图1)[24]。为了研究和改善高铌Ti-Al合金的力学性能,Nb-Ti-Al三元合金相图体系得到广泛的研究。LEONARD和VASUDEVAN [25]研究了富铌的Nb-Ti-Al合金体系在700、900及1 100 ℃的凝固路径和相平衡。然而,三元相图富铌端 的等温截面至今尚未研究清楚。LEONARD和
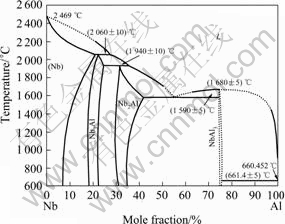
图1 Nb-Al合金相图[24]
Fig.1 Phase diagram of Nb-Al alloy[24]
VASUDEVAN[25]研究了含钛量小于 30%、温度为1 000、1 200及1 650 ℃时 Nbss/Nb3Al的相截面。虽B2有序相的范围还没有完全确定,但认为B2相存在于一个较宽的成分和温度范围。由于在Nb-Al体系中并没有观察到B2相,因此,可以认为Ti的添加有利于B2相的稳定存在。为了了解合金化元素对Nb3Al或原位复合材料Nbss/Nb3Al高温强度的影响,在1 600 ℃研究了A15相周围Nb-Al-X(X=Mo、Ta、W)的等温截面,Mo的添加形成Nb3Al-Mo3Al体系,而Ta和W在Nb3Al中的溶解度分别是30%和12%。
低对称性的晶体结构具有有限的滑移体系和破坏模式,因而大部分的金属间化合物具有本征脆性。利用合金化可以改变金属间化合物的晶体结构,提高对称性,为材料变形提供附加的滑移带,从而改善其韧性[26]。二元Nb-Al合金在1 000 ℃以上具有极高的强度,通过添加铌基固溶体(Nbss)控制其组成和相结构,可极大地改善其室温韧性和高温强度。抗氧化性能的改善可以通过在NbAl3中添加Ti、Cr、Y、Si等元素,或在Nbss中添加Ti、Al、Cr等元素实现。
RIOS等[27]研究了Nb-Al-X(Cu、Si、Ni、Ti、Cr)三元合金体系。对Al57.2Nb40.4Ni2.4共熔合金的研究表明:由NbAl3、Nb2Al和NbNiAl(C14)三相组成的Nb-Al-Ni合金的高温强度、韧脆转变温度(BDTT)和抗氧化性能可以通过调节合金成分控制。随着Ni含量的增加,即NiAl含量的增加,高温强度和BDTT降低,而断裂韧性和抗氧化性能增强。凝固工艺影响合金的相组织结构,在较低的凝固速率下形成固相规则排列的纤维状形貌的显微组织结构;反之,在非常高的凝固速率下得到非常细小的不规则共熔体[28]。由电弧熔炼定向凝固得到的Al-40.4Nb-2.4Ni三元低共熔合金,其相组织沿固化方向对齐排列,呈纤维状规则排列的微观组织结构。合金的硬度为(9.98±0.32) GPa,高于定向凝固制备的合金的硬度(8.49±0.26) GPa,共熔合金的断裂韧性为(2.18±0.24) MPa?m1/2[29]。
RONG等[30-31]研究了Nb-10Al-20V, Nb-15Al-20V 和 Nb-25Al-40V(摩尔分数,%) 3种合金。结果表明:Nb-Al-V合金可以通过适当的热处理,形成以A2或B2相为基体,沉淀析出A15和σ相的组织状态。室温时上述3种合金均为沿晶断裂,由于位错滑移和攀移导致高温破坏。JIAO等[32]在Nb-15Al-20V合金中观察到的拉伸和压缩变形行为完全不同,在拉伸时合金非常脆,而在压缩时则表现出很好的延展性。两者的不同可以用变形破坏后的位错结构来解释。在拉伸时位错的伯格斯矢量(![]() ) 沿{110}晶面滑移,位错具有很低的移动性,堆积位错队列随处可见;而在压缩时滑移面为{112},位错具有良好的移动性。
) 沿{110}晶面滑移,位错具有很低的移动性,堆积位错队列随处可见;而在压缩时滑移面为{112},位错具有良好的移动性。
MURAKAMI等[33-34]研究用机械合金化和活化等离子体烧结(SPS)制备Nb-Al、Nb-Al-W、Nb-Al-Mo及Nb-Al-N合金的方法。SPS具有烧结时间短、效率高、能有效地抑制晶粒的粗化,从而得到纳米结构材料。由于Nb-Al-W 和Nb-Al-Mo的组织不均匀,要获得均匀的组织结构,需要更高的烧结温度(1 773 K)和机械合金化活化粉体,而Nb-Al-N合金则可以通过Nb和AlN反应得到Nb2N弥散强化的高强度合金。
GNANAMOORTHY等[35]采用高频感应凝壳炉熔炼Nb-18%Al-10%Ta合金。在真空度为4×10-4Pa,温度为1 900 ℃均匀化热处理5 h后等温锻造,该合金的断裂韧性远远优于单相Nb3Al合金的断裂韧性,且断裂韧性随延性相的增加而增强。延性相Nbss促使裂纹尖端钝化,裂纹桥接和分叉极大地改善了裂纹的扩展情况。
YE等[36-39]研究了铸态Nb-15Al-xTi(x=10%、25%、40%,摩尔分数)合金的断裂韧性和疲劳裂纹生长行为。结果表明:当Ti含量为10%和25%时,合金是脆性断裂;而Ti含量为40%时,合金是韧窝断裂,断裂韧性从10~30 MPa?m1/2增加到100~110 MPa?m1/2。增韧是由于裂纹尖端的塑性变形区导致裂纹尖端屏蔽,使裂纹钝化、裂纹尾部被拉伸和形成桥联,减小裂纹尖端的应力强度因子,减缓裂纹扩展速度,阻止裂纹进一步张开,从而改善材料的断裂韧性。原子模拟结果也表明塑性的增加与钛含量较高有关。
为了改善合金的高温强度和低温断裂韧性,使用碳化铪弥散钨丝(WHfC)作为延性增强相[40]。WHfC/ NbAl3在27~1 027 ℃具有较高的强度和韧性,增韧效果通过计算四点弯曲实验下应力—位移曲线积分面积断裂功来评价。然而,由于热膨胀系数失配,延长热处理时间和热循环次数将导致力学性能的蜕变[40]。在Nb-Al相图中,当Al含量为9%~18%时,Nbss与Nb3Al两相共存,表明可以制备含有难熔金属Nbss和高熔点金属间化合物Nb3Al的原位复合材料。由脆性金属间化合物母相和延性Nbss相组成的复合材料表现出均衡的力学性能。其中,Nbss起室温增韧作用,而Nb3Al起高温增强的作用。通过真空热压和反应烧结元素粉体制备的Nbss/Nb3Al原位复合材料的断裂韧性为6.5 MPa?m1/2,而单相Nb3Al合金的断裂韧性仅为1.1 MPa?m1/2 [41]。热处理工艺对材料的性能也具有很大的影响,在快速冷却过程中保留下来的过饱和Nbss经热处理后沉淀析出NbAl金属间化合物,极大地提高了强度。另一方面,Nbss的存在明显降低了韧脆转变温度,以降低部分高温强度为代价来提高室温断裂韧性。在维氏硬度测试中发现,延性颗粒能有效地阻止小裂纹的生长,在Nb3Al/Nbss原位复合材料中添加Ti、Ta、Mo及W可在保持其室温韧性的情况下增加其高温强 度[42]。BHATTACHARYA[17]采用燃烧法合成了Nb颗粒为韧性相、Nb2Al为基体的复合材料。由于Nb燃烧反应不完全,因此,未完全反应的延性Nb颗粒相发挥着韧性强化的作用,极大地提高了该材料的断裂韧性。通过优化反应速率、控制Nb颗粒的大小、调节基体和延性颗粒的结合状态来优化材料的韧性。因此,需要进一步研究,以便在高温强度和室温韧性之间需要寻找最佳平衡,通过控制相的组成、分布及尺寸等实现固熔强化和弥散强化以提高合金的强度和韧性。
ROWC和SKELLY[43]利用高速磁控溅射原位制备了多层Nb3Al和Nb微层板结构。结果表明,这是一种制备插入式复合材料的有效方法,复合材料的室温断裂韧性证实了在Nb3Al中扩展的裂纹被延性Nb层所捕获。BLOYER等[44]研究了层状微结构Nb/Nb3Al复合材料的断裂行为和阻力曲线(R曲线)。该材料由15层500 μm层厚的脆性Nb3Al层和125 μm层厚的延性Nb金属层交错叠层排列冷压而成,然后在1 680 ℃、37 MPa热压烧结40 min,得到理论密度为98%的复合材料。在这种层状结构中,只需要20%的Nb作为增强相就可以起到很好的增韧效果。Nb3Al合金的韧性从1 MPa?m1/2增加到20 MPa?m1/2。此韧性远远高于含20 μm的Nb颗粒或1~2 μm厚的Nb微层板结构(含体积分数为4%的延性相)的Nb/Nb3Al复合材料的韧性。这种延性相增韧归结于在延性层重结晶区域的裂纹钝化,导致Nb层内裂纹尾部的桥接和塑性变形。由于延性层捕获裂纹和塑性变形受到层厚的制约,所以宏观层状结构材料具有比微观层状结构材料更好的增韧效果。以Nb作为增韧相将Nb3Al基体的韧性提高了一个数量级,其韧性也随着Nb层厚度的增加而增加[45-46]。
复合材料,尤其是金属间化合物基复合材料结合了各组成相的优异性能而受到广泛关注,被认为是超级合金的替代者。增强相通过提高弱界面来改善断裂韧性;弱界面相借助裂纹钝化和纤维拔出等增韧机制:如加入SiC晶须等脆性第二相可以在低温下增加裂纹偏折的吸收能;利用高温陶瓷增强相可抑制位错移动和晶界滑移来改善抗蠕变性能[47]。铌基合金在高温下具有较高的强度及较低的蠕变速率,铌基合金引入陶瓷基体中可改善复合材料的韧性,因而有望成为在更高温度下使用的复合材料。
GARCIA等[48-50]通过Al2O3+Nb、Al2O3+Nb+Al和Nb2O5+Al粉末混合球磨、冷等静压及真空烧结制备了Nb-Al/Al2O3复合材料。含铌体积分数为50%的铌铝基复合材料的强度为680 MPa,断裂韧性为(6.3±0.3) MPa?m1/2,硬度为6.4 GPa[47]。de PORTU 等[51]研究了Al2O3-Nb复合材料的疲劳特性,Al2O3-Nb15在高载荷下也表现出很低的磨损速率,这是由于铌是具有延性的软相,铌颗粒和基体的紧密结合,难以从基体表面脱离,铌和氧化铝有相近的热膨胀系数(8.5×10-6 ℃-1)。除了粉末冶金工艺外,利用多孔陶瓷预制体原位反应渗铝工艺也可以制备NbAl/Al2O3复合材料[52-53]。铝热原位置换反应为
![]() (1)
(1)
?Gr(1 400 ℃)=-298.197 kJ/mol
从热力学计算可知,当温度超过1 400 ℃时,铝热反应的吉布斯自由能为负值,说明此时反应能够自发进行。实验结果表明,Al和NbO2润湿充分,铝熔体能自发渗入NbO2多孔陶瓷中,铝与氧化铌反应形成氧化铝,多余的铝与还原所得的金属反应形成Nb-Al相,可以在较低的温度原位得到陶瓷增强相,制备氧化铝/Nb-Al复合材料。
快速凝固工艺可更有效地细化晶粒, 改善化学均匀性。采用该工艺制备的NbAl3和NbAl3+1%TiB2粉末[54],用热等静压制备块体材料。压缩实验结果表明,用TiB2颗粒弥散强化增韧后材料的强度得到了很大的提高。WOYTERA等[55]用真空热压工艺制备了碳化硅(SiC)晶须增强的铌铝金属间化合物复合材料。实验结果表明,SiC陶瓷增强复合材料具有良好的抗高温蠕变性能。PENG[56]在1 400 ℃下采用热压烧结工艺制备了Al2O3及TiC颗粒增韧的铌铝基复合材料。Al2O3和TiC颗粒分布在Nb-Al颗粒的界面处,该复合材料的断裂韧性为5.6 MPa?m1/2,远高于单相的铌铝金属间化合物的断裂韧性。研究结果表明,Al2O3增强Nb-Al复合材料可以改善其常温断裂韧性,提高其在高温下的抗氧化性能 [57]。同时,Al2O3相的存在有助于提高复合材料的硬度(NbAl3的硬度为(7.5±0.2) GPa,而铌铝基陶瓷复合材料的硬度为(14.6±0.2) GPa[48])。通过抑制位错移动和晶界滑移来改善高温蠕变性能,有望获得热强性能优良的陶瓷/金属(合金)复合材料,弥补Nb-Al系合金的化学环境抵御能力缺陷(抗氧化、耐腐蚀和耐冲刷等),扩大Nb-Al系合金本身的使用范围和提高其使用温度,获得具有良好综合力学性能的新型金属(合金)基陶瓷复合材料。
3 结论
1) 国内Nb-Al系金属间化合物及其复合材料的研究还非常有限,所选用的金属间化合物及与之匹配的陶瓷基体形成复合材料的体系很少,因此,寻求更多、更合理的金属间化合物与陶瓷相复合的金属(合金)基陶瓷复合材料是一项有意义和必要的工作,应开发以Nb3Al和NbAl3化合物为基体,以SiC、Al2O3和TiC 等陶瓷相为增强相强化的陶瓷/铌基合金复合材料。通过增强相提高弱界面来改善材料断裂韧性,设计新型的复合材料是进一步的发展方向。
2) 目前所报道的Nb-Al基高温结构材料的机械性能不理想,在室温韧性、高温强度、高温蠕变性能等方面的研究也十分有限,使得金属间化合物基复合材料未能得到大范围的实际应用。一些制备方法目前只适合于实验室研究,难以用于实际工业生产。因此,急需开发新工艺, 充分发挥其潜在的实用价值。
3) Nb-Al系金属间化合物及其复合材料制备工 艺-微观组织结构-力学性能之间的关系、断裂方式和强化机理等还有待进行进一步研究。
References
[1] 陈国良. 金属间化合物结构材料研究现状与发展[J]. 材料导报, 2000, 14(9): 1-5.
CHEN Guo-liang. R & D status and prospect on the ordered structural intermetallics[J]. Materials Review, 2000, 14(9): 1-5.
[2] GLOWACKI B A. Niobium aluminide as a source of high-current superconductors[J]. Intermetallics, 1999, 7(2): 117-140.
[3] GLOWACKI B A,YAN X Y, FRAY D, CHEN G, MAJOROS M, SHI Y. Niobium based intermetallics as a source of high-current/high magnetic field superconductors[J]. Physica C: Superconductivity and Its Applications, 2002, 372: 1315-1320.
[4] HANADA S, TABARU T, GNANAMOORTHY R. Processing and properties of Nb3Al alloys[J]. Acta Metallurgica Sinica: English Letters, 1995, 8(4/6): 477-487.
[5] GAUTHIER V, JOSSE C, LARPIN J P, VILASI M. High-temperature oxidation behavior of the intermetallic compound NbAl3: Influence of two processing techniques on the oxidation mechanism[J]. Oxidation of Metals, 2000, 54(1/2): 27-45.
[6] HANADA S. Niobium aluminides[J]. Current Opinion in Solid State and Materials Science, 1997, 2(3): 279-283.
[7] 林栋梁. 高温有序金属间化合物研究的新进展[J]. 上海交通大学学报, 1998, 32(2): 95-108.
LIN Dong-liang. Recent advances in high-temperature ordered intermetallics[J]. Journal of Shanghai Jiaotong University, 1998, 32(2): 95-108.
[8] OKABE T H, FUJIWARA K, OISHI T, ONO K. A fundamental study on the preparation of niobium aluminide powders by calciothermic reduction[J]. Metallurgical Transactions B: Process Metallurgy, 1992, 23(4): 415-421.
[9] KAMATA K, DEGAWA T, NAGASHIMA Y. Structural intermetallics[C]//TMS Inter Symp Proc. Warrendale, 1993: 675-678.
[10] DOLLAR A, DYMEK S. Microstructure and high temperature mechanical properties of mechanically alloyed Nb3Al-based materials[J]. Intermetallics, 2003, 11(4): 341-349.
[11] DYMEK S, DOLLAR M, LEONARD K. Synthesis and characterization of mechanically alloyed Nb3Al-base alloys[J]. Materials Science and Engineering A, 1997, 239/240: 507-514.
[12] TRACY M J, GROZA J R. Nanophase structure in Nb rich- Nb3Al alloy by mechanical alloying[J]. Nanostructured Materials, 1(5): 369-378.
[13] KACHELMYER C R, ROGACHEV A S, VARMA A. Mechanistic and processing studies in combustion synthesis of niobium aluminides[J]. Journal of Materials Research, 1995, 10(9): 2260-2270.
[14] BHATTACHARYA A K, HO C T, SEKHAR J A. Combustion synthesis of niobium aluminide and its mechanical-properties[J]. Journal of Materials Science Letters, 1992, 11(8): 475-476.
[15] NETO R M L, DAROCHA C J. Effect of the high-energy ball milling on the combustion synthesis of NbAl3[J]. Advanced Powder Technology II, 2001, 189(1): 567-572.
[16] NETO R M L, DA ROCHA C J, AMBROZIO F. Combustion synthesis of high-energy ball-milled Nb+3Al powder mixtures[J]. Advanced Powder Technology Ⅲ, 2003, 416(4): 156-161.
[17] BHATTACHARYA A K. Insitu ductile toughening of Nb2Al by combustion synthesis[J]. Journal of the American Ceramic Society, 1992, 75(6): 1678-1681.
[18] MILANESE C, MAGLIA F, TACCA A, ANSELMI- TAMBURINI U, ZANOTTI C, GIULIANI P. Ignition and reaction mechanism of Co-Al and Nb-Al intermetallic compounds prepared by combustion synthesis[J]. Journal of Alloys and Compounds, 2006, 421(1/2): 156-162.
[19] GAUTHIER V, BERNARD F, GAFFET E, JOSSE C, LARPIN J P. In-situ time resolved X-ray diffraction study of the formation of the nanocrystalline NbAl3 phase by mechanically activated self-propagating high-temperature synthesis reaction[J]. Materials Science and Engineering A: Structural Materials Properties Microstructure and Processing, 1999, 272(2): 334-341.
[20] GAUTHIER V, BERNARD F, GAFFET E, VREL D, GAILHANOU M, LARPIN J P. Investigations of the formation mechanism of nanostructured NbAl3 via MASHS reaction[J]. Intermetallics, 2002, 10(4): 377-389.
[21] YEH C L, WANG H J. Effects of sample stoichiometry of thermite-based SHS reactions on formation of Nb-Al intermetallics[J]. Journal of Alloys and Compounds, 2009, 485(1/2): 280-284.
[22] STAFFORD C R, HAARBERG C M. The electrodeposition of Al-Nb alloys from chloroaluminate electrolytes[J]. Plasmas and Ions, 1999, 1(1): 35-44.
[23] YAN X Y, FRAY D J. Synthesis of niobium aluminides by electro-deoxidation of oxides[J]. Journal of Alloys and Compounds, 2009, 486(1/2): 154-161.
[24] ABE Y, HANADA S, SAITO S, HIRANO K, KOBAYASHI N. Compositions at Nb3Al phase boundaries at 1 873 K in the Nb-Al binary phase diagram[J]. Scripta Metallurgica et Materialia, 1995, 32(1): 27-30.
[25] LEONARD K J, VASUDEVAN V K. Phase equilibria and solid state transformations in Nb-rich Nb-Ti-Al intermetallic alloys[J]. Intermetallics, 2000, 8(9/11): 1257-1268.
[26] LIU C T, STIEGLER J O. Ductile ordered intermetallic alloys[J]. Science, 1984, 226(4675): 636-642.
[27] RIOS C T, MILENKOVIC S, CARAM R. Directional growth of Al-Nb-X eutectic alloys[J]. Journal of Crystal Growth, 2000, 211(1/4): 466-470.
[28] TRIVENO RIOS C, OLIVEIRA M F, CARAM R, BOTTA F W J, BOLFARINI C, KIMINAMI C S. Directional and rapid solidification of Al-Nb-Ni ternary eutectic alloy[J]. Materials Science and Engineering A, 2004, 375/377: 565-570.
[29] RIOS C T, MILENKOVIC S, GAMA S, CARAM R. Influence of the growth rate on the microstructure of a Nb-Al-Ni ternary eutectic[J]. Journal of Crystal Growth, 2002, 237: 90-94.
[30] RONG T S, HORSPOOL D N, AINDOW M. Microstructure and mechanical behaviour of Nb-Al-V alloys with 10%-25 at. % Al and 20%-4.0 at. %. Ⅶ: mechanical behaviour and deformation mechanisms[J]. Intermetallics, 2002, 10(1): 13-21.
[31] RONG T S, HORSPOOL D N, AINDOW M. Microstructure and mechanical behaviour of Nb-Al-V alloys with 10%-25 at.% Al and 20%-40 at.%. Ⅵ: Microstructural observations[J]. Intermetallics, 2002, 10(1): 1-12.
[32] JIAO H, BARRADAS F, RONG T, JONES I P, AINDOW M. Observation of tension/compression asymmetry in an Nb-Al-V alloy[J]. Materials Science and Engineering A, 2004, 387/389: 476-480.
[33] MURAKAMI T, KITAHARA A, KOGA Y, KAWAHARA M, INUI H,YAMAGUCHI M. Microstructure of Nb-Al powders consolidated by spark plasma sintering process[J]. Materials Science and Engineering A, 1997, 239/240: 672-679.
[34] MURAKAMI T, KOMATSU M, KITAHARA A, KAWAHARA M, TAKAHASHI Y, ONO Y. Mechanical properties of spark plasma sintered Nb-Al compacts strengthened by dispersion of Nb2N phase and additions of Mo and W[J]. Intermetallics, 1999, 7(6): 731-739.
[35] GNANAMOORTHY R, HANADA S, KAMATA K. Ductile-phase toughening and fatigue crack growth in Nb3Al base alloys[J]. Scripta Materialia, 1996, 34(6): 999-1003.
[36] YE F, MERCER C, SOBOYEJO W O. An investigation of the fracture and fatigue crack growth behavior of forged damage-tolerant niobium aluminide intermetallics[J]. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science, 1998, 29(9): 2361-2374.
[37] YE F, MERCER C, FARKAS D, SOBOYEJO W O. The fracture and fatigue crack growth behavior of forged damage-tolerant niobium aluminide intermetallics[J]. Fatigue and Fracture Mechanics, 2000, 30: 477-481.
[38] YE F, FARKAS D, SOBOYEJO W O. An investigation of fracture and fatigue crack growth behavior of cast niobium aluminide intermetallics[J]. Materials Science and Engineering A: Structural Materials Properties Microstructure and Processing, 1999, 264(1/2): 81-93.
[39] YE F, DIPASQUALE J, SOBOYEJO W O. Room- and elevated-temperature fatigue crack growth in damage tolerant niobium aluminide intermetallics[J]. Creep and Fracture of Engineering Materials and Structures, 1996, 521/530: 801-804.
[40] HEBSUR M G. Studies on tungsten (WHfC) filament-reinforced NbAl3-base alloy[J]. Intermetallics, 1994, 2(1): 43-54.
[41] MURUGESH L, RAO K T V, RITCHIE R O. Crack growth in a ductile-phase-toughened in situ intermetallic composite under monotonic and cyclic loading[J]. Scripta Metallurgica et Materialia, 1993, 29(8): 1107-1112.
[42] GNANAMOORTHY R, HANADA S. Microstructure and strength of binary and tantalum alloyed two-phase Nbss/Nb3Al base alloys[J]. Materials Science and Engineering A, 1996, 207(1): 129-134.
[43] ROWC R G, SKELLY D W. Intermetallic matrix composites II[C]//Mater Res Soc Symp Proc. Pittsburgh, 1992: 411-415.
[44] BLOYER D R, RAO K T V, RITCHIE R O. Fracture toughness and R-curve behavior of laminated brittle-matrix composites[J]. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science, 1998, 29(10): 2483-2496.
[45] BLOYER D R, VENKATESWARA RAO K T, RITCHIE R O. Resistance-curve toughening in ductile/brittle layered structures: Behavior in Nb/Nb3Al laminates[J]. Materials Science and Engineering A, 1996, 216(1/2): 80-90.
[46] BLOYER D R, VENKATESWARA RAO K T, RITCHIE R O. Laminated Nb/Nb3Al composites: Effect of layer thickness on fatigue and fracture behavior[J]. Materials Science and Engineering A, 1997, 239/240: 393-398.
[47] 陈维平, 杨少锋, 韩孟岩. 陶瓷/铁基合金复合材料的研究进展[J]. 中国有色金属学报, 2010, 20(2): 257-266.
CHEN Wei-ping, YANG Shao-feng, HAN Meng-yan. Research development of ceramic/Fe-based alloy composites[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(2): 257-266.
[48] GARCIA D E, SCHICKER S, BRUHN J, JANSSEN R, CLAUSSEN N. Processing and mechanical properties of pressureless-sintered niobium-alumina: Matrix composites[J]. Journal of the American Ceramic Society, 1998, 81(2): 429-432.
[49] SCHEU C, DEHM G, KAPLAN W D, GARCIA D E, CLAUSSEN N. Microstructure of alumina composites containing niobium and niobium aluminides[J]. Journal of the American Ceramic Society, 2000, 83(2): 397-402.
[50] GARCIA D E, SCHICKER S, BRUHN J, JANSSEN R, CLAUSSEN N. Synthesis of novel niobium aluminide-based composites[J]. Journal of the American Ceramic Society, 1997, 80(9): 2248-2252.
[51] de PORTU G, GUICCIARDI S, MELANDRI C, MONTEVERDE F. Wear behaviour of Al2O3-Mo and Al2O3-Nb composites[J]. Wear, 2007, 262(11/12): 1346-1352.
[52] SCHEU C, DEHM G, KAPLAN W D, WAGNER F, CLAUSSEN N. Microstructure and phase evolution of niobium-aluminide-alumina composites prepared by melt-infiltration[J]. Physica Status Solidi A: Applied Research, 1998, 166(1): 241-255.
[53] ZHANG W, TRAVITZKY N, GREIL P. Formation of NbAl3/Al2O3 composites by pressureless reactive infiltration[J]. Journal of the American Ceramic Society, 2008, 91(9): 3117-3120.
[54] RAY R, AYER R. Microstructures and mechanical-properties of rapidly solidified niobium aluminide (NbAl3)[J]. Journal of Materials Science, 1992, 27(6): 1642-1650.
[55] WOYTERA S A, BHAGAT R B, AMATEAU M F. Development of niobium aluminide-based composites with improved toughness using treated reinforcements[J]. Acta Materialia, 1996, 44(3): 963-976.
[56] PENG L M. Synthesis and mechanical properties of niobium aluminide-based composites[J]. Materials Science and Engineering A, 2008, 480(1/2): 232-236.
[57] SCHICKER S, GARCIA D E, BRUHN J, JANSSEN R. CLAUSSEN N. Reaction synthesized Al2O3-based intermetallic composites[J]. Acta Materialia, 1998, 46(7): 2485-2492.
基金项目:国家自然科学基金资助项目(21061012, 51061015); 教育部科学技术研究重点项目(210237); 宁夏自然科学基金资助项目(NZ0916)
收稿日期:2010-03-18;修订日期:2010-06-21
通信作者:罗 民,副教授, 博士; 电话:0951-5019679; E-mail: luominjy@nxu.edu.cn


