
Comparative study of microstructure and corrosion resistance of electroless Ni-W-P coatings treated by laser and furnace-annealing
LIU Hong(刘 宏)1, 2, GUO Rong-xin(郭荣新)3, ZONG Yun(宗 云)1, HE Bing-qing(何冰清)1, LIU Zhu(刘 铸)2
1. School of Mechanical Engineering, Shandong Institute of Light Industry, Ji’nan 250100, China;
2. Corrosion and Protection Centre, School of Materials, The University of Manchester,
Manchester, M60 1QD, UK;
3 Zibo Institute of New Materials, Zibo 255040, China
Received 2 June 2009; accepted 30 October 2009
Abstract:
A comparative study of amorphous electroless Ni-W-P coatings on mild steel substrate treated by a high power diode laser and furnace annealing was presented. Effects of different laser operating parameters and furnace-annealing conditions on microstructures, in terms of crystallisation, pores formation and grain growth, were investigated using SEM/EDX and XRD. Corrosion behaviours of these coatings before and after various treatments were evaluated with anodic polarisation in 0.5 mol/L H2SO4 solution. The results show that the furnace-annealing produces either a mixture of nanocrystallined Ni and amorphous phases or precipitated Ni3P phase distributed in nanocrystallined Ni-based matrix, depending on annealing temperatures, whilst the laser treatment under the operating conditions only produces nanocrystallined Ni-based matrix with Ni3P precipitates. Corrosion performance of the coatings treated by both the laser and the furnace-annealing is dependent on the annealing temperature and laser operating conditions. Corrosion mechanisms of various treated-coatings were discussed in the consideration of phase constitutes and proportion, grain sizes of both Ni and Ni3P phases, pores formation and residual stresses.
Key words:
electroless Ni-W-P coating; laser-treatment; annealing; nano-crystallization; corrosion;
1 Introduction
Nanocrystalline materials, as a result of reduction of the grain sizes, with high volume fraction of grain boundaries and trifurcate junctions, have attracted considerable interest in the last decade[1-2]. Since BIRRINGER et al[3] succeeded in the synthesis of the first piece of bulk nanocrystalline material by means of inert gas condensation and consolidation, various synthesis techniques have been developed for producing nanocrystalline materials, including ball-milling[4], electrodeposition[5-6], severe plastic deformation[7] and crystallization of amorphous solids[2]. For nanocrystallization of amorphous solids, such as amorphous electroless-plated Ni-P base alloys and amorphous magnetic materials, most of the previous works have been focused on annealing treatment in furnace[8-11].
According to the classic corrosion theory, nano- crystalline materials might be expected to have reduced corrosion resistance since enormous amounts of intercrystalline defects such as grain boundaries may act as the preferential corrosion paths, accelerating corrosion by forming large amount of micro-electrochemical cells with the matrix[12]. However, on the other hand, nanocrystalline materials have much higher diffusivities due to the presence of large amounts of grain boundaries, which promotes the formation of a protective passive film, so that nanocrystallinity of an alloy may improve corrosion resistance as compared with its conventional polycrystalline counterpart[13]. Corrosion resistance of a wide range of nanocrystalline materials has been investigated but reported controversially. Many studies demonstrate enhanced corrosion resistance with reducing grain size to nanometer range (<100 nm), especially the remarkable increase in localized corrosion resistance is reported. For example, nanocrystalline 304 stainless steel films with a grain size of 25 nm, prepared by sputter-deposition, exhibited a more uniform corrosion and superior localized corrosion resistance as compared with the conventional polycrystalline counterparts in 0.3% (mass fraction) NaCl solution[14]. In addition, the corrosion resistance of nanocrystalline 304 type stainless steel films was larger than that of the amorphous counterpart, which was explained in terms of Cr enrichment of the surface as a result of having more rapid grain boundary diffusion paths[15]. However, some studies reported the degradation of corrosion resistance of nanocrystalline electrodeposited Ni-P coating, indicating that amorphous Ni-P displayed lower current density[16]. Up to date, only limited investigations have been conducted on the effects of nanocrystallined structures on corrosion properties of electroless Ni-P or Ni-W-P coatings[12, 17-19]. GAO et al[13] reported that, for electroless Ni-P and Ni-W-P coatings, when the coatings were heat-treated at 300 ℃ and 350 ℃, respectively, to form a single-Ni nanocrystalline structure, the coatings presented different corrosion performances. The nanostructure itself, in the absence of the third passivation element, was harmful to the corrosion resistance of Ni-P alloys. However, the addition of a third passivation element, W, markedly improved the corrosion resistance of the alloys due to the huge amount of grain boundary diffusion paths provided by the nanostructure, which favored the formation of the dense tungsten oxide film on the surface. It was also reported[17] that the corrosion resistance of amorphous electroless Ni-8%P (molar fraction) in salt-spray test was significantly reduced after heat-treatment in vacuum at 400 ℃ due to the presence of precipitated Ni3P in Ni matrix, but no investigations on other temperatures were reported. In addition, effect of heat treatment, in a temperature range of 350-550 ℃, on electroless ternary nickel-cobalt-phosphorus alloy was investigated by YOUNANL et al[19]. In 5% NaCl solution, the Ni-Co-P coatings treated at temperature higher than 400℃ showed much better corrosion resistance in comparison with that treated at 350 ℃. It was also reported[20] that the heat-treatment of amorphous electroless Ni-10.4P coating at 200℃ slightly improved its corrosion resistance in 20% H2SO4 solution, but further increase in annealing temperature from 300℃ to 500℃ deteriorated the corrosion properties. Although these investigations have demonstrated the effects of nanocrystallisation on corrosion performance of amorphous electroless Ni-P and Ni-W-P coatings, it is still lack of complete studies on the effects of coating structures at various treatment temperatures on corrosion performance, especially for Ni-W-P coatings. Since many factors might be involved in corrosion mechanism, such as degree of crystallisation (i.e. annealing temperature resulting in various degrees of crystallisation and phases), grain size and relative amount of various phases, and defects formed during annealing, further studies on such complicated cases are needed.
Laser surface engineering has been widely accepted as the means for improvement in wear, corrosion resistance and other properties of various coatings. However, most researches on electroless Ni-P coatings by laser treatment mainly concern complete melting of the coatings to enhance the substrate–coating adherence and to form amorphous or nanocrystalline surfaces on pre-plated alloys[21-23]. Unfortunately, only one publication on nanocrystallization of amorphous electroless Ni-P base coatings by laser heat-treatment has been found, in which, MATSUKAWA et al[18] applied a pulsed Nd:YAG laser beam to treat amorphous electroless Ni-P coatings and investigated the effect of such laser treatment on wear and corrosion properties. They found that the laser treatment improved both wear and corrosion resistance, and such result was compared with the heat-treated coatings at 400 ℃ in furnace only and showed the superior properties obtained by laser treatment. Since annealing temperature has crucial effects on corrosion behaviour of the coatings, their results cannot be conclusive. In addition, there has been no report so far on laser-induced nanocrystallization of amorphous electroless Ni-W-P ternary alloys.
In the present study, amorphous electroless Ni-W-P coatings were crystallized by a high power diode laser without melting, and furnace annealing, respectively. Both processes were carried out with variation of laser processing conditions and annealing temperatures in furnace, respectively, in order to perform a comprehensive comparison, in terms of microstructural characteristics and corrosion resistance, of the two processes.
2 Experimental
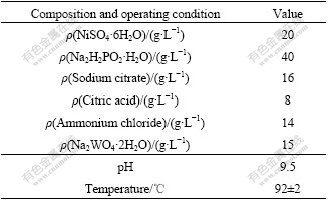
Mild steel sheets were used as substrates for electroless deposition of ternary Ni-W-P alloy in this study. The size of each sample was 10 mm×15 mm×1.1 mm. The cold-rolled substrates were then degreased in diluted NaOH solution at 40-50 ℃ for 10 min, rinsed in flowing water, cleaned in deionized water for 10 min, and activated in 50% HCl solution at 40 ℃ for 3-5 min. After being rinsed with deionized water, the substrates were placed into the electroless solution for plating. The compositions of the reaction bath used for the preparation of ternary Ni-W-P alloy are given in Table 1. Electroless plating was carried out in a 500 mL glass beaker at (92±1) ℃ for 2 h. The plating rate measured was approximately 14-16 μm/h, with a thickness in a range of 28-32 μm. After plating, the samples were rinsed again in flowing water and deionized water, dried and preserved for the following tests.
Table 1 Compositions and operating conditions of plating
A Laserline 1.5 kW continuous wave diode laser with a rectangular beam of 2.5 mm×3.5 mm was used to scan, along the short edge, over the coated surfaces with argon protection. Laser scanning velocities were varied from 6 mm/s to 11 mm/s, with a fixed power level of 150 W. It was observed that when the velocity was 6 mm/s, the surface was melted which was beyond the scope of the current investigation. Therefore, in this study, laser scanning velocities of 7, 8, 9, 10 and 11 mm/s were considered without surface melting. For comparison, the as-plated samples were also treated by heating at 200, 300, 400, 500 and 600 ℃ for 1 h in a muffle furnace under argon atmosphere to protect the samples from oxidation. Microstructures of the coatings, in terms of phase constituents and grain size, were characterized by X-ray diffraction (XRD) using Philips X’pert MRD diffractometer with monochromatic Cu Kα radiation. The grain sizes of the nanocrystalline structures were estimated using the Scherrer formula. The peaks of XRD patterns were fitted using Profit Program after subtracting the background. In addition, residual stresses were measured using X-ray diffraction techniques with a Proto XRD-3000 residual stress analyzer. An EVO50 scanning electron microscope (SEM) with energy dispersive analysis X-ray spectroscope (EDX) attachment was used to determine the surface morphology and elemental compositions of the coatings.
Anodic potentiodynamic polarization in 0.5 mol/L H2SO4 solution at 25 ℃ was carried out to study the corrosion behaviour of the coatings, using a Gill AC potentiostat. A conventional three-compartment glass cell, with a platinum rod as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode, was employed. Each sample with a defined area of about 0.5 cm2 was exposed to the electrolyte. Polarisation curves were recorded by sweeping the electrode potential from -500 mV to +1 000 mV at a scan rate of 75 mV/min. Tafel plot was made from the data and the corrosion current density (Jcorr) was determined by extrapolating the straight-line section of the anodic and cathodic Tafel lines. Each polarisation test for each sample was repeated three times.
3 Results and discussion
3.1 Microstructural characteristics
Chemical compositions of the as-plated Ni-W-P alloy coatings and those with different treatment conditions were determined by EDX, as listed in Table 2, suggesting that the coating compositions are, in principle, not changed with different treatment conditions. Fig.1 shows the XRD patterns of the as-plated and furnace-annealed Ni-W-P coatings. It is evident that the structures of both as-plated and annealed coatings at 200 ℃ are amorphous, while the structure of the coating annealed at 300 ℃ is a mixture of amorphous and nanocrystalline Ni. The nanocrystalline Ni phase, which is a supersaturated solid solution with P and W, precipitates from the Ni-W-P coatings. The grain size of the Ni phase estimated from the peak broadening of its X-ray pattern is 10.5 nm. The peak broadening is the superimposition of the sharp peak from the (111) diffraction of nanocrystalline Ni and the broad background peak from the diffraction of amorphous phase in the coatings. However, other two peaks of the crystalline Ni phase such as the Ni (200) and Ni (220) are not obvious in the diffraction pattern. This may be due to the ultra-fine grains of the Ni phase further widening the peaks and increasing the random scattering intensity; and the limited amount of nanocrystalline Ni phase in the mix-structure also weakens the peaks.
Table 2 Chemical compositions of Ni-W-P coatings after various treatments
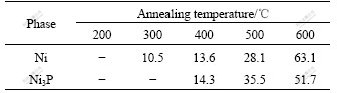
When the annealing temperature reaches 400 ℃, a new crystalline phase of Ni3P precipitates from the coatings in addition to the Ni phase, in which the grain size of Ni3P phase is larger than that of Ni phase. Further increasing the annealing temperature results in further increase in grain sizes of both phases; however, the grain size of Ni phase becomes larger than that of Ni3P phase as shown in Table 3. From the XRD patterns shown in Fig.1, it is evident that the peak intensities of both Ni3P and Ni phases vary with annealing temperature. At the annealing temperature of 400 ℃, the peak intensities of both phases are relatively small; while over 500 ℃, they are sharply increased. This implies that degree of phase transformation of amorphous coating increases, namely the amounts of Ni and Ni3P phases increase.
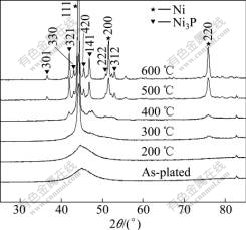
Fig.1 XRD patterns of as-plated deposit and deposits annealed at various temperatures
Table 3 Estimated average grain sizes of coatings after furnace annealing at various temperatures (nm)

On the other hand, it is understood that the crystalline phase in the Ni-W-P coatings is a supersaturated Ni solid solution with P and W. The incorporation of the larger W atom in the face-centered cubic Ni matrix causes a lattice change and peak shift. In fact, the peaks of the Ni phase in the XRD patterns have been observed to be slightly shifted to a lower angle for both Ni (111) and Ni (200). Therefore, in this study, Ni (111) could be suggested to be Ni-W (111), since no extra phase containing W or Ni3P peak shift is observed. The shift of both Ni (111) and Ni (200) peaks was also reported in previous work[18].
Fig.2 shows the XRD patterns of the electroless Ni-W-P coatings after laser treatment at different scanning velocities. With increasing laser scanning velocities, which corresponds to decreasing the treatment temperatures, both peaks of the Ni crystalline phases and Ni3P precipitated from Ni-W-P coatings become broadened in the diffraction patterns. This indicates that the grain sizes of both phases, as shown in Table 4, become smaller with the increase in scanning velocities. From the XRD patterns, it can also be revealed that the peak intensities of both Ni3P and Ni phases vary with the laser scanning velocities. At the fastest scanning velocity of 11 mm/s, the peak intensity is the minimum while the scanning velocity is 7 mm/s or 8 mm/s, the peak intensity enhances and remains almost constant. However, compared with the furnace annealing at 600 ℃, the peak intensities obtained by laser heat-treatments are lower, which might suggest less degree of Ni3P phase transformation occurring in the laser process.
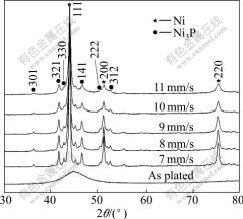
Fig.2 XRD patterns of electroless Ni-W-P coatings before and after laser treatment at various scanning velocities
Table 4 Estimated average grain sizes of coatings after laser-treatment at various scanning velocities (nm)
In order to understand the differences of microstructural characteristics between the laser and annealing treatments, thermal histories in both processes need to be considered, in terms of temperature and time. Firstly, the temperatures in the laser treatment are much higher than those in the furnace-annealing. Since the surface is melted at the laser scanning velocity of 6 mm/s, the surface temperature at the velocity of 7 mm/s should be close to the melting temperature of the coating. Secondly, the heating time, which is equivalent to the interaction time between the laser beam and the coating surface (short beam edge, 2.5 mm/s scanning velocity), is much shorter, in a range of 227-357 ms, than the holding time of 1 h in the furnace-annealing. Therefore, the laser heat-treatment is a process with much higher heating and cooling rates than the furnace-annealing. Since crystallization is affected by heating rates, the crystallization temperature should be higher in laser process. Therefore, the nanocrystallization of the amorphous electroless Ni-W-P coatings by laser treatment occurs at higher temperature within much shorter time. This might be the reason for less degree of Ni3P phase transformation in the laser process.
In addition, the grain growth is controlled by temperature, heating and cooling rates, as a result, the grain sizes obtained at the temperature of 400 ℃ and at the fastest scanning velocity of 11 mm/s present the minimum values, respectively. Although the grain sizes attained by furnace-annealing and laser treatment are all in the nano-scale, relative size of both phases is different. It is noticed that, when the laser scanning velocity is 10 mm/s or furnace-annealing is conducted at 400 ℃ and 500 ℃, the grain size of nanocrystalline Ni phase is smaller than that of Ni3P precipitates. This characteristic in grain size is in contrast to the results achieved in other treatment conditions.
3.2 Surface morphology
Fig.3 presents the surface morphologies of Ni-W-P coatings treated under different treatment conditions. The SEM micrograph of the coating annealed at 400℃ (Fig.3(b)) reveals a significant amount of dark, spherical submicron pores. According to the Ref.[23], the crystallization temperature of electroless Ni-W-P alloy is about 400 ℃. Therefore, at this temperature, a large amount of fine Ni3P phases precipitate out of the supersaturated Ni solid solution, leading to the volume contraction of the coatings[24]. Such volume contraction might be associated with the formation of pores within the coatings. On the other hand, during the electroless plating process, the bubbles of hydrogen might be trapped in the coatings to form very fine pores as observed in Fig.3(a). When the coating is heated up, the hydrogen intends to escape from the surface. This process can be beneficial by higher temperature due to the faster diffusion. Therefore, further increase of annealing temperature results in the reduction of pores (Fig.3(c)). For the laser treatment, at the low scanning velocity of 7 mm/s, almost no pores are observed (Fig.3(d)), while the increase in scanning velocities increases the tendency of pores formation. Pores formation is mostly presented for the coating treated at the scanning velocity of 10 mm/s, but then reduced at the velocity of 11mm/s. Apart from the processing temperature, formation of pores in laser process might also be considered by the degree of Ni3P phase transformation and interaction time. At low velocities, higher temperature and relatively longer interaction time might be beneficial to promoting the mergence of pores, to reduce pores formation; at high velocities, less degree of Ni3P phase transformation results in less volume contraction, so that less pores are formed. Therefore, it may imply that when the velocity is between the two regions, i.e. 10 mm/s in this case, the most pores can be formed.
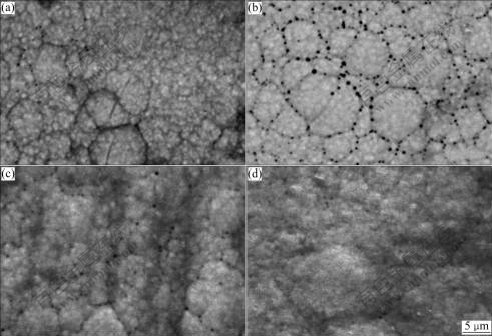
Fig.3 SEM micrographs of Ni-W-P coatings under different conditions: (a) As-plated; (b) Furnace-annealed at 400 ℃; (c) Furnace- annealed at 600 ℃; (d) Laser treated at 7 mm/s
3.3 Corrosion behaviour
Fig.4 shows the anodic polarization curves of the as-plated, furnace-annealed and laser-treated Ni-W-P coatings, respectively, in 0.5 mol/L H2SO4 solution. No passivation can be seen in any circumstance, suggesting that the Ni-W-P coatings in either amorphous state or treated by various conditions present no active-passive behaviour. The relationships of the corrosion current density, Jcorr, with annealing temperatures and laser scanning velocities are shown in Fig.5(a). It is evident that the corrosion resistance of the as-plated Ni-W-P coating is not the highest, although it is in amorphous state. For furnace-annealing, the coating treated at 200 ℃ presents the lowest value of Jcorr, while the Jcorr of the coating treated at 400 ℃ reaches the highest value, and then drops down over 400 ℃. In order to explain the variation of corrosion resistance with annealing temperature, apart from the microstructural characteristic and surface morphologies described above, the state of residual stress within the coatings should be considered. According to the early work by SONG and YU [25] and XIE et al[26], the internal stress produced in the process of electroless deposition is tensile. Corrosion resistance of coatings is affected by the stress state of the coating; and larger tensile stress causes greater tendency for corrosion to occur. During electroless plating, the whole deposition reaction process is accompanied by hydrogen evolution. The hydrogen atoms are occluded in as-plated deposits, generating internal tensile stress within the coatings. Therefore, when the coating is subjected to a heating process, relief of the tensile stress is expected, which may enhance corrosion resistance of the coatings. This could explain the finding that annealing at 200 ℃for the coating, remaining amorphous state, further enhances the corrosion resistance. Increase in annealing temperature to 300 ℃ exhibits no further increase in corrosion resistance, but is similar to the as-plated state. This suggests that the formation of nanocrystallined Ni phase is not detrimental to corrosion performance.
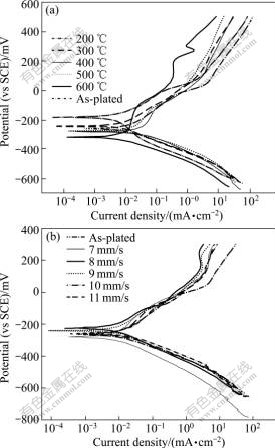
Fig.4 Anodic polarization curves of electroless Ni-W-P coatings in 0.5 mol/L H2SO4 solution: (a) Furnace-annealed; (b) Laser-treated
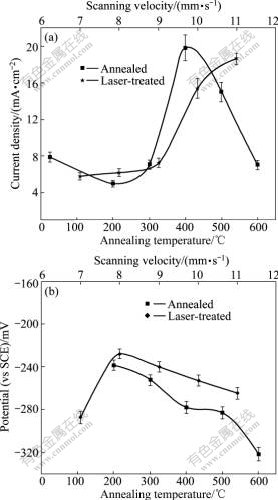
Fig.5 Effects of annealing temperature and laser scanning velocity on corrosion current densities (a) and corrosion potential (b) of coatings
Fig.6 shows the variation of residual stress in furnace annealing and laser treatments. The residual stress in coatings from 400℃ to 600℃ is mainly transformation stress. When the temperature is 400 ℃, the transformation stress is the largest, and then it decreases with increment of temperature. This just accords with the phenomenon that the value of Jcorr is the highest at 400 ℃. In addition, it is also a reason that the precipitation of Ni3P occurring at this temperature results in the formation of micro-galvanic cells between the Ni3P and the matrix, along with the formation of crystalline imperfection, such as grain boundaries and phase boundaries in the coatings. Another important factor associated with the worst corrosion resistance at 400 ℃ is the appearance of the maximum porosity at this particular temperature. Combined effects from several factors result in the lowest corrosion resistance for the coating annealed at 400℃. When the annealing temperature is higher than 400 ℃, reduction of Jcorr for the coatings treated at 500 ℃ and 600 ℃ might be attributed to the significant reduction of porosity. Furthermore, surface oxidation of the samples during cooling process in air, at and above 500℃ could promote the formation of Ni-oxide and W-oxide, which may be beneficial to the reduction of corrosion current densities. On the other hand, although the increase in annealing temperature reduces the content of P in Ni-matrix due to the precipitation of Ni3P phase, which shifts the Jcorr to more negative direction, as shown in Fig.5(b), hence enhancing the formation of micro-galvanic cells between the two phases, it does not seem to play an important role in determining the corrosion behaviour. In addition, the effect of the growth of grain size for both Ni3P and Ni phases with rising processing temperature on corrosion performance is similar to the finding reported for nanocrystalline electrodeposited Ni-P coating[17].
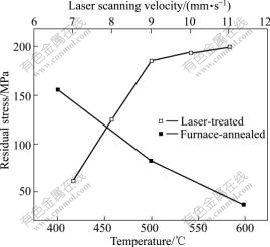
Fig.6 Variation of residual stress in furnace annealing and laser treatments
For the laser-treated coatings, the value of Jcorr at scanning velocities from 7 mm/s to 9 mm/s are lower than that of the as-plated Ni-W-P coating; and it goes up at scanning velocity of 10 mm/s and 11 mm/s. Although the corrosion performance of laser-treated coatings is also determined by the same factors as those described earlier, such as formation of pores and Ni3P phase, grain sizes of both Ni and Ni3P phases, and the residual stress, each factor may play a different role for laser-treated coatings from the furnace-treated ones. Firstly, all the laser-treated Ni-W-P coatings consist of nanocrystalline Ni-based matrix with distribution of Ni3P phase, but the amount of Ni3P phase produced at scanning velocities from 7 mm/s to 9 mm/s in the laser-treated samples is less than that treated at temperatures of 500 ℃ and 600 ℃ in the furnace annealing. Since Ni3P is cathodic to Ni-base matrix, the increase in area ratio of anode to cathode can effectively reduce corrosion current density. Therefore, the corrosion resistance of the laser-treated coatings at these scanning velocities is better than the best one observed at the annealing temperature of 600 ℃ in furnace. Although the coating treated at 400 ℃ may show a similar amount of Ni3P phase, the formation of most pores at this particular temperature should be mainly responsible for the worst corrosion resistance. Secondly, as described earlier, when the scanning velocity is 10 mm/s, the most pores are found, thereby leading to poorer corrosion resistance; when the scanning velocity is 11 mm/s, although less pores within the coating are formed, the corrosion resistance becomes the worst because of larger residual stress produced at higher scanning velocity (shown in Fig.6). For the velocities of 7 mm/s and 8 mm/s, the best corrosion resistance is achieved since there are almost not pores in coatings, in addition to the relatively small residual stress.
Due to the rapid heating and cooling rates involved in laser process, the following results would be reasonable to deduce. 1) The amount of P remained in the Ni-matrix by laser-treatment should be higher than that by the furnace-treatment, so that the driving force for electrochemical reactions between the Ni3P and the Ni-matrix in laser-treated coatings would be smaller than that in the furnace treated coatings. 2) The relationship between φcorr and laser scanning velocity is unexpected in Fig.5(b). This might be associated with the phenomenon that the residual stress of coatings treated by laser is higher than that of coatings annealed in furnace. 3) Whether being furnace-annealed or laser-treated, grain size of Ni3P exceeding that of Ni is a great disadvantage to corrosion resistance of a coating in 0.5 mol/L H2SO4 solution. Maybe the size combination of big cathode and small anode offers promoting condition for electrochemical corrosion.
4 Conclusions
1) For furnace-annealed coatings, amorphous electroless Ni-W-P coatings can be transformed into a mixture of amorphous and nanocrystalline Ni phase, or nanocrystalline phases consisting of Ni and Ni3P. The grain sizes of both phases increase with the increase in annealing temperature.
2) Amorphous Ni-W-P coatings annealed at 200 ℃ and 300 ℃ show better corrosion resistance than its amorphous counterpart. However, corrosion resistance of the coating annealed at 400 ℃ is the worst. With further increasing the annealing temperature, the corrosion resistance of the coatings is improved.
3) For laser-treated coating, amorphous electroless Ni-W-P coatings treated by the diode laser are transformed into nanocrystalline phases consisting of Ni and Ni3P. The grain sizes of both phases increase with decrease in scanning velocity.
4) Corrosion performance of the laser-treated coatings at low scanning velocities between 7 mm/s and 9 mm/s is close to the best performance of the furnace- annealed coating at 200 ℃ and 300 ℃. Further increase in scanning velocity results in the deterioration of corrosion resistance.
5) Corrosion mechanism of the amorphous electroless Ni-W-P coatings treated by both laser and furnace-annealing can be considered as a result of combined effects from porosity, phase constitutes and proportion, residual stress, grain sizes of both Ni and Ni3P phases. However, each factor plays a different role in different cases. Compared with conventional furnace- annealing, laser treatment offers significant advantages and great potentials for industrial applications.
References
[1] REVESZ. A, LENDVAI J, LORANTH J, PADAR J. Nanocrystallization studies of an electroless plated Ni-P amorphous alloy [J]. Journal of the Electrochemical Society, 2001, 148 (11): 715-720.
[2] LU K. Nanocrystalline metals crystallized from amorphous solids: Nanocrystallization, structure, and properties [J]. Materials Science and Engineering R: Reports, 1996, 16 (4): 161-221.
[3] BIRRINGER R, GLEITER H, KLEIN H P, MARQUQRDT P. Nanocrystalline materials: An approach to a novel solid structure with gas-like disorder? [J]. Physics Letters A, 1984, 102 (8): 365- 369.
[4] KOCH C C. Synthesis of nanostructured materials by mechanical milling: Problems and opportunities [J]. Nanostructured Materials, 1997, 9(1/8): 13-22.
[5] ERB U, ELSHERIK A M, PALUMBO G, AUST K T. Synthesis, structure and properties of electroplated nanocrystalline materials [J]. 1993, 2(4): 383-390.
[6] BALARAJU J N, RAJAM K S. Superhard nanocomposite coatings of TiN/a-C prepared by reactive DC magnetron sputtering [J]. Surface and Coatings Technology, 2005, 195(2/3): 147-153.
[7] VALIEV R Z, ISLAMGALIEV R K, ALEXANDROV I V. Bulk nanostructured materials from severe plastic deformation [J]. Progress in Materials Science, 2000, 45(2): 103-189.
[8] TSAI Y Y, WU F B, CHEN Y I, PENG P J, DUH J G, TSAI S Y. Thermal stability and mechanical properties of Ni-W-P electroless deposits [J]. Surface and Coatings Technology, 2001, 146/147(9/10): 502-507.
[9] WANG Tian-xu, MENG Ji-long, HU Yong-jun, RAO Qian-yang. Influence of heating temperature on the microstructure and phase transformation behaviour of electroless plating Ni-W-P on aluminium alloy [J]. Metal Heat Treatment, 2005, 30(6): 21-25. (in Chinese)
[10] SHA W. Thermodynamic analysis of crystallisation in amorphous solids [J]. Journal of Alloys and Compounds, 2001, 322: 17-18.
[11] CREMASCHI V, MATERIALIA S, AVRAM I. Electrochemical studies of amorphous, nanocrystalline, and crystalline FeSiB based alloys [J]. Scripta Materialia, 2002, 46(1): 95-100.
[12] WANG X Y, LI D J. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel [J]. Electrochemical Acta, 2002, 47(24): 3939-3947.
[13] GAO Y, ZHENG Z J, ZHU M, LUO C P. Corrosion resistance of electrolessly deposited Ni-P and Ni-W-P alloys with various structures [J]. Materials Science and Engineering A, 2004, 381(1/2): 98-103.
[14] ZANDER D, K?STER U. Corrosion of amorphous and nanocrystalline Zr-based alloys [J]. Materials Science and Engineering A, 2004, 375/377: 53-59.
[15] INTURI R B, SZKLARSKA S Z. Localized corrosion of nanocrystalline 304 type stainless steel films corrosion [J]. Electrochemica Acta,1992, 48(4): 398-403.
[16] THORPE S J, RAMASWAMI B, AUST K T. Corrosion and Auger studies of a nickel-base metal-metalloid [J]. J Electrochem Soc, 1988, 135: 2162-2170.
[17] ROFAGHA R, ERB U, OSTRANDER D, PALUMBO G, AUST K T. The effects of grain size and phosphorus on the corrosion of nanocrystalline Ni-P alloys [J]. Nanostructured Materials, 1993, 2: 1-8.
[18] MATSUKAWA K, KATAOKA M, MORINUSHI K. The effect of pulsed laser annealing on wear and corrosion properties of electroless Ni-P plating [J]. Tribology Transactions, 1994, 37: 573-381.
[19] YOUNANL M M, ALY I H M, NAGEEB M T. Effect of heat treatment on electroless ternary nickel-cobalt-phosphorus alloy [J]. Journal of Applied Electrochemistry, 2002, 32: 439-446.
[20] ZHANG X Y, DENG Z G. Effects of phosphorus content and heat treatment on the corrosion behaviours of Ni-P alloys [J]. Surface Technology, 1994, 23 (6): 254-259. (in Chinese)
[21] GARCIA-ALONSO M C, ESCUDERO M L, LOPEZ V, MAC?AS A. The corrosion behaviour of laser treated Ni-P alloy coatings on mild steel [J]. Corrosion Science, 1996, 38(3): 515-530.
[22] KOSTER U, ZANDER Z. Environmental properties of Zr-based metallic glasses and nanocrystalline alloys [J]. Scripta Materialia, 2001, 44(8/9): 1649-1654.
[23] LU G J, ZANGARI G. Corrosion resistance of ternary Ni-P based alloys in sulfuric acid solutions [J]. Electrochemical Acta, 2002, 47(18): 2969-2979.
[24] PALANIAPPA M, SESHADRI S K. Structural and phase transformation behaviour of electroless Ni-P and Ni-W-P deposits [J]. Materials Science and Engineering A, 2007, 460/461: 638-644.
[25] SONG J.Y, YU J. Residual stress measurements in electroless plated Ni-P films [J].Thin Solid Films, 2002, 415: 167-172.
[26] XIE H, CHEN W Z, QIAN K W. Effects of heat treatment on the internal stress and adhesion of Ni-P coating [J]. Plating and Environmental protection, 2004, 24(3): 13-15.
Foundation item: Project(Y2006F40) supported by the Natural Science Foundation of Shandong Province, China; Project(N00003) supported by UK Northwest Science Council through Northwest Laser Engineering Consortium (NWLEC)
Corresponding author: L1U Hong; Tel: +86-531-88523981; E-mail: hongshuoxin@163.com
DOI: 10.1016/S1003-6326(09)60252-1
(Edited by YANG Bing)
Abstract: A comparative study of amorphous electroless Ni-W-P coatings on mild steel substrate treated by a high power diode laser and furnace annealing was presented. Effects of different laser operating parameters and furnace-annealing conditions on microstructures, in terms of crystallisation, pores formation and grain growth, were investigated using SEM/EDX and XRD. Corrosion behaviours of these coatings before and after various treatments were evaluated with anodic polarisation in 0.5 mol/L H2SO4 solution. The results show that the furnace-annealing produces either a mixture of nanocrystallined Ni and amorphous phases or precipitated Ni3P phase distributed in nanocrystallined Ni-based matrix, depending on annealing temperatures, whilst the laser treatment under the operating conditions only produces nanocrystallined Ni-based matrix with Ni3P precipitates. Corrosion performance of the coatings treated by both the laser and the furnace-annealing is dependent on the annealing temperature and laser operating conditions. Corrosion mechanisms of various treated-coatings were discussed in the consideration of phase constitutes and proportion, grain sizes of both Ni and Ni3P phases, pores formation and residual stresses.
Superhard nanocomposite coatings of TiN/a-C prepared by reactive DC magnetron sputtering [J]. Surface and Coatings Technology, 2005, 195(2/3): 147-153." target="blank">[6] BALARAJU J N, RAJAM K S. Superhard nanocomposite coatings of TiN/a-C prepared by reactive DC magnetron sputtering [J]. Surface and Coatings Technology, 2005, 195(2/3): 147-153.
Corrosion resistance of electrolessly deposited Ni-P and Ni-W-P alloys with various structures [J]. Materials Science and Engineering A, 2004, 381(1/2): 98-103." target="blank">[13] GAO Y, ZHENG Z J, ZHU M, LUO C P. Corrosion resistance of electrolessly deposited Ni-P and Ni-W-P alloys with various structures [J]. Materials Science and Engineering A, 2004, 381(1/2): 98-103.


