Trans. Nonferrous Met. Soc. China 23(2013) 3280-3285
Effect of surface microstructure of aluminum coating on corrosion properties of magnetic refrigerant gadolinium
Hong-yan WU1, Jian LIU1, Hao-feng ZHAO1, Qiong JIANG2, Yi XU2, Jia XU1
1. School of Physics and Optoelectronic Engineering, Nanjing University of Information Science and Technology, Nanjing 210044, China;
2. School of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China
Received 15 November 2012; accepted 13 March 2013
Abstract:
Al coatings with different microstructures were prepared on the surface of Gd using the magnetron sputtering technique to improve its corrosion resistance. The corrosion behaviors for the pure Gd and Gd with Al coating in distilled water were studied using the mass loss and electrochemical performance. As a result, pure Gd without coating shows a certain amount of surface cracks under water flow conditions, whereas the polygonal Al coating decreases the path of the corrosive medium to body due to the existence of eroding pits structure. Compared with the polygonal structure Al coating and pure Gd, the lamellar structure of Al coating exhibits a higher electrochemical protection performance (e.g., a lower corrosion current and higher self-corrosion potential) and no occurrence of pitting corrosion. Due to an effective physical shield, the formation of the lamellar structure protected the inner Gd part from being corroded, and prolonged the duration of cathodic protection.
Key words:
corrosion resistance; lamellar aluminum coating; polygonal aluminum coating; magnetic refrigeration; pure gadolinium;
1 Introduction
Since 1976, the first magnetic refrigeration operated at room temperature was established by Brown, the research on room-temperature magnetic refrigerator has been carried out all over the world [1,2]. The rare earth metal Gd has been chosen as one of the best magnetic refrigerant materials due to its large magnetic moment and Curie temperature near room temperature (TCurie= 294 K) [3]. Water is normally used as the heat transfer of steam in many magnetic refrigerators demonstrators, but water may cause a serious corrosion problem in rare earth metal Gd [4]. Although the magnetocaloric effect of Gd and other promising room-temperature refrigerants were widely investigated, few studies on the relationship between the corrosion resistant coating for pure Gd and corrosion mechanisms in water cycling systems have been reported [5].
Surface modification is one of effective methods to improve corrosion resistance of Gd [6]. It is a major choice of coating materials with high thermal conductivity for magnetocaloric effect and corrosion resistance in heat transfer medium. Both copper and aluminum exhibit high thermal conductivity, but high purity copper is less employed due to its relatively high price and mass [7]. By contrast, Al and its alloys are applied widely to heat transfer medium due to their excellent diathermancy, tactility, toughness, high intensity relations as well as light specific mass [8]. Al and its alloys are passivated in neutral solution, thus they can be applied as protective coating for magnetic refrigerant Gd. Still, if correctly fabricated, constructions of aluminum may be reliable and have long service life [9]. In the literature, protective coatings for Al and Cu on the surface of Gd by magnetron sputtering technology were investigated. It was demonstrated that Al coating can improve the corrosion resistance of Gd, whereas Cu coating cannot benefit the corrosion proof of Gd for its bad cohesion with Gd [10]. Even if Al coating can improve corrosion, different surface microstructures attribute to difference in performance [11].
In this work, two different structures of Al coatings on pure Gd were prepared by the magnetron sputtering technique. The polarization curves of different structures of Al coatings and pure Gd were analyzed. The effects of Al coating with two different structures on the corrosion resistance were compared. The corrosion mechanisms of coatings and pure Gd in heat transfer medium were discussed.
2 Experimental
2.1 Material and process
The Gd specimens with the purity of 99.9% in mass fraction were spark cut into plates in dimensions of 10 mm×10 mm×5 mm. The Gd plates were mechanically polished, cleaned with acetone and then blown dried.
The magnetic sputtering target of Al plate (d100 mm×5 mm) with purity of 99.9%, which was prepared by powder metallurgy, was used as the source electrode for supplying alloying elements. The base pressure of the system was lower than 5×10-4 Pa prior to each coating deposition. The coating was grown in Ar (99.999% purity) atmosphere. During the deposition, the substrate bias and magnetron power were varied, as shown in Table 1. The substrates were held at a bias voltage of 200-230 V with a deposition rate of 0.8 nm/s. The coatings were grown at a substrate temperature of 500-600 °C. Such a deposition condition results in a coating thickness of 8-15 μm.
2.2 Corrosion properties
Neutral solution test was employed to evaluate the corrosion resistance of the coating and rare earth Gd.
Distilled water was chosen as the electrolyte solution to study the mass loss and electrochemical performance. Polarization curve and electrochemical impedance chart measurements were carried out on an electrochemical working station (CHI660D Shanghai). The exposed surface area of electrochemical specimen was encased by epoxy resin with a test area of 1 cm2. Polarization curves were obtained through a three-electrode system at a scan rate of 10 mV/min. An electrode made by Ag/AgCl (in saturated KCl) and a platinum electrode were used as the reference and auxiliary electrodes, respectively.
For the detection of mass loss, the specimens were carefully ground with silicon carbide papers up to No.1000 before immersion test, degreased in acetone and dried in air. After immersion,the specimens were cleaned. The surface of specimen was ground again and new flesh solution was used for each measurement. The mass loss was determined using a precision electronic balance with a resolution of 0.1 mg.
The samples surface morphologies before and after the corrosion tests were analyzed on a high resolution field emission scanning electron microscope (FESEM, Hitachi S-4800).
3 Results and discussion
3.1 Microscopic analysis of coatings
Typical resulting microstructures for polygonal and lamellar Al coatings are shown in Fig. 1. There is no doubt that two coatings uniformly distribute over the pure Gd as seen from the low magnification SEM images. The distribution of the lamellar Al coating has a microstructure characterized with lamellar of the similar thickness of 20-30 nm, as shown in Fig. 1(a) (red arrow at a red area pointing to lamellar structure with high magnification). The lamellar Al coating possesses a homogeneous and dense microstructure and has no pores direct to substrate. Figure 1(b) shows the surface morphology of polygonal Al coating, which exhibits a polygonal-like uniform microstructure with a low degree of porosity on the surface.
Table 1 Process parameters of two different aluminum coatings
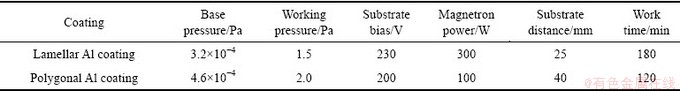

Fig. 1 SEM images of lamellar Al coating (a) and polygonal Al coating (b)
3.2 Corrosion resistance
3.2.1 Static corrosion test
As can be seen in Fig. 2, the mass loss increased with the increase of immersion time. The mass loss of the polygonal Al coating was approximately 10 times more than that of the lamellar Al coating, and the pure Gd corroded more quickly than the polygonal Al coating in distilled water. Figure 3(a) shows the surface features of the lamellar Al coating that was immersed in distilled water. After 10 d, the corrosion film grew as a coherent, compact crystalline layer and can be acted as the protection layer. A typical pitting corrosion formed on surface of the polygonal Al coating is present in Fig. 3(b).
However, the eroded pits are relatively small, homogeneous and compact. Serious corrosion appears after removing the corrosion products formed on the surface of pure Gd due to the number of corroding cracks, as shown in Fig. 3(c). It can be seen from Fig. 3(d) that many calcite-like corrosion products in seemingly random forms distribute on the surface of Gd plate. The oxidized film looses and shows evident micro-cracks, which are easy to be separated. Thus, the film is not protective enough for the Gd matrix.
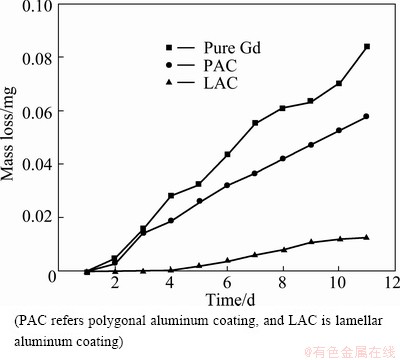
Fig. 2 Relationship between mass loss and immersion time
From above, the lamellar Al coating might be the more proper corrosion inhibitors for Gd than the polygonal Al coating.
3.2.2 Electronical chemical corrosion
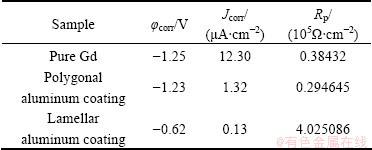
Typical polarization curves of three samples in distilled water are shown in Fig. 4. The corrosion rate is proportional to the density of the current in agreement with Faraclay’s law. Variation in corrosion current density is listed in Table 2. From polarization curves of three samples, lamellar and polygonal Al coatings have a similar corrosion current (marked in red area). Corrosion current density (Jcorr) values of two types of Al coating are much smaller compared with the pure Gd (12.3 μA/cm2). The corrosion current density for polygonal Al coating is an order of magnitude less than pure Gd. Jcorr of the lamellar Al coated Gd is the minimum.

Fig. 3 SEM images showing surface corrosion features of LAC (a), PAC (b), and pure Gd with low (c) and high (d) magnification immersed in distilled water, respectively
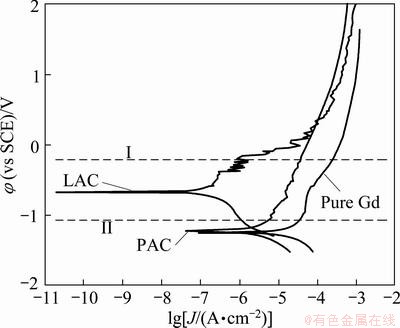
Fig. 4 Typical polarization curves of three samples in distilled water, and crossing point of anodic Tafel slopes of LAC and PAC
Table 2 Corrosion parameters obtained from polarization curves
Polarization studies are shown in Fig. 4 that lamellar Al coating decreases corrosion current by up to two orders of magnitude compared with pure Gd, which is presumably due to the uniform lamellar distribution of Al coating as discussed previously. When the corrosion potential is taken into account, it is the difference in potential between the anodic and cathodic sites. The free corrosion potential (φcorr ) of the Al coating with lamellar structure (-0.62 V), is higher compared with the polygonal structure (-1.25 V), and the curve of the latter at potential value is similar with that of substrate. Corrosion of polygonal Al coating occurs at the area of low-oxygen concentration with the release of hydrogen gas (anode, shown in II), while corrosion of lamellar Al coating forms at the cathode, and will not take place. In contrast, polygonal Al coating forms at the anode (shown in I), and causes serious corrosion. Because the anode current of lamellar Al is significantly smaller than that of polygonal Al; the corrosion rate of lamellar Al coating is lower than that of polygonal Al coating. Therefore, a lamellar Al coating due to surface lamellar stacking structure prevents the internal diffusion from surface ions. The best corrosion resistance exhibits on lamellar Al coating.
It can be also seen from other electrochemical parameters in agreement to corrosion current. The polarization resistance is also apparent in Table 2. The polarization resistance (Rp) of the lamellar Al coating is about 4.0×105 Ω/cm-2. It is approximately 10 times higher than that of polygonal Al coating and 100 times higher that of pure Gd.
Two types of Al coatings are in the active state and could provide sufficient driving force of cathodic protection to substrate. Anodic Tafel slopes (ba) of two Al coatings are significantly smaller compared with cathodic Tafel slope (bc). φcorr is higher and Jcorr is lower for the lamellar Al coating, which indicates that the anodic process of corrosion is inhibited for the Al coating with lamellar structure. The results suggest that the consumption rate of the lamellar Al coating is much slower than that of the other samples. When coupling with the same cathode, the higher the free corrosion potential and polarization resistance of the material are, the lower the corrosion current density is [11]. Therefore, it is expected that lamellar Al coating can increase the corrosion resistance of pure Gd. All these results demonstrated that the electrochemical corrosion behaviors of the samples were dominated by cathode. It is not beneficial to forming a stable passivation film on the surface, so the protective mechanisms of Al coatings are mainly attributed to sacrificial anode protection.
3.2.3 Corrosion mechanisms
Under water flow conditions, pitting, cracks and cavitations may be more severe than those responsible for corrosion [12,13].
The corrosion products of Gd in the water are Gd2O3 floccules and hydrogen. The reaction is as follows [5]:
2Gd+3H2O→Gd2O3↓+3H2↑
SEM image for corrosion appearance of the pure Gd in distilled water indicates that cracks with various sizes on the uneven surface are so much that the nearby corrosion products are easy to fall off the pure Gd surface.
A number of corrosion cracks of pure Gd seen from Fig. 3(c) attribute to stresses difference between corrosion products and pure Gd. The stresses generated by the collapse of bubbles are much greater than those associated with corrosion [14]. It is often sufficient not only to remove protective corrosion product films but actually to tear out small fragments of metal from the surface. Pure Gd freshly exposed as a result of this action will of course be subject to corrosion and the resultant damage is due to a combination of corrosion and the mechanical forces associated with the bubble collapse. Hence, some parts of pure Gd surface are out of protection and not immune to corrosion. Pure Gd leads to more mass loss than polygonal Al coating. The results illustrate that pure Gd is corroded more seriously due to the formation of loose corrosion products and hydrogen precipitation.
Pitting is a highly localized type of corrosion on the surface of Al coating in neutral aqueous solutions. Pitting corrosion is not observed on the lamellar Al coating, but pitting corrosion appears on the surface of the polygonal Al coating in the water. Pits are initiated at weak sites in the oxide by ion attack [14]. Pits propagate according to the reactions:
Al=Al3++3e (1)
Al3++3H2O=Al(OH)3+3H+ (2)
Hydrogen evolution is an important reduction processes at the intermetallic cathodes.
2H++2e=H2 (3)
O2+2H2O+4e=4OH- (4)
According to reaction (2) the pH will decrease and small vapor bubbles will form in the water, which causes pitting corrosion in regions where the flow conditions produce low pressures and subsequent violent collapse of these bubbles on the surface of the materials in neighboring areas where the local pressure is higher [15].
From the above mentioned analysis, it can be known that the lamellar Al coated Gd exhibits the better corrosion resistance than polygonal Al coated Gd. It is related to lamellar structure which has a much lower dissolution rate than polygonal-like Al coating. In addition, the lamellar structure forms a more effective physical shield, extending the path of the corrosive medium to body.
4 Conclusions
1) The corrosion rate of pure Gd greatly decreased due to preparation of Al coating with the different structures. Al coating with high density lamellar microstructure was successfully synthesized by magnetron sputtering technology.
2) The electrochemical corrosion data obtained from different structures of Al coatings and pure Gd in the distilled water were analyzed. Two structures of Al coatings are both of benefit to improving corrosion resistance of Gd. The lamellar Al coating has a better excellent resistance to pitting corrosion compared with polygonal Al coating in the distilled water. The corrosion mechanisms of three specimens are related to lamellar structure which has a much lower dissolution rate and forms a more effective physical shield, extending the path of the corrosive medium to body. The corrosion products of pure Gd reacting with water cause the formation of stresses and a number of cracks, which accelerate corrosion rate of pure Gd.
References
[1] YU B, LIU M, EGOLF P W, KITANOVSKI A. A review of magnetic refrigerator and heat pump prototypes built before the year 2010 [J]. Int J Refrigeration, 2010, 33: 1029-1060.
[2] CANEPA F, CIRAFICI S, NAPOLETANO M, CIMBERLE M R, TAGLIAFICO L, SCARPA F. Ageing effect on the magnetocaloric properties of Gd, Gd5Si1.9Ge2.1 and on the eutectic composition Gd75Cd25 [J]. J Phys D: Appl Phys, 2008, 41(15): 1-10.
[3] GILDAS D, LIN G X, CHEN J C. Performance characteristics of magnetic Brayton refrigeration cycles using Gd, Gd0.74Tb0.26 and (Gd3.5Tb1.5)Si4 as the working substance [J]. International Journal of Refrigeration, 2012, 35: 1035-1042.
[4] ZHANG Ze-yu, LONG Yi, WEN Da, YE Rong-chang, WAN Fa-rong. Corrosion behavior of commercial magnetic refrigerant gadolinium in water [J]. Journal of Rare Earths, 2004, 22: 99-102.
[5] CHEN J, CHEN Y G, WU C L, TANG Y B. Corrosion behavior of magnetic refrigerant gadolinium in oxalic acid solution [C]//Second IIF-IIR International Conference on Magnetic Refrigeration at Room Temperature. Portoroz, 2007: 209-216.
[6] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. Journal of Alloys and Compounds, 2002, 336(1): 88-113.
[7] LYUBINA J, HANNEMANN U, COHEN L F, RYAN M P. Novel La(Fe,Si)13/Cu composites for magnetic cooling [J]. Adv Energy Mater, 2012, 11(2): 1323-1327.
[8] ZHENG Xiao-qiu, WU Wei, YI Rong-xi, WAN Jian-xin, LONG An-wen. Effect of process parameters on the adhesion of magnetron sputtered copper and aluminum coatings on gadolinium substrate [J]. Journal of Materials Protection, 2009, 42(6): 29-31. (in Chinese)
[9] ADRIANA E L, JOHN E S. Characterization of surface structure in sputtered Al films: Correlation to microstructure evolution [J]. Journal of Applied Physics, 1999, 85(2): 876-882.
[10] YANG Yong, WU Wei. Research for the corrosion behavior of Gd sputtered by protective coating [J]. Journal of Xiamen University of Technology, 2008, 16(2): 41-49. (in Chinese)
[11] ZHANG J, YANG D H, OU X B. Microstructures and properties of aluminum film and its effect on corrosion resistance of AZ31B substrate [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 312-317.
[12] DARREN A L, MALLIKARJUNA N N. A comprehensive investigation of copper pitting corrosion in a drinking water distribution system [J]. Corrosion Science, 2010, 52(6): 1927–1938.
[13] KIM S J, HAN M S, KIM S K, JANG S K. Improvement of hydrogen embrittlement and stress corrosion cracking by annealing for Al-4.4Mg-0.6Mn alloy [J].Transactions of Nonferrous Metals Society of China, 2011, 21: 17-22.
[14] LIANG Chen-hao, ZHANG Wei. Pitting corrosion mechanisms and characteristics of aluminum in solar heating systems [J]. Journal of the Chinese Chemical Society, 2006, 53(2): 313-318.
[15] SZKLARSKA-SMIALOWSKA Z. Pitting corrosion of aluminum [J]. Corrosion Science, 1999, 41: 1743-1767.
磁控溅射铝膜的微观结构对磁致冷钆腐蚀性能的影响
吴红艳1,刘 剑1,赵浩峰1,蒋 琼2,徐 一2,徐 佳1
1. 南京信息工程大学 物理与光电工程学院,南京 210044;
2. 南京航空航天大学 材料科学与工程学院,南京 210016
摘 要:研究了表面镀铝和未镀铝的钆的静态和电化学腐蚀试验。结果表明,未镀铝的钆在水作用下表面形成大量裂痕,而多角状的铝膜结构由于点蚀坑的存在比片状铝膜结构的耐腐蚀性能差。片状铝膜由于与基体之间形成有效的物理阻挡,且片状结构致密,延长了阴极保护。因此,制备表面层状铝膜结构是显著提高磁制冷钆表面耐腐蚀性能行的有效方法。
关键词:耐腐蚀性;片状铝膜;多角状铝膜;磁制冷;纯钆
(Edited by Hua YANG)
Foundation item: Project (BK2012463) supported by the Natural Science Foundation of Jiangsu Province of China; Project (51245010) supported by Special Funds of the National Natural Science Foundation of China; Project (11047143) supported by the National Natural Science Foundation of China; Projects (12KF069, 12KF036) supported by Opening Found of Laboratory of Nanjing University of Information Science and Technology, China
Corresponding author: Hong-yan WU; Tel: +86-25-84454995; E-mail: whyc3w3@126.com
DOI: 10.1016/S1003-6326(13)62864-2
Abstract: Al coatings with different microstructures were prepared on the surface of Gd using the magnetron sputtering technique to improve its corrosion resistance. The corrosion behaviors for the pure Gd and Gd with Al coating in distilled water were studied using the mass loss and electrochemical performance. As a result, pure Gd without coating shows a certain amount of surface cracks under water flow conditions, whereas the polygonal Al coating decreases the path of the corrosive medium to body due to the existence of eroding pits structure. Compared with the polygonal structure Al coating and pure Gd, the lamellar structure of Al coating exhibits a higher electrochemical protection performance (e.g., a lower corrosion current and higher self-corrosion potential) and no occurrence of pitting corrosion. Due to an effective physical shield, the formation of the lamellar structure protected the inner Gd part from being corroded, and prolonged the duration of cathodic protection.


