
Grain refining mechanism of Al-3B master alloy on hypoeutectic Al-Si alloys
LIU Yuan1, 2, DING Chao2, LI Yan-xiang1, 2
1. Key Laboratory for Advanced Materials Processing Technology of Ministry of Education,
Tsinghua University, Beijing 100084, China;
2. Department of Mechanical Engineering, Tsinghua University, Beijing 100084, China
Received 17 June 2010; accepted 15 August 2010
Abstract:
Al-3B master alloy is a kind of efficient grain refiner for hypoeutectic Al-Si alloys. Experiments were carried out to evaluate the effect of undissolved AlB2 particles in Al-3B master alloy on the grain refinement of Al-7Si. It is found that the number and the settlement of AlB2 particles in the melt all have effect on the grain refining efficiency. On the basis of experiments and theoretical analysis, a new grain refinement mechanism was proposed to explain the grain refinement action of Al-3B on hypoeutectic Al-Si alloys. The formation of ‘Al-AlB2’ shell structure is the direct reason for grain refinement and the undissolved AlB2 particles is the indirect nucleating base for subsequent α(Al) phase.
Key words:
Al-3B master alloy; hypoeutectic Al-Si alloys; nucleation mechanism; grain refinement;
1 Introduction
Hypoeutectic Al-Si alloys are the most important foundry aluminum alloys which have wide applications in marine, automobile and aircraft industries due to their excellent casting characteristics, pressure tightness and corrosion resistance [1]. Hypoeutectic Al-Si alloys have a large volume fraction of primary α(Al) in their microstructure. Quality of the castings can be improved by grain refinement that reduces the size of primary α(Al) grains in the casting. A fine equiaxed grain structure leads to several benefits, such as high yield strength, high toughness, improved machinability and excellent deep drawability of the products [2]. The aluminum alloys contain Si [3-4] as alloying elements which make them respond poorly to grain refinement by Al-5Ti-1B master alloy, which is usually termed as poisoning effect. It is in general believed that the poisoning elements interact with the grain refining constituents of the Al-Ti-B master alloys (Al3Ti and TiB2) and make them ineffective or less effective [5-6].
In 1981, LU and WANG [7] found for the first time that Al-4B master alloy is an efficient grain refiner for A356 alloy with 7%Si (mass fraction). From then on, other researchers [8-11] found that Al-B system is one kind of efficient and long-acting grain refiner for Al-Si alloys. In addition, it can overcome the poisoning of high level of Si concentration. Thirty years have passed and a lot of research about this has been done; however, the grain refinement mechanism of Al-B master alloys on hypoeutectic Al-Si alloys is still a controversial subject. Up to now, there are mainly two kinds of grain refinement mechanisms. One is called boride theory proposed by SIGWORTH and GUZOWSKI [9]; the other is called Al-B eutectic theory proposed by MOHANTY and GRUZLESKI [10]. In boride theory, the AlB2 particles introduced from Al-B master alloys are thought to act as nucleating substrates for α(Al) because the AlB2 particle just has a lower crystallographic disregistry of 4.96% with α(Al). While in Al-B eutectic theory, as seen from Al-B phase diagram (shown in Fig. 1), there exists a eutectic reaction: L→Al(s)+AlB2(s), in Al-B binary alloy at 659.7 °C, which is higher than the nucleation temperature of α(Al). So the solid Al created by this reaction can act directly as the nucleation substrate of subsequent α(Al) so as to cause the grain refinement. MOHANTY and GRUZLESKI [10] also thought that the undissolved AlB2 did not participate in the grain refining process because the experiments showed that Al-B master alloy has no any grain refining action to pure aluminum. Therefore, the sedimentation and conglomeration of the undissolved AlB2 particles cannot affect the grain refining efficiency, so Al-B master alloys are long-acting grain refiners for hypoeutectic Al-Si alloys. The deviation between these two theories is that if the undissolved AlB2 particles introduced from Al-B master alloys participate in the grain refinement process.
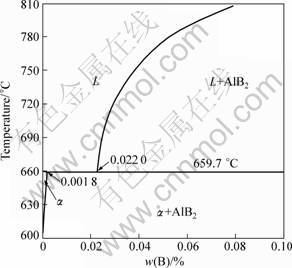
Fig. 1 Al-rich side of Al-B phase diagram [6]
Generally, B exists as AlB2 and AlB12 particles in Al-3B master alloy, as shown in Fig. 2. AlB12 is not stable when B content is low in the alloy melt. So after Al-3B is added into the Al-Si alloys melt, AlB12 particles will react with Al to form AlB2 particles by peritectic reaction [9, 12]: AlB12(S)+5Al(L)→6AlB2(S). At the same time, some of the AlB2 particles will dissolve in the melt. In other words, B exists as the form of undissolved AlB2 particles and the dissolved B atoms in the melt. Naturally, the grain refinement is caused by the undissolved AlB2 particles or the dissolved B or both of them.
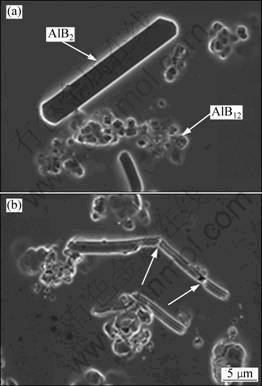
Fig. 2 Microstructures of Al-3B before rolling (a) and after rolling (b) at reduction level of 40%
In the present work, experiments were conducted to show the grain refining ability of Al-3B master alloy to commercial pure aluminum and hypoeutectic Al-7Si alloys. The emphasis was focused on the action of AlB2 particles in the grain refining process. At last, a new grain refinement mechanism was proposed to explain the grain refining action of Al-3B master alloy on hypoeutectic Al-Si alloys.
2 Experimental
Binary Al-7Si alloy was made in an electrical resistance furnace by mixing commercial pure aluminium (99.7%) and commercial pure silicon (99.6%). Chemical analyses were performed by emission spectrometric analyzer with a measurement accuracy of 0.001%.
Al-7Si alloy for the grain refinement studies was remelted in an electrical resistance furnace and held at a constant temperature of 720 °C. Grain refinement was achieved by adding Al-3B master alloy rods. The contact time of the grain refiner was 20 min and the melt was stirred for 30 s. In 5 min, the melt was poured into the standard sample cup (30 mm×55 mm). After solidification the samples were sectioned midway from the bottom of the cylinder. One section was macroetched and the other was anodised. Grain sizes were measured by the lineal intercept method on the anodised samples in an optical microscope under polarised light.
Considering that the number and sizes of the undissolved AlB2 particles in the melt must inherit the number and sizes of the AlB2 particles in the original Al-3B master alloy, some Al-3B blocks were rolled at a reduction level of 40%, then, their grain refinement ability to Al-7Si alloy was tested. The microstructures of Al-3B before and after rolling are shown in Fig. 2. It is obvious that the bulky AlB2 particles ruptured (pointed by the arrowheads) after rolling, correspondingly, the number will increase.
In order to prove if the undissolved AlB2 particles participate in the grain refinement of Al-7Si, an experiment was made. The detailed experimental procedures are as follows: after adding Al-3B and stirring the melt for 30 s, a steel plate with support shelves was put into melt and stand at the half height of the melt to check the effect of settlement of AlB2 particles close to the plate on the final grain size. After that, the melt was held at 700 °C for 2 h without any disturbing, then power off the furnace and let the melt cooled with the furnace and solidified directly in the crucible. In the last, the distribution of grain sizes in the longitudinal section of Al-7Si cast was examined.
3 Results and discussion
3.1 Effect of B content on grain size of Al-7Si
Different amount of Al-3B master alloys was added into Al-7Si alloy for grain refinement. The relation between grain size and B content is shown in Fig. 3 and microstructures of corresponding alloys are shown in Fig. 4. It can be seen from Fig. 3 that, when mass fraction of B is less than 0.01%, there is no grain refinement; while the grain size decreases distinctly with the increase mass fraction of B between 0.01% and 0.03%; when the mass fraction of B increases up higher than 0.03%, the grain size of Al-7Si does not decrease further. This result can be explained with Al-B binary phase diagram shown in Fig. 1 in which AlB2 phase exists in the melt (above 659.7 °C) just when mass fraction of B is higher than 0.02%.
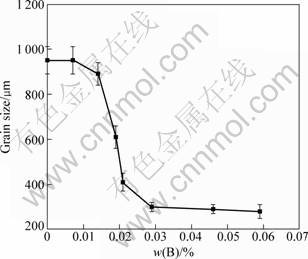
Fig. 3 Relation between grain size of Al-7Si and mass fraction of B
3.2 Effect of rolling on grain refinement ability of Al-3B
Figure 5 shows the grain size of Al-7Si with addition of rolled and unrolled Al-3B master alloy. It is obvious that the rolled Al-3B master alloy always leads to a smaller grain size, which means that the grain refinement ability of Al-3B master alloy is improved after rolling. Considering that the rolling process just influences the form and average number of the AlB2 particles, so it is surmisable that the undissolved AlB2 particles participate in the grain refinement process of Al-7Si.
3.3 Effect of Al2B settlement on grain size distribution of Al-7Si ingot
This experiment was made as follows: after adding Al-3B(0.03%B) and stirring the melt for 30 s, a steel plate with support shelves was put into melt and stand at the half height of the melt. After that, the melt was held at 700 °C for 2 h without any disturbing, then power off the furnace and let the melt cooled with the furnace and solidified directly in the crucible. In the last, the macro-grain distribution in the longitudinal section of Al-7Si ingot was examined with a chemical agent of v(HCl):v(HNO3)=3:1. Figure 6 shows the entire grain distribution in the longitudinal section of Al-7Si ingot, together with the measured B content at four selected zones. It is apparent that the grains are very coarse in these zones of about 10 mm in width close to the top of the ingot and underside of the steel plate. But the grain is fine in other zone. Because there is not a burning loss of B in the inner part (close to the steel plate) of the melt, it is reasonable that the coarser grain size is caused by the settlement of AlB2 particles in this zone. As shown in Fig. 6, the measured B contents at four zones also show the settlement phenomenon of AlB2 particles because the bottom of the ingot and the upper part close to the steel plate all present a higher B content than other positions. From the experiment result, we can conclude that the undissolved AlB2 particles play an important role in the grain refinement; at least, they are necessary for grain refinement of Al-7Si.
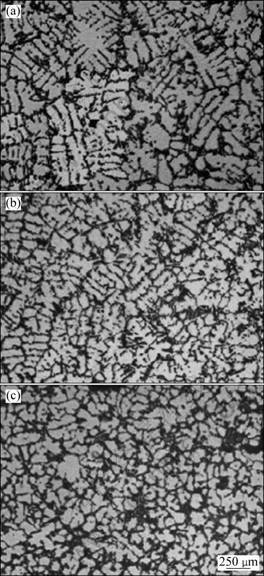
Fig. 4 Microstructures of alloys: (a) Without B; (b) With 0.017%B; (c) With 0.046%B
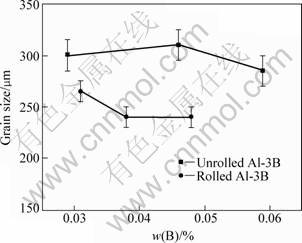
Fig. 5 Grain size of Al-7Si with addition of rolled and unrolled Al-3B master alloy
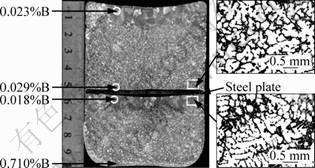
Fig. 6 Grain structure in longitudinal section of Al-7Si ingot together with measured B mass fraction at four selected zones
It is well known that the solid particles may rise or settle in the liquid if its density is different from that of the liquid. The settling distance can be calculated as Stokes’ law [13]:
![]() (1)
(1)
where S is the moving distance of the particle; t is the time for settling; g is the gravity; ρs and ρl are the densities of the particles and liquid, respectively; d is the diameter of the particle; and η is the dynamic viscosity of the liquid. So the undissolved solid AlB2 particles should subside in Al-7Si melt because its density (3.1 g/cm3 [14]) is higher than that of the melt (2.4 g/cm3).
The SEM observation of the Al-3B master alloy shows that the cylindrical AlB2 particles have an average diameter of 2 μm and length of 5 μm. If we suppose that they are globular particles with an equivalent diameter of 3.1 μm and substitute the other parameters: t=7 200 s, g=9.8 m/s2, ρs=3.1 g/cm3, ρl=2.4 g/cm3, η=2.8×10-3 Pa·s into Eq. (1), the calculated settling distance of the particles is 9.4 mm, which is similar to the width of the coarse-grain area close to the top of the ingot and underside of the steel plate, as shown in Fig. 6.
3.4 Grain refining ability of Al-3B to commercial pure aluminum
Table 1 lists the grain size of commercial pure aluminum with different B contents and microstructures of corresponding alloys are shown in Fig. 7. It is can be seen that there is not grain refinement with 0.04%B addition. But when the mass fraction of B is as high as 0.3%, the average grain size reduces at a certain extent but grains still are coarse. This experiment proves that the grain refining ability of Al-3B to the commercial pure aluminum is very poor, which also has been approved by other literates [10, 14-15]. In other words, AlB2 is not a good nucleating substrate for α(Al).
Table 1 Grain size of commercial pure aluminum with different B contents
![]()
4 Grain refining mechanism of Al-3B
From the experiment results above, we can conclude that the undissolved AlB2 particles are necessary for the grain refinement of Al-7Si. The traditional Al-B eutectic theory cannot explain these results. But the grain refining experiment on pure aluminum also prove that AlB2 particle is not an effective nucleating substrate for α(Al), so the traditional boride theory also cannot explain the above experiments. In other words, the excellent grain refining ability of Al-3B to Al-7Si is not because of the direct nucleating action of undissolved AlB2 particles to α(Al) phase.
Based on the previous experimental results and analysis, a new mechanism is put forward to explain the refining action of Al-B master alloy on Al-Si hypoeutectic alloy. This new mechanism is described in Fig. 8 in which a layer of solid aluminum first form on the undissolved AlB2 particle from Al-B eutectic reaction at 659.7 °C, then during the following cooling process, when the melt is cooled down to the liquidus temperature of Al-Si alloy (the temperature for α(Al) phase to form), the subsequent α(Al) phase will nucleate directly on the pre-formed solid Al, finally lead to the grain refinement. In other words, the existence of AlB2 particles accelerate the eutectic reaction at 659.7 °C and leads the ‘Al-AlB2’ shell structure to form. The ‘Al-AlB2’ shell structure is the direct reason for grain refinement. The existence of AlB2 particles is an indispensable pre-condition and AlB2 particle is the indirect nucleating base for subsequent α(Al) phase.
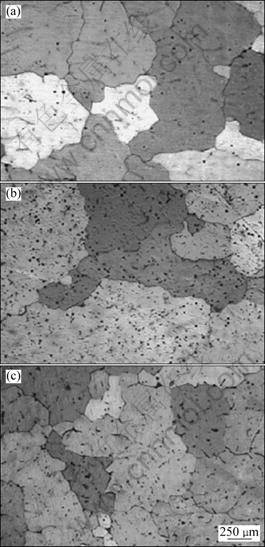
Fig. 7 Microstructures of alloys with different B contents: (a) Without B; (b) 0.04%B; (c) 0.30%B
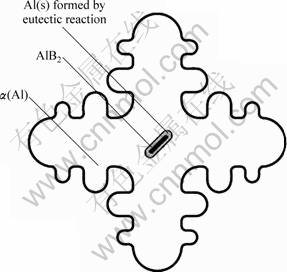
Fig. 8 Sketch for describing grain refining mechanism of Al-3B to Al-Si alloy
As shown in Fig. 10, AlB2 particles were found within α(Al) grains of Al-7Si alloy refined by Al-3B master alloy (mass fraction is 0.029%). TONDEL and HALVORSEN [11] also found AlB2 particles in the α(Al) of Al-9.6Si grain refined by Al-4B. This is just because undissolved AlB2 particles act indirectly as the nucleation base of α(Al).
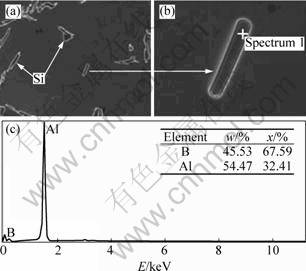
Fig. 9 AlB2 particles located within α(Al) (Al-7Si) grain by SEM observation (a, b) and EDS analysis (c)
When mass fraction of B in Al-7Si is less than 0.01%, no grain refinement can be achieved. TONDEL and HALVORSEN [11] studied the grain refinement behavior of Al-4B master alloy on Al-9.6Si alloy and also found when mass fraction of B is less than the critical value (<0.02%), there is no grain refinement. It is easy to understand that, when mass fraction of B is less than the solubility limit of 0.022%, as shown in Fig. 1, no AlB2 particles exist in the Al-Si alloys melt, correspondingly, there will have no grain refinement phenomenon.
The undissolved AlB2 particles are tiny and their density (3.1 g/cm3 [16]) is low so that the sedimentation rate is low. We think that one of the main reasons why Al-B master alloys are longer-acting grain refiners for hypoeutectic Al-Si alloys compared with Al-Ti-B master alloys, is the useful phase of TiAl3 with the density of 4.5 g/cm3 [16].
The new mechanism combines the traditional boride theory and eutectic reaction theory. It can explain all experimental phenomena. However, it is an inferential model up to now because no direct experimental proof has been observed, so further study will be made in the future.
5 Conclusions
1) The effect of grain refiner additions, Al-3B master alloy, on grain size was investigated in hypoeutectic Al-7Si alloy.
2) When mass fraction of B is less than 0.01%, there is no grain refinement; while the grain size decreases distinctly with the increase of mass fraction of B between 0.01% and 0.03%; when mass fraction of B increases up higher than 0.03%, the grain size does not decrease further.
3) The grain refinement ability of Al-3B master alloy improves after rolling and the settlement of AlB2 particles has effect on the grain refining efficiency.
4) The existence of AlB2 particles is an indispensable pre-condition and AlB2 particle is the indirect nucleating base for subsequent α(Al) phase. The undissolved AlB2 particles accelerate the eutectic reaction at 659.7 °C and lead the ‘Al-AlB2’ shell structure to form. The subsequent α(Al) phase will nucleate directly on the ‘Al-AlB2’ shell structure, finally lead to the grain refinement.
References
[1] ATXAGA G, PELAYO A, IRISARRI A M. Effect of microstructure on fatigue behaviour of cast Al-7Si-Mg alloy [J]. Materials Science and Technology, 2001,17: 446-450.
[2] MaCARTNEY D G. Grain refining of aluminum and its alloys using inoculants [J]. Int Mater Rev, 1989, 34: 247-260.
[3] SRITHARAN T, LI H. Influence of Ti to B ratio on the ability to grain refine Al-Si alloy [J]. J Mater Process Technol, 1997, 63: 585-589.
[4] MOHANTY P S, SAMUEL F H, GRUZLESKI J E. Studies on addition of inclusions to molten aluminum using a novel technique [J]. Metall Trans B, 1995, 26: 103-109.
[5] KORI S A, AURADI V, MURTY B S, CHAKRABORTY M. Poisoning and fading mechanism of grain refinement in Al-7Si alloy [J]. Materials Forum, 2005, 29: 387-393.
[6] QIU D, TAYLOR J A, ZHANG M X, KELLY P M. A mechanism for the poisoning effect of silicon on the grain refinement of Al-Si alloys [J]. Acta Materialia, 2007, 55: 1447-1456.
[7] LU H T, WANG L C. Grain refining in A356 alloys [J]. J Chin Foundryman’s Assoc, 1981, 29: 10-18.
[8] KORI S A, MURTY B S, CHAKRABORTY M. Development of an efficient grain refiner for Al-7Si alloy [J]. Mater Sci Eng A, 2000, 280: 58-61.
[9] SIGWORTH G K, GUZOWSKI M M. Grain refinement of hypoeutectic Al-Si alloys [J]. AFS Transaction, 1985, 93: 907-912.
[10] MOHANTY P S, GRUZLESKI J E. Grain refinement mechanisms of hypoeutectic Ai-Si alloys [J]. Acta Mater, 1996, 44(9): 3749-3760.
[11] TONDEL P A, HALVORSEN G. Grain refinement of hypoertectic Al-Si Foundry alloys by addition of boron containing silicon metal [J]. Light Metasl, 1993: 783-790.
[12] WANG X M. The formation of AlB2 in an Al-B master alloy [J]. Journal of Alloys and Compounds, 2005, 403: 283-287.
[13] SCHAFFER P L, DAHLE A K. Settling behaviour of dirrerent grain refiners in aluminium [J]. Mater Sci Eng A, 2005, 413: 373-378.
[14] EASTON M, STJOHN D. Grain refinement of aluminum alloys: Part 1. The nucleant and solute paradigms-a review of the literature [J]. Metall Trans A, 1999, 30: 1613-1623.
[15] MARCANTONIO J A, MONDOLFO L F. Nucleation of aluminium by several intermetallic compounds [J]. J Inst Metals, 1970, 98(1): 23-27.
[16] SHAHROOZ N, REZA G. Boron-based refiners: Implications in conventional casting of Al-Si alloys [J]. Mater Sci Eng A, 2007, 452: 445-453.
Al-3B中间合金对亚共晶Al-Si合金的晶粒细化机制
刘 源1, 2,丁 超2,李言祥1, 2
1. 清华大学 先进成形制造教育部重点实验室,北京 100084;
2. 清华大学 机械工程系,北京 100084
摘 要:Al-3B中间合金是亚共晶Al-Si合金的高效细化剂之一。实验研究了Al-3B中间合金中未溶AlB2颗粒对Al-7Si合金晶粒细化的影响。结果表明,AlB2颗粒的数目和沉降对晶粒细化效果都有重要影响。在实验结果和理论分析基础上,提出了Al-3B中间合金对亚共晶Al-Si合金晶粒细化的新机制,认为通过共晶反应形成的“Al-AlB2”包覆结构是导致晶粒细化的直接原因,未溶的AlB2颗粒是α(Al)相的间接行核基底。
关键词:Al-3B中间合金;亚共晶Al-Si合金;行核机制;晶粒细化
(Edited by YANG Hua)
Foundation item: Project supported by Tsinghua-Wuxi Science Foundation, China
Corresponding author: LIU Yuan; Tel: +86-10-62789328; Fax: +86-10-62773637; E-mail: yuanliu@tsinghua.edu.cn
DOI: 10.1016/S1003-6326(11)60878-9


