
Synthesis and characterization of aluminum particles coated with uniform silica shell
CHENG Zhi-peng(程志鹏), YANG Yi(杨 毅), LI Feng-sheng(李凤生), PAN Zhen-hua(潘振华)
National Special Superfine Particle Engineering Research Center,
Nanjing University of Science and Technology, Nanjing 210094, China
Received 18 April 2007; accepted 10 June 2007
Abstract:
The silica coated aluminum composite particles were prepared by hydrolysis–condensation polymerization of tetraethylorthosilicate(TEOS) on the surface of aluminum particle. The structure, morphology, and properties of the silica coated aluminum were studied. The peaks of Si—O—Si are presented in the Fourier transform infrared (FT-IR) spectrum of the composite particles. The thickness of the silica shell is about 80 nm according to the results of transmission electron microscopy(TEM) and laser particle size analysis, while the mean diameter of the aluminum particle is 7.13 μm. The mass fraction of silica in the sample was determined by fluorescent X-ray spectrometry(XRF). Result of the thermogravimetric analysis(TGA) indicates that thermal stability of silica coated aluminum particles is better than that of pure aluminum particles at low temperature while more reactive at high temperature.
Key words:
silica; aluminum particle; coating; core-shell; oxidation;
1 Introduction
Recently, core-shell particles have been attracted much interest due to their fantastic properties, different from those of single-component materials, and their synthesis has opened new directions for material research[1-5]. Coating the particles with a thin shell of a compatible material makes it possible to control the core particles. The structure, size, and composition of core particles can be altered in a controllable way to tailor their magnetic, optical, mechanical, thermal, electrical, and catalytic properties[6-10].
Aluminum particle, with a high enthalpy of combustion, is commonly used in rocket propellant formulations. However, aluminum oxide may rapidly form when aluminum surfaces are exposed in air, especially superfine aluminum particles[11-13]. The Al2O3 layer generally does not constructively contribute to the uses for the metal and is often considered “dead weight”, whose reduction or elimination would enhance the energy of the aluminum. So protecting aluminum particles against oxidation to obtain a material with higher active (metallic) aluminum content is of great significance, and some of the work has been reported. KWON et al[14] reported that oxide-free aluminum particles can be achieved by aluminum diboride coating. FOLEY et al[15] pointed out that transition metal coating can inhibit oxide formation on aluminum particles. JOUET et al[16] suggested using stearic acid can improve the oxidation-resistance of the aluminum particles.
Due to their chemical and thermal durability, silica coatings are of interest for protection of materials against oxidation, such as iron[17], carbon fibers[18] and Co[19]. Though a number of studies have been reported on the coating of various materials with a silica layer, rare report regarding the coating of silica layer on the aluminum particle appears in literature. In this work, the coating of silica on the aluminum surface was performed using sol-gel route. The characterization of aluminum particle and silica coated aluminum particle was investigated using FT-IR, XRD, TEM, XRF and TGA.
2 Experimental
2.1 Materials
In the preparation of SiO2 coated aluminum particles, aluminum particles (A.R., 99%, Anshan Angang Corporation), tetraethylorthosilicate(A.R., TEOS: Si(OC2H5)4, 99.9%, Shanghai Chemical Corporation), ethanol (99.9%. Shanghai Chemical Corporation) and ammonium hydroxide (A.R., NH4OH, 28%, Shanghai Shiyi Chemical Corporation) were used.
2.2 Preparation of silica coated aluminum composite particles
The SiO2 coated aluminum composite particles were prepared by hydrolysis-condensation polymerization of TEOS on the surface of aluminum particles. The aluminum particle was dispersed into the mixture of TEOS and 200 mL ethanol with the mole ratio of TEOS to Al of 1?50, then 10 mL NH4OH was added slowly, and the mixture was vigorously stirred at 40 ℃ for 6 h. After the reaction, the silica coated aluminum particle was collected by centrifuge and washed with ethanol followed by deionized water for several times to remove dissociative polysiloxane, unreacted monomer, and polysiloxane oligomers. The composite particles were then dried at 40℃ in vacuum oven.
2.3 Characterization
The chemical structure of the composites was measured with a Bruker vector-22 Fourier transform infrared(FT-IR) using KBr pellet technique. Phase identification via X-ray diffraction (XRD) was performed on a Bruker Advance D8 X-ray diffractometer using Cu Kα radiation. The particle size distribution of the composites was measured by particle size analyzer (Malvern Instruments). Transmission electron micrographs(TEM) were taken with a JEOL TEM-200CX microscope. The composition was detected using fluorescent X-ray spectrometry with a ARL-9800. Thermogravimetric analysis(TGA) was carried out. Instrument SDTQ-600 was used at a heating rate of 20 ℃/min in air with Al2O3 as reference. The resistance to corrosion by acids was measured comparatively by dispersing aluminum particles and composite particles to hydrochloric acid of same concentration, respectively was tested, and then the pH value (PHS-3C) of the solution was tested as a function of time.
3 Results and discussion
The hydrolysis of TEOS would happen in the presence of water, and Si(OH)4 was formed as one of the hydrolysates (Eqn.(1)). The purpose of adding NH4OH was to promote the hydrolysis of TEOS. Then, Si(OH)4 molecule would polymerize with other Si(OH)4 or TEOS molecule (Eqn.(2) or Eqn.(3)). The product of this step was the monomer or oligomer of polysiloxane. Finally, the monomers or oligomers of polysiloxane continue to polymerize and form a film of high relative molecular mass polysiloxane with a three-dimensional network structure (see Eqn.(4)). Because water and TEOS are immiscible, a mutual solvent such as ethanol is normally used as a homogenizing agent.
Fine particles can provide nucleation centers and decrease the kinetic barrier to nucleation of a supersaturated solution. Furthermore, the monomers or oligomers of polysiloxane along with Si(OH)4 molecules have very high activities, so they can be adsorbed to the surface of the aluminum particles rapidly. The polymerization of Eqn.(4) occurs on the surface of the aluminum particles and the silica film thus covers around the particles tightly.
Si(OC2H5)4+4H2O→Si(OH)4+4C2H5OH (1)
Si(OH)4+Si(OH)4→(HO)3Si—O—Si(OH)3+H2O (2)
Si(OH)4+(C2H5O)4Si→
(HO)3Si—O—Si(C2H5)3+C2H5OH (3)
![]() (4)
(4)
The infrared spectra of the particles were analyzed in the range of 500-4 000 cm-1, as indicated in Fig.1. It can be seen that the spectrum of blank silica powder shows absorption bands including 800 cm-1 of Si—O—Si stretching, 980 cm-1 of Si—OH stretching, and 1 100 cm-1 of Si—O—Si asymmetric stretching. By comparing the spectra of uncoated with silica coated aluminum particles, the coating of the Al particles with silica evidently induces the presence of new absorption bands at 800, 980 and 1 100 cm-1. In addition, in Fig.1 the absorption bands near 3 400 and 1 630 cm-1 refer to the vibration of remainder water in the samples.

Fig.1 FT-IR spectra of aluminum particle, blank silica particle and silica coated aluminum particle
Fig.2 shows the XRD patterns of aluminum particle, blank silica particle, and the silica coated Al particle. There is a broaden peak located in the range of 22? which is the characteristic of amorphous silica. This implies that there is amorphous silica in composite particles.
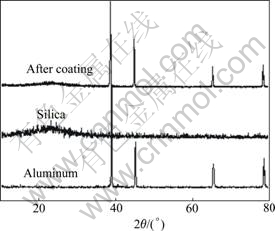
Fig.2 XRD patterns of aluminum particle, blank silica particle and silica coated aluminum particle
The morphology of the silica coated aluminum particle is shown in Fig.3. It can be seen that the silica layer is continuously coated on the core particle surface. The thickness of the silica layer is estimated to be about 80 nm.
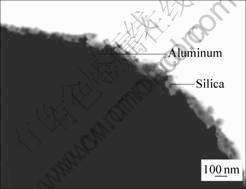
Fig.3 TEM image of silica coated aluminum particle
The particle size distribution of the aluminum particle and silica coated aluminum particle is shown in Fig.4. The mean particle size of aluminum particle is 7.13 μm, and that of the silica coated aluminum particle is about 7.22 μm according to the results of laser particle size analysis. The thickness of the shell can be calculated roughly to be less than 100 nm, which is in accordance with the TEM image. The particle size distribution of the silica coated aluminum particles.

Fig.4 Particle size distribution of aluminum particle before and after coating
The composition of the composite particles was detected by fluorescent X-ray spectrometry. The mole ratio of SiO2-to-Al is 1.52%. So the mass fraction of SiO2 can be calculated by
![]() (5)
(5)
where ![]() is the mass fraction of SiO2,
is the mass fraction of SiO2, ![]() and MAl are the relative molecular masses of SiO2 and aluminum, respectively.
and MAl are the relative molecular masses of SiO2 and aluminum, respectively.
The mass fraction of SiO2, w, obtained from Eqn.(5) is 3.26 %.
Meanwhile, the efficiency of the silica can be characterized by using the mole ratio of deposited silica to total one, which can be calculated according Eqn.(6):
![]() (6)
(6)
where ![]() is the efficiency of silica, Ru is the mole ratio of SiO2 to Al in ultimate products, and Rp is the mole ratio of TEOS to Al.
is the efficiency of silica, Ru is the mole ratio of SiO2 to Al in ultimate products, and Rp is the mole ratio of TEOS to Al.
Compact SiO2 shell rather than the lacunary was expected to obtain on the aluminum particle’s surface to prevent aluminum particle oxidation. The acid soluble property method was used to examine the film structure on the particle surface. Fig.5 shows the resistance of the aluminum particle to corrosion by acids before and after SiO2 coating. The original pH value of the hydrochloric acid in both samples is 0.5. The pH values of the solution at different time show the extent of reaction between aluminum and hydrochloric acid, which indicates the resistance to corrosion by acids of the aluminum particle. It can be found that pH values of the solution added with SiO2 coated aluminum particle increase to 0.74 step by step at the initial 40 min, and then stay around 0.75 steadily. The possible reason is that some of the aluminum particles are not coated compactly that can still react with hydrochloric acid. Comparatively, pH values of the solution added with aluminum particles increase to 1.24 at the initial 40 min and then reach an equilibrium value of 3.02. As shown in Fig.5, the resistance of the aluminum particles to corrosion by acids is obviously improved after being coated with SiO2. This indicates that most of the aluminum particles are compactly coated with SiO2 shell.

Fig.5 Resistance of aluminum particles to corrosion by acids before and after coating
Fig.6 shows the TGA curves of pure aluminum particle and SiO2 coated aluminum particle heated over the temperature range of 50-1 050 ℃ at a rate of 20 ℃/min under air. As shown in Fig.6, the oxidation processes of both pure and silica coated aluminum particle are characterized by two stages. During the first slow oxidation stage below about 850 ℃, the mass growth rate of the pure aluminum is 105% while it is only 102% for the coated aluminum, demonstrating the oxidation resistance ability of aluminum particles can be enhanced by silica coating. But an interesting phenomenon is observed during the second stage above about 850 ℃, showing that the mass of silica coated aluminum particles grows faster than pure aluminum does. When the temperature goes up to 1 050 ℃, the mass growth rate of silica coated particles reaches 140%, which is distinctly higher than that of pure aluminum (about 130%). This indicates silica coating can enhance the oxidation of aluminum during this stage, which could be probably explained by the facts that aluminum has melted above 660 ℃ and the thermal expansion makes the fresh aluminum break out the silica shell. As the coating materials weaken the natural aluminum oxide layer, this happens more easily than with untreated aluminum. The silica coating can prevent aluminum particles from oxidizing at low temperature while enhance oxidation at high temperature, which makes the heat release of aluminum more concentrated and fast, followed by a remarkably improved exothermal efficient.

Fig.6 TGA curves of aluminum particle before and after coating
4 Conclusions
1) The SiO2 coated aluminum particles were prepared by hydrolysis-condensation polymerization of TEOS on the surface of aluminum particles. The structure of the composite coating particles was confirmed by FT-IR and XRD.
2) The thickness of the silica layer is about 80 nm according to the results of TEM and laser particle size analysis.
3) The TGA results show that the silica coating can prevent aluminum particles from oxidizing at low temperature while enhance oxidation at high temperature, which makes the heat release of aluminum more concentrated and fast.
References
[1] ZHANG H Z, LUO X H, XU J, WANG B, YU D P. Synthesis of TiO2/SiO2 core/shell nanocable arrays [J]. J Phys Chem B, 2004, 108(39): 14866-14869.
[2] PRAKASH A, MCCORMICK A V, ZACHARIAH M R. Tuning the reactivity of energetic nanoparticles by creation of a core-shell nanostructure [J]. Nano Lett, 2005, 5(7): 1357-1360.
[3] ZHAO H L, WENG K R, GUAN S K, LOU L H, LI Y A, ZHAO H T, HU Z Q. Microstructure of Al2O3/SiO2 ceramic core nano- composites [J]. Trans Nonferrous Met Soc China, 2004, 14(3): 501-504.
[4] WANG S B, SHI G. Q. Uniform silver/polypyrrole core-shell nanoparticles synthesized by hydrothermal reaction [J]. Mater Chem Phys, 2007, 102: 255-259.
[5] XIAO X X, HUANG K L, YAN J H, HE Q Q. Synthesis and characterization of CoFe2O4 nanoparticles [J]. Trans Nonferrous Met Soc China, 2005, 15(5): 1172-1177.
[6] JEONG U, KIM J U, XIA Y A. Monodispersed spherical colloids of Se@CdSe: Synthesis and use as building blocks in fabricating photonic crystals [J]. Nano Lett, 2005, 5(5): 937-942.
[7] LU D L, DOMEN K, TANAKA K I. Electrodeposited Au-Fe, Au-Ni, and Au-Co alloy nanoparticles from aqueous electrolytes [J]. Langmuir, 2002, 18(8): 3226-3232.
[8] ZHANG S C, LI X G. Synthesis and characterization of CaCO3@SiO2 core–shell nanoparticles [J]. Powder Technol, 2004, 141: 75-79.
[9] ZHU M W, QIAN G D, WANG Z Y, WANG M Q. Fabrication of nanoscaled silica layer on the surfaces of submicron SiO2-Ag core-shell spheres [J]. Mater Chem Phys, 2006, 100: 333-336.
[10] PHUNG X, GROZA J, STACH E A, WILLIAMS LN, RYTCHEY S B. Surface characterization of metal nanoparticles [J]. Mater Sci Eng A, 2003, 359: 261-268.
[11] Shafirovich E, Bocanegra P E, Chauveau C, I. Gokalp, Goldshleger U, Rosenband V, Gany A. Ignition of single nickel-coated aluminum particles [J]. Proceedings of the Combustion Institute, 2005, 30: 2055-2062.
[12] Politzer P, Lane P, Grice M E. Energetics of aluminum combustion [J]. J Phys Chem A, 2001, 105(31): 7473-7480.
[13] Alavi S, Mintmire J W, Thompson D L. Molecular dynamics simulations of the oxidation of aluminum nanoparticles [J]. J Phys Chem B, 2005, 109(1): 209-214.
[14] Kwon Y S, Gromov A A, Ilyin A P. Reactivity of superfine aluminum powders stabilized by aluminum diboride [J]. Comb Flame, 2002, 131: 349-352.
[15] Foley T J, Johnson C E, Higa KT. Inhibition of oxide formation on aluminum nanoparticles by transition metal coating [J]. Chem Mater, 2005, 17(16): 4086-4091.
[16] Jouet R J, Warren A D, Rosenberg D M, Belitto V J, Park K, Zachariah M R. Surface passivation of bare aluminum nanoparticles using perfluoroalkyl carboxylic acids [J]. Chem Mater, 2005, 17(11): 2987-2996.
[17] WANG G H, Harrison A. Preparation of iron particles coated with silica [J]. J Colloid Interface Sci, 1999, 217: 203-207.
[18] Hoffman W P, Phan H T. The deposition of silica on carbon as a model system for oxidation protection coatings [J]. Carbon, 1995, 33(4): 509-524.
[19] Kobayashi Y, Horie M, Konno M, Rodriguez- Gonzalez B, Liz-Marzan L M. Preparation and properties of silica-coated cobalt nanoparticles [J]. J Phys Chem B, 2003, 107(30): 7420-7425.
Foundation item: Project(50306008) supported by the National Natural Science Foundation of China
Corresponding author: LI Feng-sheng; Tel: +86-25-84315942; E-mail: nano301@126.com


