
Growth of straight carbon nanotubes by simple thermal chemical vapor deposition
ZOU Xiao-ping(邹小平) 1,2,3,4, H. ABE 3, T. SHIMIZU 3, A. ANDO 5, H. TOKUMOTO 3,6, ZHU Shen-ming(朱申敏)4, ZHOU Hao-shen(周豪慎)4
1.Research Center for Sensor Technology, Beijing Information Technology Institute, Beijing 100101, China;
2.Beijing Key Laboratory for Sensor, Beijing 100101, China;
3.Nanotechnology Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibalaki 305-8562, Japan;
4.Energy Electronics Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Umezono, Tsukuba, Ibalaki 305-8568, Japan;
5.Nano-electronics Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Umezono, Tsukuba, Ibalaki 305-8568, Japan;
6.Nanotechnology Research Center, Research Institute for Electronic Science, Hokkaido University, Sapporo 001-0021, Japan
Received 10 April 2006; accepted 25 April 2006
Abstract:
Straight carbon nanotubes (CNTs) were achieved by simple thermal chemical vapor deposition(STCVD) catalyzed by Mo-Fe alloy catalyst on silica supporting substrate at 700 ℃. High-resolution transmission electron microscopy images show that the straight CNTs are well graphitized with no attached amorphous carbon. Mo-Fe alloy catalyst particles play a very crucial role in the growth of straight CNTs. The straight carbon nanotubes contain much less defects than the curved nanotubes and might have potential applications for nanoelectrical devices in the future. The simple synthesis of straight CNTs may have benefit for large-scale productions.
Key words:
straight carbon nanotubes; simple thermal chemical vapor deposition; Mo-Fe alloy catalyst;
1 Introduction
Since the carbon nanotube was discovered in 1991 by IIJIMA[1], it has attracted much attention due to fundamental science and potential applications. According to theoretical predictions, carbon nanotubes have many unique properties such as remarkable electronic properties[2-4] and high mechanical strength, and all of which suggest a wide range of potential applications in the future. Because defects severely limit the electrical conductivity[5] and mechanical strength of the material, it is important to grow defect-free nanotubes in order to examine the theoretical predictions and use them in applications. So far, several methods have been developed to synthesize carbon nanotubes[6, 7]. Among them, chemical vapor deposition(CVD) technique has received a great deal of attention since carbon nanotubes(CNTs) can be synthesized in low-cost and large-scale production. But in the usual catalytic growth of CNTs by CVD, CNTs are often curved and tangled together, and have a great of structural defects. These will limit the basic research and potential applications. How to synthesize the straight CNTs is a challenge.
In the prensent work the growth of straight nanotubes was investigated by using Mo-Fe/silica as catalyst. These straight nanotubes grown by CVD may offer the possibility to use them in future applications. The results show that the straight CNTs are well graphitized with no attached amorphous carbon. Mo-Fe alloy catalyst particles play a very crucial role in the growth of straight CNTs. The straight carbon nanotubes contain much less defects than the curved nanotubes and might have potential applications for nanoelectrical devices in the future.
2 Experimental
The straight carbon nanotubes were synthesized in the catalytic decomposition of ethanol at 700 ℃ over Mo-Fe/silica gel support materials. For the catalyst preparation, the silica powder was impregnated into a mixed solution of iron nitrate and ammonia molybdenate. Then, silica was dried overnight at 110 ℃. Finally, it was calcinated at 450 ℃ for 1 h. No reduction was required.
The growth of straight carbon nanotubes was carried out in a horizontal tube furnace at atmospheric pressure. The reaction chamber was a quartz tube with a 50 mm in diameter and 500 mm in length. One end of the tube was connected to an air vent; the other was closed. Outside and adjacent to the middle part of the quartz tube there was a tubular electric furnace (about 300 mm length). In the central part of the tube there was an isothermal area and the substrate was located in this area in a rectangular quartz boat. So, the temperature of the substrate was the same as that of growth for carbon nanotubes (CNTs). Ethanol vapor was passed over the catalyst and heated by the electric furnace. Scanning electron microscope FE-SEM, S-4300, HITACHI and a high-resolution transmission electron microscope HRTEM, JEM 2000F, JEOL were employed to characterize the morphologies and microstructures of CNTs formed on the surface of silica. A drop of ethanol solution of carbon product was directly dropped on the copper grids for specimens for transmission electron microscopy characterization.
3 Results and discussion
The silica supported catalysts were found to be active in the formation of carbon nanotubes as shown in Fig. 1. In this image the silica powder (white contrast) is present with nanotubes and no amorphous carbon is observed. Most of the nanotubes are straight and up to about several micrometers long. These carbon nanotubes have diameters around 10 nm.
When a single crystal of metal is used as a catalyst, different crystal planes have different catalytic performances[8]. As for the growth of carbon nanotubes in previous reports[9], when just one kind of transition metal is used as a catalyst, due to different catalytic activities on the surface of the nanoparticles [10, 11], the growth rate along the circumference is not uniform; and this causes the nanotube to curve towards the lower growth rate side.
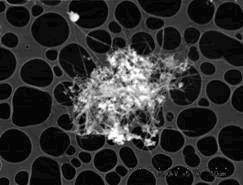
Fig.1 SEM image of carbon nanotubes grown on Mo-Fe/silica at growing time of 0.5 h
During pretreatment, these metal atoms diffuse out of the supercages of the silica and aggregate to form Mo-Fe alloy nanoparticles on the outer surface of the silica. These nanoparticles of this alloy are the catalysts for the growth of the carbon nanotubes. As a matter of fact, the Mo-Fe alloy nanoparticles are very much like spheres. This regularity in morphology indicates that the surface of the Mo-Fe alloy is nearly uniform. In the case of a Mo or Fe single metal catalyst, TEM examination indicates that Mo and Fe metal nanoparticles are nearly polygonal. In addition, when a Mo or Fe single metal catalyst is used to grow carbon nanotubes in the same condition as that of the Mo-Fe alloy, no similar results can be obtained, even when the growth conditions are changed. Therefore, it can be concluded that the catalytic activity on the surface of the Mo-Fe alloy is nearly uniform, which results in a uniform growth rate along the circumference. This might be the reason why the growth of straight nanotubes can be realized. The TEM image(Fig. 2) shows the straght nanotubes have clear fringes and a little amorphous carbon. The carbon nanotube is well graphitized and no amorphous carbon is seen around the periphery of the carbon nanotube.
It is interesting to note that this nanotube is filled with graphic fragment during the growth process. TEM examination reveals that the filled nanotubes are about 2 nm in diameter. The inner diameter of these nanotubes (about 2 nm) is much smaller than the usual results in Ref.[9]. We think that the much smaller inner diameter is due to the surface chemistry of the Mo-Fe alloy and not just the difference in the size of the catalyst grains. Furthermore, we should mention some differences between the present results and previous work about aligned carbon nanotubes[9]. Except for different substrates and catalysts, the growth temperature is about 700 ℃, which is the same as that of previous work. In
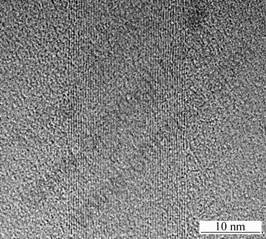
Fig.2 High-resolution TEM image of carbon nanotube
addition, the carbon nanotubes are obtained in exactly the same conditions as that of in Ref.[9] except for the substrate and catalyst; but we can only obtain a large amount of curved carbon nanotubes. The reasons and details of these unique nanotubes are under further investigation.
4 Conclusions
By using a Mo-Fe catalyst a large amount of straight CNTs are obtained, which means that the Mo-Fe alloy is a good catalyst for the growth of CNTs; and the CNTs have several interesting characteristics: straightness, less defects, and good graphitization. All these features may make this method useful in the research of the properties of CNTs and for potential applications of carbon nanotubes.
Acknowledgements
This work was partially supported by Japan Science Promotion Society (JSPS) and New Energy and Industrial Technology Development Organization (NEDO) as a part of Nano Carbon Technology (NCT) Project, Japan.
References
[1] IIJIMA S, Helical microtubules of graphitic carbon[J]. Nature, 1991, 354: 56-58.
[2] MINTMIRE J W, DUNLAP B I, WHITE C T. Are fullerene tubules metallic?[J]. Phys Rev Lett, 1992, 68: 631-634.
[3] HAMADA N, SAWADA S, OSHIYAMA A. New one-dimensional conductors: graphitic microtubules[J]. Phys Rev Lett, 1992, 68: 1579-1581.
[4] HARIGAYA K, FUJITA M. Dimerization structures of metallic and semiconducting fullerene tubules[J] Phys Rev B, 1993, 47(24): 16563-16569.
[5] CHICO L, CRESPI V H, BENEDICT L X, LOUIE S G, COHEN M L. Pure carbon nanoscale devices: nanotube heterojunctions[J]. Pyhs Rev Lett, 1996, 76: 971-974.
[6] DRAVID V P, LIN X, WANG X K, YEE A, KETTERSON J B, CHANG R P H. Buckytubes and derivatives: their growth and implications for buckyball formation[J]. Science, 1993, 259: 1601-1604.
[7] HATTA N, MURATA K, Very long graphitic nano-tubules synthesized by plasma-decomposition of benzene[J]. Chem Phys Lett, 1994, 217: 398-402.
[8] STRONG D R, SOMORJAI C A. Catalytic Ammonia Synthesis[M]. New York: Plenum, 1991.
[9] ZOU X P, ABE H, SHIMIZU T, ANDO A, NAKAYAMA Y, TOKUMOTO H, ZHU S M, ZHOU H S. Simple thermal chemical vapor deposition synthesis and electrical property of multi-walled carbon nanotubes[J]. Physica E, 2004, 24: 14-18.
[10] IVANOV V, NAGY J B, LAMBIN Ph, LUCAS A, ZHANG X B, ZHANG X F, BERNAERTS D, TENDELOO G V, AMELINCKX S, LAUDUYT J V. The study of carbon nanotubules produced by catalytic method[J]. Chem Phys Lett, 1994, 223: 329-335.
[11] AMELINCKX S, ZHANG X B, BERNAERTS D, ZHANG X F, IVANOV V, NAGY J B. A formation mechanism for catalytically grown helix-shaped graphite nanotubes[J]. Science, 1994, 265: 635-639.
Foundation item: Project(KM200510772013) supported by the Science and Technology Development Program of Education Committee of Beijing City; Project (2005-2007) supported by the Academic Innovative Team Program(Novel Sensor and Materials: Nanodevice and Nanomaterials) of Education Committee of Beijing City
Corresponding author: ZOU Xiao-ping; Tel: +86-10-64884673-812; Fax: +86-10-64879486-812; E-mail: xpzou2005@gmail.com


