Trans. Nonferrous Met. Soc. China 25(2015) 3959-3966
Formation mechanisms of Ni-Al intermetallics during heat treatment of Ni coating on 6061 Al substrate
Mohsen ADABI1,2, Ahmad Ali AMADEH1
1. School of Metallurgy and Materials Engineering, College of Engineering, University of Tehran, P.O. Box 11155-4563, Tehran, Iran;
2. Department of Metallurgy and Materials Engineering, Faculty of Engineering, Roudehen Branch, Islamic Azad University, P.O. Box 189, Roudehen, Tehran, Iran
Received 7 March 2015; accepted 17 June 2015
Abstract:
The formation mechanisms and growth kinetics of Al3Ni and Al3Ni2 in Ni-Al diffusion couple prepared by electrodeposition of Ni on Al substrate were investigated. The nickel coating with 20 μm thickness was applied on 6061 aluminum alloy by direct current electroplating. The samples were then heat-treated for different durations at 450, 500 and 550 °C under argon atmosphere. The intermetallic phases were identified by means of scanning electron microscopy (SEM), energy dispersive spectrometry (EDS) and X-ray diffraction (XRD). The results showed that the formation of intermetallic phases consisted of two important steps. The first step was the lateral growth of intermetallic phase from separate sites, resulting in the formation of a continuous layer. The second step was the growth of the continuous intermetallic layer in the direction perpendicular to the interface. However, excessive increase in thickness of intermetallic phases led to the detachment of reaction products, i.e., Al3Ni and Al3Ni2, from the substrate. It was also observed that aluminum was the dominant diffusing element during Al3Ni growth, while nickel diffusion was dominant during Al3Ni2 growth. The growth kinetics of both Al3Ni and Al3Ni2 phases obeyed a parabolic law.
Key words:
Ni-Al intermetallics; electrodeposition; heat treatment; formation mechanism; growth kinetics;
1 Introduction
Nickel aluminade compounds due to their low density, good corrosion resistance and high electrical and thermal conductivities are promising materials for industrial applications as bulk or coating [1,2]. Hence, the formation sequences and growth kinetics of nickel aluminade phases including Al3Ni, Al3Ni2, AlNi, Al3Ni5 and AlNi3 [3] have been extensively studied by using diffusion couples [1,4-8]. Based on Gibbs free energy (ΔG), AlNi is the preferred phase while the initial phase observed experimentally is Al3Ni [9]. Therefore, many attempts have been performed to present a good rule predicting the formation of the first phase [10]. PRETORIUS et al [11] used the effective heat of formation concept for prediction of first phase formation. In fact, the phase with the most negative effective heat of formation is the first intermetallic phase which is formed during interaction of metal-metal couple. The effective heat of formation can be obtained by the multiplication of a coefficient in heat of formation. This coefficient is calculated by dividing the concentration of the limiting element of the compound which has the lowest eutectic temperature in the binary system to the concentration of the limiting element in the compound to be formed.
It has been reported that some factors such as type of diffusion couple (e.g., Al-Ni, AlNi-Ni, Al3Ni-Ni), thickness of reacted zones, temperature and time of heat treatment can affect the formation of Al-Ni intermetallics. For example, in the study of bulk diffusion, where the thickness of reacted zones could reach 100 μm, four intermetallic phases including Al3Ni, Al3Ni2, AlNi and AlNi3 were observed after heat treatment of Al-Ni couple at 600 °C for 340 h [12]. Nevertheless, thin film reactions studies indicated that only Al3Ni phase with thickness of 100 nm was formed by annealing of Al-Ni couple at 300 °C [9]. This difference in the number of growing phases between bulk and thin film reactions, resulting from reaction temperature and thickness of reacted zones, led to the development of lateral diffusion couple. Due to unlimited supply of atoms from Al-Ni lateral couple and the other growing phase in the thin film, the thickness of reacted zone could reach 10 μm and more than one phase can grow. Therefore, lateral diffusion couple has a combination characteristic of both thin film and bulk diffusion couples [7]. LIU et al [13] observed that in lateral diffusion couples, Al3Ni and Al3Ni2 phases simultaneously grew from 350 to 425 °C.
There are some inconsistencies in the data on growth kinetics of intermetallic phases. According to JUNG et al [4], the growth kinetic of Al3Ni phase does not obey the parabolic law, whereas MICHAELSEN and BARMAK [14] have reported that the growth of Al3Ni phase shows a parabolic dependence on the annealing time. Furthermore, the dominant diffusing element during the formation of intermetallic phases is still not entirely clear. Some researchers believe that nickel is the dominant diffusing element during formation of Al3Ni and Al3Ni2 [15], while according to LIU et al [7], diffusion of aluminum is dominant during the formation of the same intermetallic phases. Therefore, a more detailed examination of the formation mechanisms of intermetallic phases can be interesting and the aim of this work was to investigate the formation mechanisms and growth kinetics of intermetallic phases in Ni-Al diffusion couple prepared by electrodeposition of nickel on 6061 Al alloy.
2 Experimental
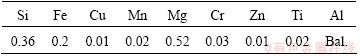
The Ni coatings were electrodeposited from a modified Watts bath using a direct current at the current density of 2 A/dm2. The composition of the bath is presented in Table 1. The pH and temperature of the bath were fixed at 4 and 50 °C, respectively. During electrodeposition, the bath was agitated by means of a magnetic stirrer at 250 r/min. Plates of 6061 aluminum alloy were used as the cathode. The chemical composition of the alloy is given in Table 2. The plates with the dimensions of 2 cm × 3 cm × 0.2 cm were mechanically ground to 2000 grit SiC papers. The anode was a nickel plate with 99.9% purity. Prior to electroplating, the aluminum substrates were firstly degreased in 50 g/L NaOH solution at 70 °C for 15 s. They were rinsed with distilled water and dipped in 65% nitric acid solution for 5 s. They were then cleaned with distilled water and dipped in a zincate bath (30 g/L Ni(SO4)2, 40 g/L ZnSO4, 106 g/L NaOH, 10 g/L KCN, 40 g/L KHC4H4O6, 5 g/L CuSO4 and 2 g/L FeCl2). Finally, the specimens were placed in the electrolyte for electrodeposition of Ni coatings. The coated samples were then heat-treated at 450, 500 and 550 °C in a tube furnace under argon protective atmosphere. The duration of heat treating varied from 1 to 24 h depending on diffusion temperature and then the specimens were cooled in the furnace to room temperature.
Table 1 Composition of electroplating bath (g/L)
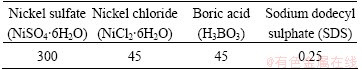
Table 2 Composition of 6061 aluminum alloy used in this research (mass fraction, %)
After heat treatment, the specimens were sectioned, ground and polished using 1 μm diamond paste. Then, the cross sections of the samples were characterized using a VegaTescan scanning electron microscope (SEM) equipped with energy dispersive X-ray spectrometer (EDS). Phase analysis was carried out by means of X-ray diffraction (XRD) technique using a Philips Xpert instrument at 40 kV and 30 mA with Cu Kα radiation (λ=1.54056  ) at a step size of 0.02 (°)/s in the range of 30°-80°.
) at a step size of 0.02 (°)/s in the range of 30°-80°.
3 Results and discussion
3.1 Microstructural evolution
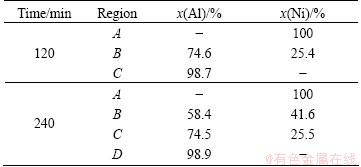
Backscattered electrons images of the cross sections of as-deposited and heat-treated specimens at 450 °C for various times are presented in Fig. 1. The SEM image of as-deposited nickel coating (Fig. 1(a)) shows that the deposited layer is uniform with about 20 μm thickness. The heat treatment of the specimens leads to the formation of one or two layers between Ni coating and Al substrate. In fact, there is only one product layer between the coating and the substrate after 120 min heat treatment (Fig. 1(c)). By increasing the heat treating time, two distinct reaction layers are formed (Figs. 1(e) and (f)). The EDS results of these layers listed in Table 3 show that the layer adjacent to the Al substrate contains approximately 25% Ni and 75% Al corresponding to Al3Ni phase. The chemical composition of the zone located between Al3Ni and residual nickel (Zone B in Fig. 1(e)) is also close to Al3Ni2 phase. XRD patterns of the specimens heat-treated at 450 °C for different time shown in Fig. 2 confirm the results obtained by EDS analysis. According to XRD patterns, the specimen heat- treated at 450 °C for 120 min includes residual Ni, Al3Ni intermetallics (Fig. 2(a)) whereas after 240 min of heat treatment or more, it is composed of residual Ni, Al3Ni and Al3Ni2 intermetallics (Fig. 2(b)). Similar results are also reported for heat treatment of Ni-P coating on Al-12%Si substrate [15]. The peaks corresponding to Al substrate are also present in XRD patterns.
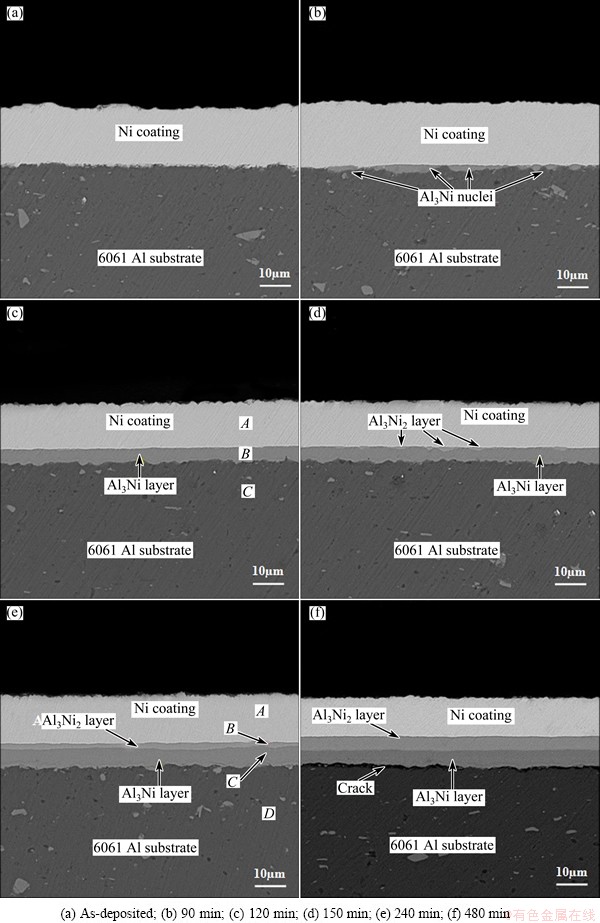
Fig. 1 SEM images of cross-sectioned samples heat-treated at 450 °C for different time
Table 3 EDS analyses results of cross section of heat-treated specimens at 450 °C for different time
It should be noted that long time heat treatment of the specimens decreases the adherence of the coating to the substrate, and consequently, the reaction products are detached from the substrate as seen in Fig. 1(f). It can be due to the following reasons:
1) The heat treatment of Ni-Al diffusion couple can lead to the formation of Kirkendall voids at the interface between Al3Ni intermetallic and Al substrate. Indeed, the formation of Kirkendall voids resulting from coalescence of vacancies at the interface can cause the separation of product phases from Al substrate. The Kirkendall voids were also observed by SEQUEIRA and AMARAL [16] for Ni-Ti system.
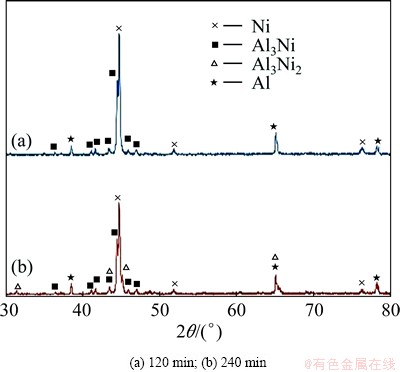
Fig. 2 XRD patterns of heat-treated specimens at 450 °C for different time
2) The difference in the coefficient of thermal expansion (CTE) of aluminum substrate and the intermetallics [17] can lead to the detachment of intermetallic layers from the Al substrate. In fact, the critical condition to initiate cracking at the intermetallic layer/Al substrate interface is given as [18]
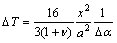 (1)
(1)
where x is the intermetallic thickness, ΔT is the temperature change to initiate cracking and detachment of layers, Δα is the difference in CTEs of aluminum substrate and intermetallic, ν is the Poisson ratio and a is the length of crack formed during temperature decrease at the interface. During a given heat treatment, some parameters like v, ΔT and Δα are constants. Therefore, with increasing the thickness of intermetallic layer, the crack length is increased.
The heat treatment process was performed at different temperatures (450, 500 and 550 °C) for various time (15, 60 and 240 min) to investigate the effect of heat treating temperature on thickness of intermetallic layer. The results of these experiments are presented in Fig. 3. As shown in this figure, the thickness of intermetallic layers is almost the same. It can therefore be concluded that 50 °C increase in temperature is approximately equal to 240 min increase in heat treatment time.
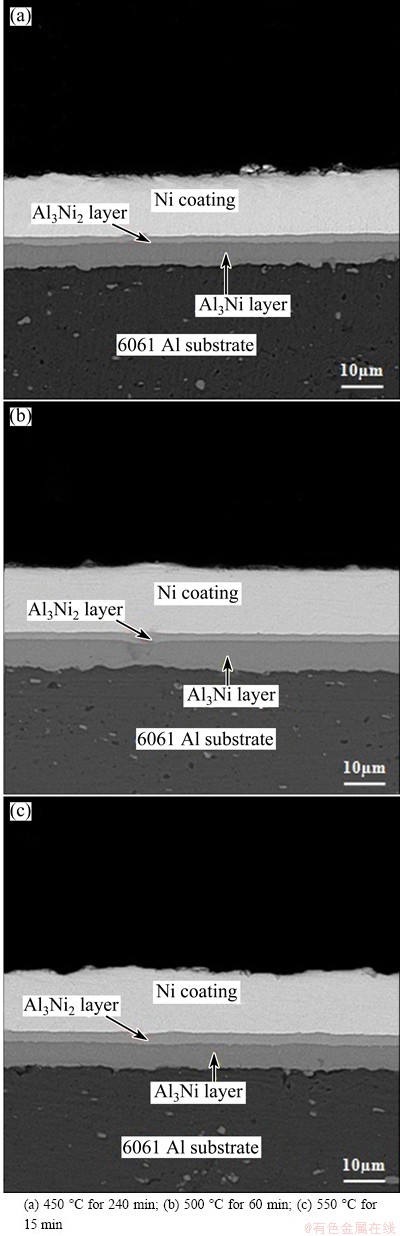
Fig. 3 SEM images of cross sections of heat-treated specimens
3.2 Formation mechanisms of Ni-Al intermetallics
To investigate the mechanisms of the formation of Ni-Al intermetallics during heat treatment, at first, the dominant diffusing element in the diffusion couple should be determined. There are contrary reports about the dominant diffusing species in Ni-Al couple. COLGAN [19] reported that aluminum was the dominant diffusing species during the formation of Al3Ni and Al3Ni2, whereas VOJTECH et al [15] found that nickel was the dominant diffusing species in Ni-P/Al couple. To determinate the dominant diffusing species, the couple of Ni-Al2O3/Al substrate was prepared by electrodeposition of Ni-Al2O3 composite coating on 6061 Al alloy from a modified Watts bath containing suspended Al2O3 particles. Afterward, the coated samples were heat-treated at 450 °C for 120 and 240 min, respectively. Figure 4 shows the cross sections of as- deposited and heat-treated specimens. As shown in Fig. 4(a), the Ni-Al2O3 composite coating has a thickness about 22 μm. By annealing the sample at 450 °C for 2 h, the Al3Ni layer is formed between the Ni-Al2O3 coating and Al substrate. It is also observed that the thickness of Ni-Al2O3 layer decreases in comparison with the as- deposited sample (Fig. 4(b)). Since the Al3Ni intermetallic phase is observed around the ceramic particles, it can be suggested that the outward diffusion of Al is dominant during the formation of this layer. This is in good agreement with the result reported by CASTLEMAN and SEIGLE [5]. With increasing the time of heat treatment up to 240 min at the same temperature, both Al3Ni and Al3Ni2 phases are formed while the thickness of residual Ni-Al2O3 coating remains approximately constant. Hence, it can be proposed that the inward diffusion of Ni is dominant during the formation of Al3Ni2 layer. This finding is consistent with the results reported by IP et al [20].

Fig. 4 SEM images of Ni-Al2O3composite coatings
The successive steps occurring during heat treatment of Ni-Al couple are also shown in Fig. 1. As seen in Fig. 1(b), the formation of Al3Ni is initiated by nucleation at separate sites. This is in line with the results obtained by SWAIN et al [21] and QIU and WANG [22] showing that Al3Ni forms by heterogeneous nucleation at preferred sites during early stage phase transformation. It is also observed that more Al3Ni nuclei are located in Al substrate with respect to nickel coating because the diffusion coefficient of Ni in Al at 450 °C (5.2×10-13 cm2/s) is higher than that of Al in Ni (3.7×10-16 cm2/s) [23,24]. After the connection of discrete sites to each other and the formation of a thin continuous Al3Ni layer, its subsequent growth occurs in the direction perpendicular to the interface towards nickel coating (Fig. 1(c)). When the thickness of Al3Ni layer reaches a critical value and the Al3Ni-Ni interface is also saturated from nickel, Al3Ni intermetallic reacts with nickel to form Al3Ni2 phase (Fig. 1(d)). The nucleation and lateral growth of this phase is the same as those of Al3Ni (Fig. 1(e)). The diffusion of nickel through Al3Ni2 layer leads to the increase in the thickness of Al3Ni2 layer (Fig. 1(f)), whereas the thickness of Al3Ni layer, due to its consumption for the formation of Al3Ni2 layer, remains approximately constant. It is noted that at the interface of Al3Ni and Al substrate where Kirkendall voids are coalesced into disk voids, the Al3Ni layer becomes thinner, because the voids can decrease the rate of Al supply to the Al3Ni/Al interface. Consequently, the thickness of Al3Ni layer due to the reaction with nickel diffused through Al3Ni2 layer decreases gradually, while the thickness of Al3Ni2 layer increases until the consumption of all Al3Ni.
3.3 Growth kinetic
According to Fig. 1, the thickness of intermetallic layers increases by increasing the time of heat treatment. Similar behavior is observed at all heat treatment temperatures. In general, the thickness of intermetallic layers can be estimated by the parabolic rate law [25]:
x=ktn (2)
where x is the thickness of intermetallic layer, k is the rate constant, t is the heat treatment time and n is the time exponent. In fact, t=tt-t0, where tt and t0 are the total heat treatment time and delay time (or the time required for initiation of intermetallic phases), respectively. It is empirically found that n takes the value of 0.5 when the diffusion reaction is controlled by volume diffusion [4]. The variation of intermetallic layer thickness heat-treated at 450, 500 and 550 °C as a function of heat treatment time (t0.5) is presented in Fig. 5. As shown in this figure, for the specimen heat-treated at 550 °C, the linear relationship is observed between the thicknesses of intermetallic layers and the square root of heat treatment time. The linear increasing in the thickness of layers with square root of heat treatment time indicates a volume diffusion-controlled process for the growth of both layers [26]. However, the lines related to the specimens heat-treated at 450 and 500 °C do not pass from the experimental values, because the growth of intermetallic layers at these temperatures is controlled by both volume and grain boundary diffusions. This observation is in agreement with results reported by RASHIDI [27].
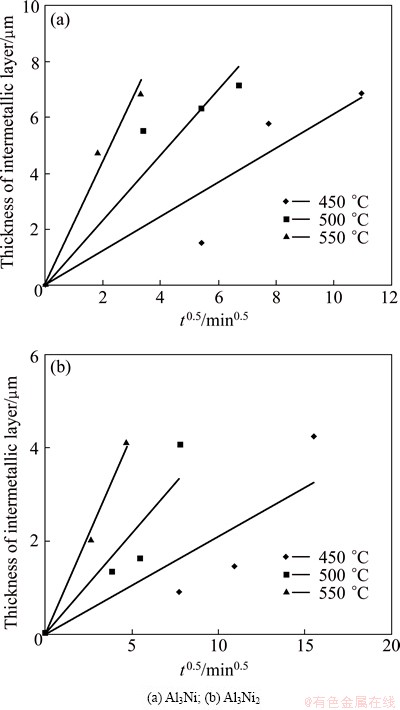
Fig. 5 Growth curves of intermetallic layers in Ni-Al couple
The activation energy, which is the energy required for the solid state diffusion of Ni and/or Al in the intermetallic layers, is usually determined by Arrhenius equation [28]:
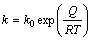 (3)
(3)
where k0 is the frequency factor, Q is the activation energy, R is the gas constant and T is the temperature. From the temperature dependence of the logarithmic values of rate constants, the values of frequency factor and activation energy for phase growth were determined from the intercept and slope of the plot as shown in Fig. 6. The activation energy (Q) obtained from this work and the previous studies are listed in Table 4. As seen in Table 4, in the present work, the activation energies for the growth of Al3Ni and Al3Ni2 phases are 56 and 62 kJ/mol, respectively. These values are lower than those presented in other researches. Similar trend has also been reported by TANG et al [30] for the growth activation energy of Cu6Sn5 during heat treatment of Cu-Sn couple prepared by electrodeposition. The reported activation energy is lower than the values reported by other researchers. This difference is attributed to the initial abnormally rapid growth of Cu6Sn5 in the annealed Cu/Sn couples. Furthermore, other parameters such as preparation method of diffusion couple can affect the activation energy of the growth of intermetallic layers. In this research, the Ni-Al couple was prepared by electrodeposition of Ni on Al substrate whereas the other researchers used the diffusion couples produced by hot dipping [12,26,29] or spot welding procedure [5]. In the first technique, pure nickel sheet is dipped in an aluminum melt while in the second technique, aluminum is placed between two nickel disks and melted by spot welding [5]. It seems that the microstructure of diffusion couple can influence the activation energy of the growth of intermetallic phases.
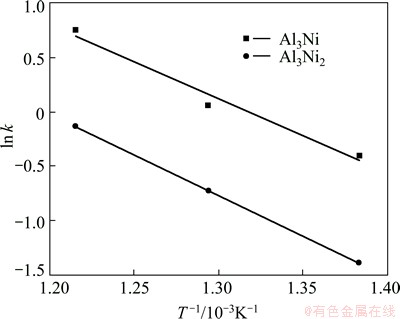
Fig. 6 Temperature dependence of growth rate constant in Ni-Al couples
Table 4 Comparison of activation energy for growth of Al3Ni and Al3Ni2 phases in present work and other researches (kJ/mol)
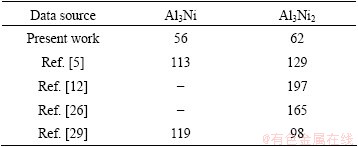
The electroplated layers are fine grained, rich in defects such as vacancies and non-fully dense microstructures that could encourage the volume and short-circuit diffusion of metal atoms and improve the interface reactions [31]. Consequently, the activation energies for the growth of Al3Ni and Al3Ni2 phases are lower than the values presented in other researches.
4 Conclusions
1) Heat treatment of Ni-coated Al alloy at suitable time and temperature resulted in the formation of Al3Ni and Al3Ni2.
2) Excessive increase in thickness of intermetallic layers led to their detachment from the substrate.
3) The diffusion of aluminum was dominant during formation of Al3Ni phase, while nickel was the dominant diffusing element during Al3Ni2formation.
4) The growth kinetics of both intermetallic phases obeyed the parabolic law.
References
[1] WU Liang, HE Yue-hui, JIANG Yao, ZENG Yi, XIAO Yi-feng, NAN Bo. Effect of pore structures on corrosion resistance of porous Ni3Al intermetallics [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3509-3516.
[2] CUI Hong-zhi, WEI Na, ZENG Liang-liang, WANG Xiao-bin, TANG Hua-jie. Microstructure and formation mechanism of Ni-Al intermetallic compounds fabricated by reaction synthesis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1639-1645.
[3] ROMANOWSKA J. Aluminum diffusion in aluminide coatings deposited by the CVD method on pure nickel [J]. CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry, 2014, 44: 114-118.
[4] JUNG S B, MINAMINO Y, YAMANE T, SAJI S. Reaction diffusion and formation of Al3Ni and Al3Ni2 phases in the Al-Ni system [J]. Journal of Materials Science Letters, 1993, 12: 1684-1686.
[5] CASTELMAN L, SEIGLE L. Layer growth during interdiffusion in the aluminum-nickel alloy system [J]. Transaction of Metallurgical Society of AIME, 1967, 212: 590-598.
[6] ZADOROZHNYY V, KALOSHIN S, TCHERDYNTSEV V, GORSHENKOV M, KOMISSAROV A, ZADOROZHNYY M. Formation of intermetallic Ni-Al coatings by mechanical alloying on the different hardness substrates [J]. Journal of Alloys and Compounds, 2014, 586: s373-s376.
[7] LIU J, MAYER J, BARBOUR J. Phase formation of NiAl3 on lateral diffusion couples [J]. Journal of Applied Physics, 1988, 64: 651-655.
[8] KONIECZNY M. Microstructural characterisation and mechanical response of laminated Ni-intermetallic composites synthesised using Ni sheets and Al foils [J]. Materials Characterization, 2012, 70: 117-124.
[9] COLGAN E, NASTASI M, MAYER J. Initial phase formation and dissociation in the thin-film Ni/Al system [J]. Journal of Applied Physics, 1985, 58: 4125-4129.
[10] PRETORIUS R. Phase sequence of silicide formation at metal-silicon interfaces [J]. Vacuum, 1990, 41: 1038-1042.
[11] PRETORIUS R, VREDENBERG A, SARIS F, DEREUS R. Prediction of phase formation sequence and phase stability in binary metal-aluminum thin-film systems using the effective heat of formation rule [J]. Journal of Applied Physics, 1991, 70: 3636-3646.
[12] JANSSEN M, RIECK G. Reaction diffusion and Kirkendall-effect in the nickel-aluminum system [J]. Transactions of Metallurgical Society of AIME, 1967, 239: 1372-1385.
[13] LIU J, MAYER J, BARBOUR J. Kinetics of NiAl3 and Ni2Al3 phase growth on lateral diffusion couples [J]. Journal of Applied Physics, 1988, 64: 656-662.
[14] MICHAELSEN C, BARMAK K. Calorimetric determination of NiAl3 growth kinetics in sputter-deposited Ni/Al diffusion couples [J]. Journal of Alloys and Compounds, 1997, 257: 211-214.
[15] VOJTECH D, NOVAK M, ZELINKOVA M, NOVAK P, MICHALCOVA A, FABIAN T. Structural evolution of electroless Ni-P coating on Al-12wt.% Si alloy during heat treatment at high temperatures [J]. Applied Surface Science, 2009, 255: 3745-3751.
[16] SEQUEIRA C A C, AMARAL L. Role of Kirkendall effect in diffusion processes in solids [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 1-11.
[17] MONDOLFO L F. Aluminum alloys: Structure and properties [M]. London: Butterworths London, 1976.
[18] EVANS H, LOBB R. Conditions for the initiation of oxide-scale cracking and spallation [J]. Corrosion Science, 1984, 24: 209-222.
[19] COLGAN E. A review of thin-film aluminide formation [J]. Materials Science Reports, 1990, 5: 1-44.
[20] IP S, SRIDHAR R, TOGURI J, STEPHENSON T, WARNER A. Wettability of nickel coated graphite by aluminum [J]. Materials Science and Engineering A, 1998, 244: 31-38.
[21] SWAIN M, SINGH S, BASUS, GUPTA M. Effect of interface morphology on intermetallics formation upon annealing of Al-Ni multilayer [J]. Journal of Alloys and Compounds, 2013, 576: 257-261.
[22] QIU X, WANG J. Experimental evidence of two-stage formation of Al3Ni in reactive Ni/Al multilayer foils [J]. Scripta Materialia, 2007, 56: 1055-1058.
[23] HIRANO K, AAGRWALA R, COHEN M. Diffusion of iron, nickel and cobalt in aluminum [J]. Acta Metallurgica, 1962, 10: 857-863.
[24] HASAKA M, MORIMURA T, UCHIYAMA Y, KONDO S, WATANABE T, HISATSUNE K, FURUSE T. Diffusion of copper, aluminum and boron in nickel [J]. Acta Metallurgica, 1993, 29: 959-962.
[25] MIRJALILI M, SOLTANIEH M, MATSUURA K, OHNO M. On the kinetics of TiAl3 intermetallic layer formation in the titanium andaluminum diffusion couple [J]. Intermetallics, 2013, 32: 297-302.
[26] JAIN M, GUPTA S. Formation of intermetallic compounds in the Ni-Al-Si ternary system [J]. Materials Characterization, 2003, 51: 243-257.
[27] RASHIDI A. Activation energy for formation of nickel-aluminide thin film on nanocrystalline andmicrocrystalline nickel [J]. Vacuum, 2013, 95: 35-42.
[28] BALOGH Z, SCHMITZ G. Diffusion in metals and alloys [M]. Amesterdam: Elsevier, 2014.
[29] REN X, CHEN G, WU C, ZHANG J. Formation and growth kinetics of intermediate phases in Ni-Al diffusion couples [J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2009, 24: 787-790.
[30] TANG Wen-ming, HE An-qiang, LIU Qi, IVEY D G. Solid state interfacial reactions in electrodeposited Cu/Sn couples [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 90-96.
[31] TANG W, HE A, LIU Q, IVEY D G. Room temperature interfacial reactions in electrodeposited Au/Sn couples [J]. Acta Materialia, 2008, 56: 5818-5827.
6061铝基体上镍涂层热处理过程中Ni-Al金属间化合物的形成机理
Mohsen ADABI1,2, Ahmad Ali AMADEH1
1. School of Metallurgy and Materials Engineering, College of Engineering, University of Tehran, P.O. Box 11155-4563, Tehran, Iran;
2. Department of Metallurgy and Materials Engineering, Faculty of Engineering, Roudehen Branch, Islamic Azad University, P.O. Box 189, Roudehen, Tehran, Iran
摘 要:采用电沉积方法在Al基体上沉积Ni制备Ni-Al扩散偶,并研究扩散偶中Al3Ni和Al3Ni2的形成机理和生长动力学。在6061铝基体上采用直流电沉积方法制备20 μm厚的Ni涂层。然后在Ar气气氛下,样品在450,500和550 °C下热处理不同时间。采用扫描电子显微镜、能谱仪和X射线衍射仪对金属间化合物进行表征。结果表明,Ni-Al金属间化合物的形成可分为两个重要步骤。首先,金属间化合物在不同位置侧面生长,形成连续金属间化合物层;其次,连续金属间化合物层在垂直于界面方向继续生长。随着金属间化合物厚度的增长,Al3Ni和Al3Ni2等反应产物将与基体发生分离。Al是Al3Ni生长的主要扩散元素,而Ni是Al3Ni2生长的主要扩散元素。Al3Ni和Al3Ni2相的生长动力学遵循抛物线方程。
关键词:Ni-Al 金属间化合物;电沉积;热处理;形成机理;生长动力学
(Edited by Yun-bin HE)
Corresponding author: Ahmad Ali AMADEH; Tel: +98-21-82084609; Fax: +98-21-88006076; E-mail: amadeh@ut.ac.ir
DOI: 10.1016/S1003-6326(15)64073-0
Abstract: The formation mechanisms and growth kinetics of Al3Ni and Al3Ni2 in Ni-Al diffusion couple prepared by electrodeposition of Ni on Al substrate were investigated. The nickel coating with 20 μm thickness was applied on 6061 aluminum alloy by direct current electroplating. The samples were then heat-treated for different durations at 450, 500 and 550 °C under argon atmosphere. The intermetallic phases were identified by means of scanning electron microscopy (SEM), energy dispersive spectrometry (EDS) and X-ray diffraction (XRD). The results showed that the formation of intermetallic phases consisted of two important steps. The first step was the lateral growth of intermetallic phase from separate sites, resulting in the formation of a continuous layer. The second step was the growth of the continuous intermetallic layer in the direction perpendicular to the interface. However, excessive increase in thickness of intermetallic phases led to the detachment of reaction products, i.e., Al3Ni and Al3Ni2, from the substrate. It was also observed that aluminum was the dominant diffusing element during Al3Ni growth, while nickel diffusion was dominant during Al3Ni2 growth. The growth kinetics of both Al3Ni and Al3Ni2 phases obeyed a parabolic law.


