
Galena-pyrolusite co-extraction in sodium chloride solution and
its electrochemical analysis
LONG Huai-zhong(龙怀中)1, CHAI Li-yuan(柴立元)1, QIN Wen-qing(覃文庆)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 12 December 2008; accepted 6 May 2009
Abstract:
The co-extraction behavior of galena-pyrolusite in a sodium chloride solution and the electrochemical mechanism of this process were investigated, and some factors affecting the leaching rate of Pb and Mn were optimized. The results show that all the factors such as the concentration of NaCl, HCl and pyrolusite ore, reaction time, temperature, adding times of HCl, affect the leaching rate of Pb. The main affecting factors are the concentration of NaCl, reaction time and temperature. The Tafel polarization curves and EIS plots of the galena and pyrolusite in the NaCl solution demonstrate that during the oxidation process of galena mineral electrode, film forms on the galena surface, which prevents galena from deeper oxidation. However, the film resistance can be greatly reduced in the presence of sodium chloride, thus promoting the reaction rate of galena.
Key words:
galena; pyrolusite; co-extraction behavior; electrochemical mechanism;
1 Introduction
Lead has a wide range of application in different fields of the world. The absolute amount of lead usage in the storage battery field accounts for more than 70% of the total lead consumption. With the increasing demand of environmental protection and use of electric vehicles, the consumption of lead will inevitably increase[1].
The galena is the main mineral to extract lead metal. Considering the environmental pollution and bad effects on the human health caused by the extraction of lead metal, preparation of lead materials and chemical products[2-3], researches have been widely conducted to find a hydrometallurgical process which is more environmentally friend, low-energy consumption in the lead extraction and lead materials preparation[4-6]. The leaching processes include leaching with FeCl3[7-12], Fe2(SO4)3[13] or Cl2 as the oxidants, bioleaching[13-14] and co-extraction[15]. The results show that the formation of S0 membrane is an important factor to affect the lead leaching rate and galena reaction rate.
The studies on the pyrolusite-galena co-extraction are seldom reported so far.
Thus the co-extraction of galena and pyrolusite and electrochemical mechanism of the process are studied in this work.
2 Experimental
2.1 Materials
The galena flotation concentrate was acquired from Mengzi Mineral Processing Plant in Yunnan Province of China. The chemical composition of the concentrate is listed in Table 1. The contents of Pb, Zn, Fe, CaO, MgO and Al2O3 were analyzed by titrimetric analysis. The element S was analyzed by combustion analysis, and SiO2 was analyzed by gravimetric analysis. Microscopic examination of the thin slides showed that the Pb minerals were mainly galena (PbS).
Table 1 Chemical composition of lead concentrate (mass fraction, %)

The XRD pattern of the galena concentrate is shown in Fig.1. The elemental analysis shows that Pb and Fe are present in the forms of PbS and FeS2, respectively.
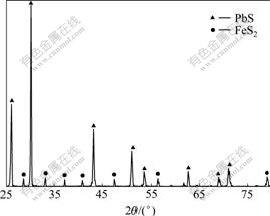
Fig.1 XRD pattern of galena concentrate
The proper sample cut from the good mineralized crystallization galena, with 97.87% purity of the galena, was used to make a mineral electrode, which was polished smoothly by 0.3 μm and 0.05 μm alumina. Then the mineral electrode was put into a plexiglass cylinder with diameter of 7 mm, sealed around by epoxy resin. The exposing area of the electrode was polished as the working surface with apparent contact area of 1 cm2. The electrode was soldered to a copper wire as the electrode cable.
The pyrolusite in the test was chemical manganese powder, and the amount of particles smaller than 0.14 mm accounted for 96%. The elemental analysis results of pyrolusite are listed in Table 2.
Table 2 Chemical composition of pyrolusite (mass fraction, %)

The XRD pattern of the pyrolusite is shown in Fig.2. The elemental analysis shows that Mn is present in the
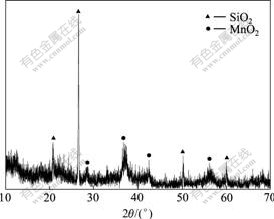
Fig.2 XRD pattern of pyrolusite
form of MnO2, while the Fe phase is relatively low and complex. Besides, a certain amount of SiO2 (α-quartz) is also involved in the sample.
In the electrochemical test, the high purity sample of pyrolusite was got from Mengzi, Yunnan Province of China. The sample containing 93.42% MnO2 by elemental analysis, after sliding and polishing, was made into pyrolusite electrode.
2.2 Methods
The dosage of galena flotation concentrate was 200 g in each leaching experiment. The galena concentrate, pyrolusite and sodium chloride were added into the hydrochloric acid solution with a certain concentration and temperature according to experimental conditions. As soon as the reaction completed, the solution was filtered and the filter cake was dried in the blast oven. Phase composition and microstructure of the samples were tested by XRD and SEM, respectively. The leaching recoveries of Pb and Mn were then calculated and the reaction mechanisms were studied by phase, morphology and chemical analysis.
The electrochemical measurement was performed with the EG&G M283A galvanostat system produced by Princeton company, America. The graphite was used for auxiliary electrode and Ag/AgCl electrode was used as reference electrode with 0.10 mol/L KCl solution as the supporting electrolyte. The Tafel polarization measurement was carried out by the 352 Softcorr Ⅲ software with the scanning speed of 10 mV/s. The AC impedance test was performed by the M398 Electrochemical Impedance 1.3 Software with frequency ranging from 100 kHz to 10 MHz and with the scanning voltage of 197.3 mV (vs Ag/AgCl).
3 Mechanism of galena extraction
In the acid solution, thermodynamically, galena reacts with pyrolusite according to the following chemical reactions:
PbS+MnO2+4H+→Mn2++Pb2++S+H2O (1)
PbS+4MnO2+9H+→4Mn2++Pb2++![]() +4H2O (2)
+4H2O (2)
PbS+4MnO2+8H+→4Mn2++Pb2++![]() +4H2O (3)
+4H2O (3)
In mild acid, the main reaction is Eq.(3), while in strong acid the main reaction is Eq.(1), and under such condition, the surface contact of galena and pyrolusite was impeded by the elemental sulfur produced by the reaction.
In the presence of Cl-, the lead salt produced by reaction is mostly in the form of insoluble PbCl2. While in the presence of NaCl, PbCl2 will coordinate with Cl- to produce lead coordinate compounds which are soluble in water:
Pb2++Cl-![]() PbCl+ (4)
PbCl+ (4)
PbCl++Cl-![]() PbCl2 (5)
PbCl2 (5)
PbCl2+Cl-![]()
![]() (6)
(6)
![]() +Cl-
+Cl-![]()
![]() (7)
(7)
4 Results and discussion
4.1 Co-extraction of galena-pyrolusite in NaCl solution
4.1.1 Effect of reaction time on leaching
Fig.3 shows the effect of the reaction time on the leaching rates of Pb and Mn. It can be seen from Fig.3 that the leaching rate of Pb is improved remarkably before 30 min and maintains at about 95% after 60 min. But the leaching rate of Mn increases greatly with time going, and reaches the peak value of 85% when the reaction time is 60 min.
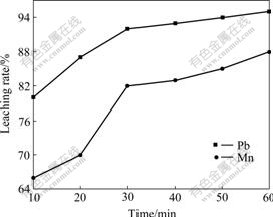
Fig.3 Effect of reaction time on leaching rates of Pb and Mn
4.1.2 Effect of reaction temperature on leaching rate
Fig.4 shows the effect of reaction temperature on the leaching rates of Pb and Mn. It can be seen from Fig.4 that, when the temperature is below 70 ℃, the
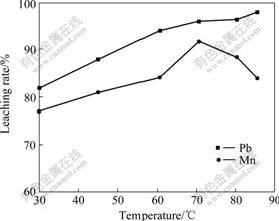
Fig.4 Effect of temperature on leaching rates of Mn and Pb
leaching rate of Mn increases with temperature increasing, while the temperature exceeds 70 ℃, the leaching rate decreases. The leaching rate of Pb increases with temperature going up. The temperature mainly affects the leaching rates of Pb, Mn by changing the reaction rates. Under 80 ℃, a part of HCl volatilizes, which makes the reduction of HCl in the total hydrochloric acid.
4.1.3 Effect of HCl dosage on leaching rates of Pb and Mn
Fig.5 shows the results obtained in the presence of different dosages of HCl, and its concentration is 1.5 mol/L. Apparently, the more the dosage of HCl used in the reaction, the higher the recoveries of Pb and Mn. But the effect of HCl dosage on Pb recovery is not obvious, while Mn recovery is influenced greatly.
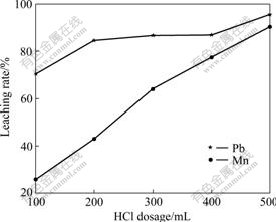
Fig.5 Effect of HCl dosage on leaching rates of Pb and Mn
4.1.4 Effect of HCl addition method on leaching rate
Based on the analysis above, the addition method of 1.5 mol/L, 500 mL HCl was investigated. HCl was added by one, two or three times, and the results are shown in Table 3.
Table 3 Influence of adding times of HCl on leaching rate

It can be seen that, when HCl is added two times, the leaching rate of Pb increases from 72.37% to 92.86%, the leaching effect is the best. While the effect of HCl addition method on the leaching rate of Mn is not the same as that of Pb.
4.2 Electrochemical analysis of galena and pyrolusite electrodes in NaCl solution
4.2.1 Electrochemical behavior of galena electrode
Fig.6 shows the Tafel plots of galena in different concentrations of NaCl solution. The electrochemical parameters obtained by data fitting and processing of Fig.6 are listed in Table 4.
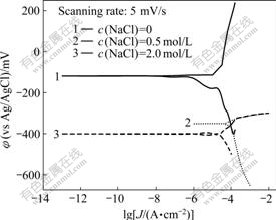
Fig.6 Tafel polarization plots of galena in different sodium chloride concentrations
Table 4 Fitting parameters of Tafel polarization plots

It can be seen from Table 4 that, the corrosion current density significantly increases from 2.149 ?A/cm2 to 203.500 ?A/cm2 and the corrosion potential greatly decreases from -112.60 mV to -400.40 mV (vs Ag/AgCl) with the concentration of sodium chloride changing from 0 to 2.0 mol/L. The results show that the electrode reaction exacerbates and the corrosion rate of galena is obviously accelerated.
The electrochemical impedance spectroscopy (EIS) of galena in different concentrations of NaCl solution is shown in Fig.7.
It can be seen from Fig.7 that the surface resistance of galena decreases from 2.9 kΩ/cm2 to 1.2 kΩ/cm2 with increasing NaCl concentration, which means that the surface is activated. Meanwhile, in the low-frequency side (right side), the capacitance arcs show different signs in different NaCl concentrations. When the NaCl concentration is 0.5 mol/L, there exist adsorption effects characterized by emerging signs of a small semicircle and diffusion and transmission characterized by the emergency of Warburg Impedance linear, respectively. When the NaCl concentration increases to 2.0 mol/L, there is no such signal mentioned above. Accordingly, it can be speculated from the EIS plots that during the oxidation process of galena electrode, the insoluble
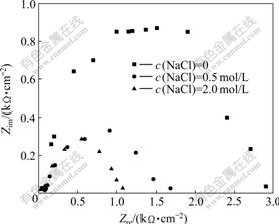
Fig.7 EIS plots of galena electrode in different sodium chloride concentrations (Scanning voltage: 197.3 mV (vs Ag/AgCl); Frequency range: 100 kHz-10 MHz)
products generated attach to the galena surface and reduce the oxidation rate of galena. When NaCl is added, the products would dissolve to the sodium chloride solution from the galena surface in the form of coordination compounds. Then the diffusion of the oxidation products is not the dominant step to determine the leaching process any more.
It can be seen from Fig.6 that when there is no sodium chloride in the electrolyte solution, the cathodic reaction of galena generates insoluble products which hinder deeper reaction of electrode reaction. But if the electrode surface is put in sodium chloride solution, the electrode reaction intensifies. The equations are as follows:
PbS+Cl-+2e→PbCl2+S (8)
PbCl2+Cl-→![]() (9)
(9)
![]() +Cl-→
+Cl-→![]() (10)
(10)
4.2.2 Electrochemical behaviors of pyrolusite electrode
The Tafel plots of pyrolusite in different concentrations of NaCl solution are indicated in Fig.8. Table 5 lists the electrochemical parameters which are obtained by data fitting and processing.
It can be seen from Table 5 that, the corrosion potential of pyrolusite electrode has a significant negative shift, which decreases from 500 mV to 120 mV (vs Ag/AgCl) when the concentration of sodium chloride changes from 0 to 2.0 mol/L. This indicates that the anodic reaction of pyrolusite electrode is intensified with the NaCl concentration increasing. The corrosion current density reaches the maximum value of 16.78 ?A/cm2 when the NaCl concentration is 0.5 mol/L, which indicates that the corrosion rate of pyrolusite electrode surface increases. However, the corrosion rate decreases when the current density increases to a certain value.
Fig.9 shows the electrochemical impedance plot of pyrolusite.
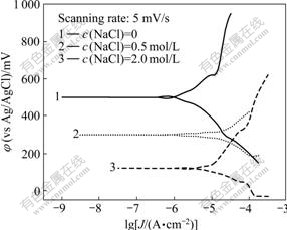
Fig.8 Tafel plots of pyrolusite in different sodium chloride concentrations
Table 5 Fitting parameters of Tafel polarization plots

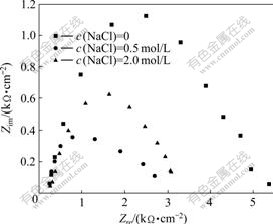
Fig.9 EIS plots of pyrolusite electrode in different sodium chloride concentrations (Scanning voltage: 197.3 mV (vs Ag/AgCl), Frequency range: 100 kHz-10 MHz)
It can be seen from Fig.9 that, the impedance value is small when the NaCl concentration is 0.5mol/L. This impedance value is smaller than that in the solution when the NaCl concentration is 2.0 mol/L, but only with a change of 380 Ω/cm2, which means that there are no products with greater resistance generating on the surface of pyrolusite electrode. The small change of impedance value is caused by a certain degree of “resistance layer” formed on the surface, which results from the increase of concentrations of MnCl2 and manganese coordination compounds near the electrode surface. Therefore, the diffusion of oxidation products of the pyrolusite electrode is a dominant reaction step.
The results of the Tafel polarization plots and EIS plots of galena and pyrolusite electrodes in different concentrations of NaCl solution show that, in the galena- pyrolusite redox leaching system, NaCl mainly affects the corrosion reaction of galena electrode, and can coordinate with PbCl2, then the coordinate compounds dissolve from galena surface into NaCl solution. Thus the speeds of galena oxidation and deoxidization of pyrolusite are improved significantly.
4.3 XRD and SEM analyses of leaching residues
The XRD patterns of the leaching residue are shown in Fig.10. The concentrations of NaCl are 0.34 and 2.4 mol/L.
It can be seen from Fig.10 that, when the NaCl concentration is 0.34 mol/L, most PbCl2 is kept in the residue; when the concentration of NaCl increases to 2.40 mol/L, there is few PbCl2 left in the residue. On one hand, the PbCl2 of the residue covers the surface of unreacted PbS and hinders the oxidation reaction. On the other hand, it is extremely difficult for PbCl2 to dissolve in water, accordingly reducing the leaching rate of Pb.
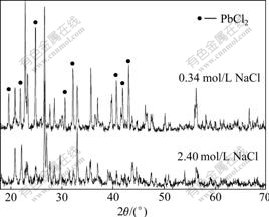
Fig.10 XRD patterns of leaching residue
The SEM images of galena and pyrolusite concentrates and residue samples leached in the NaCl solution of 2.4 mol/L are shown in Fig.11. It can be seen from Fig.11 that, there are large pores in the leaching residue, which indicates that the leaching products dissolve into the sodium chloride solution from the original mineral surface.
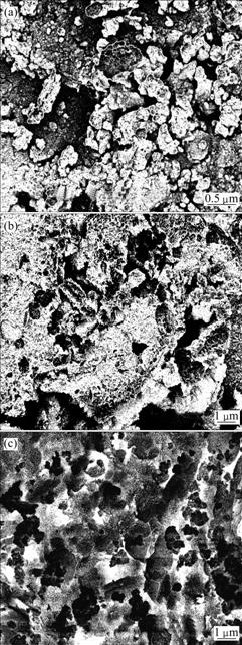
Fig.11 SEM images of galena(a), pyrolusite(b) and residue samples(c)
5 Conclusions
1) In the co-extraction of galena-pyrolusite in a sodium chloride solution, some factors affecting the leaching rate of Pb and Mn were optimized. All the factors such as the concentration of NaCl, HCl and pyrolusite ore, reaction time, temperature, adding times of HCl, affect the leaching rate of Pb. The main affecting factors are the concentration of NaCl, reaction time and temperature.
2) The electrochemistry tests on galena and pyrolusite electrodes in NaCl solution demonstrate that the formation of PbCl2 of insoluble film in the co-extraction of galena and pyrolusite blocks the redox process; while the ligand (NaCl) can make the PbCl2 film to “peel off”, thus promoting the reaction rate significantly.
References
[1] CELWYN H E. What are we going to do with lead?[J]. The Battery Man, 1996, 38: 22-31.
[2] ANDERSON S, CHAPPELKA A H, FLYNN K M, ODOM J W. Lead accumulation in Quercus nigra and Q-velutina near smelting facilities in Alabama, USA[J]. Water Air and Soil Pollution, 2000, 118: 1-11.
[3] HETTWER K, DEICKE M, RUPPERT H. Fens in Karst sinkholes—Archives for long lasting 'immission' chronologies[J]. Water Air and Soil Pollution, 2003, 149: 363-384.
[4] KARTIO I J, LAAJALEHTO K, SUONINEN E J, BUCKLEY A N, WOODS R. The initial products of the anodic oxidation of galena in acidic solution and the influence of mineral stoichiometry[J]. Colloids and Surfaces A, 1998, 133: 303-311.
[5] de GIUDICI G, ZUDDAS P. In situ investigation of galena dissolution in oxygen saturated solution: Evolution of surface features and kinetic rate[J]. Geochimica Et Cosmochimica Acta, 2001, 65: 1381-1389.
[6] KHOLMOGOROV A, PASHKOV G, MIKHLINA E, MIKHLIN Y. Electrochemical aspects of the nitric acid leaching of lead sulfide concentrates: On the way to a highly effective method for Pb recovery[J]. Electrochemistry in Mineral and Metal Processing, 2006(2): 221-330.
[7] QIN W Q, LIU H, TANG S H, SUN W. Preparation of lead sulfate powder directly from galena concentrates[J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 479-483.
[8] LONG H Z, CHAI L Y, LIU H, QIN W Q. Hydro-chemical conversion of galena in FeCl3-KCl solution[J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 1331-1335.
[9] PARAMGURU R K, MISHRA K G, KANUNGO S B. Electrochemical phenomena in MnO2-FeS2 leaching in dilute HCl (Part 2): Studies on polarization measurements[J]. Canadian Metallurgical Quarterly, 1998, 37: 395-403.
[10] PARAMGURU R K, KANUNGO S B. Electrochemical phenomena in MnO2-FeS2 leaching in dilute HCl (Part 3): Manganese dissolution from Indian Ocean nodules[J]. Canadian Metallurgical Quarterly, 1998, 37: 405-417.
[11] CORREIA M J N, CARVALHO J R. Chloride leaching of Portuguese lead concentrates[J]. Minerals Engineering, 1992(5): 245-253.
[12] BAL? P. Influence of solid state properties on ferric chloride leaching of mechanically activated galena[J]. Hydrometallurgy, 1996, 40: 359-368.
[13] da SILVA G. Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena[J]. Hydrometallurgy, 2004, 75: 99-110
[14] JIANG L, ZHOU H Y, PENG X T, DING Z H. Bio-oxidation of galena particles by Acidithiobacillus ferrooxidans[J]. Particuology, 2008(6): 99-105.
[15] DUTRIZAC J E. The leaching of sulphide minerals in chloride media[J]. Hydrometallurgy, 1992, 29: 1-45.
(Edited by YUAN Sai-qian)
Foundation item: Project(50774094) supported by the National Natural Science Foundation of China
Corresponding author: LONG Huai-zhong; Tel: +86-731-88876765; E-mail: ysxblong@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60233-8


