DOI:10.19476/j.ysxb.1004.0609.2019.06.07
骨移植多孔钛材料制备方法与发展概况
贾建刚,井勇智,高昌琦,刘 畅,季根顺,郭铁明
(兰州理工大学,甘肃省有色金属先进加工与再利用国家重点实验室,兰州 730050)
摘 要:
多孔钛是一种具有三维连通孔结构的金属材料,与致密生物金属材料相比,由于自身的开孔性和连通性使其弹性模量大幅度降低,从而能够与人体骨模量较好匹配,同时使体液自由流通,加之钛具有良好的生物相容性,因而被用作骨科移植材料。而如何控制多孔结构,获得性能优异的多孔钛基体是开展相关研究和应用的前提与基础。已开发出的多孔钛及其合金的制备方法有气体成泡法、有机海绵复制法、浆料发泡法、凝胶柱模法、冷冻铸造法、自蔓延高温合成法、放电等离子烧结法、添加造孔剂法、增材制造法、纤维合成法等。不同制备方法具有各自优点,但普遍存在制备成本较高或性能偏低等问题;另外,针对不同患者的实际骨质情况,需要不同力学性能及孔隙结构的骨移植材料。因此,在较大范围内对多孔钛骨移植材料结构和模量进行调节仍然需继续研究。目前,多孔钛骨移植材料已实现临床应用,国内机构也已经推出多孔钛椎体植入材料用于临床实践。未来,多孔钛作为骨移植材料的研究和临床应用仍有很大空间。
关键词: 多孔钛;泡沫金属;骨移植材料;多孔制备;生物相容性
文章编号:1004-0609(2019)-06-1187-11 中图分类号:TG146.2 文献标志码:A
泡沫金属(也称多孔金属)研究始于20世纪中期,随着多孔材料在不同领域的应用,其优异性能受到广泛关注。多孔钛作为一种骨科移植材料近年来得到快速发展,自20世纪90年代以来,已开发出了多种制备方法和工艺,并对多孔钛结构、性能、加工方式及应用进行了大量研究,取得一些有益结果。
多孔钛具有优异的生物相容性和耐蚀性,易灭菌及良好的人体安全性等优点,因此大量应用于填充支撑骨组织缺陷或骨组织修复固定[1]。人体骨是一种外表致密内部较为疏松多孔的组织,致密骨与疏松骨的弹性模量分别在3~30 GPa和0.02~0.5 GPa[2],抗压强度在2~200 MPa之间[3]。多孔钛的开孔性不仅能够大大降低其弹性模量,而且当连通孔的尺寸为50~200 μm时,可为体液的流通及骨组织的长入提供良好的生长环境[4],最终形成一体的整合骨,从而彻底解决由于模量失配导致的应力屏蔽现象。多孔钛作为骨科移植材料发展至今经历了工艺研究、表面仿生改性及细胞培养实验、动物活体移植和临床应用4个阶段。其中如何控制多孔结构,获得性能优异的多孔钛基体是开展其他研究和应用的前提与基础。
ASHBY等[5]将泡沫金属的制备归纳成4个大类(气相发泡法,水溶液电沉积法,液态处理法,固态处理法)包括了9种工艺方法。其中,医用多孔钛及其合金的制备方法有气体成泡法、有机海绵复制法、浆料发泡法、凝胶注模法、冷冻铸造法、自蔓延高温合成法、放电等离子烧结法、添加造孔剂法、增材制造法、纤维合成法等。本文将以多孔钛的制备和研究现状进行分类综述,以期能为相关领域的工作者提供 参考。
1 多孔钛的制备方法及性能特点
1.1 气相成孔法
由于熔点高、活性大,很难用常规气体起泡法制备多孔钛。OPPENHEIMER等[6]以氩气作为起泡气体,将Ti6Al4V合金粉末装入金属容器中,抽真空后再充入0.33 MPa的氩气,100 MPa热等静压(HIP)并在840~1030 ℃下循环保温使氩气在粉末中鼓泡形成孔洞,同时粉末经高温烧结,最终形成孔隙率8%~52%,屈服强度170~670 MPa,弹性模量28~120 GPa的泡沫钛合金。
该法制备的多孔钛成本昂贵且内部孔结构分布不均匀,导致产品最终的开孔率较低,孔洞之间连通窗口的尺寸较小,仅有几十微米,不利于骨组织向内部孔洞的长入,其形貌如图1所示。因此,该方法在制备多孔钛方面的研究和应用近年也很少有所报道。
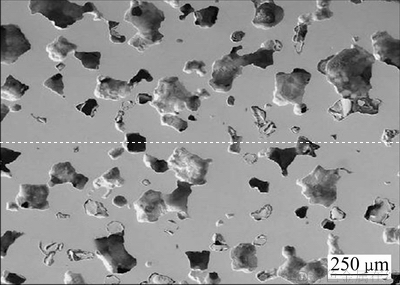
图1 气相成孔法制备多孔钛的SEM像[6]
Fig. 1 SEM image of porous titanium prepared by gas phase pore formation [6]
1.2 发泡剂成孔法
1.2.1 有机海绵复制法
有机海绵复制法是通过配浆-浸渍-干燥-高温烧结4个步骤制备多孔钛。先将TiH2粉末、水、胶凝剂、稳定剂按比例配制成液浆,再将有机泡沫材料在液浆中充分浸蘸,取出后低压挤出多余液浆并干燥去除水份,最后真空烧结获得连通性良好的泡沫钛。
CACHINHO等[7]利用该方法制备出的多孔钛孔径为100~600 μm,孔隙率75%,抗压强度(23.72±1.12) MPa,弹性模量为(0.30±0.003) GPa,利用溶胶凝胶法(Sol-gel)对其表面制备羟基磷灰石(HA)涂层,在模拟体液中表现有良好的抗蚀性。LEE等[8]采用微弧氧化法(MAO)在多孔钛表面制备了HA/TiO2混合涂层,经体外细胞培养实验表明这种涂层具备较好的生物活性。高勇等[9]也有相近结果的文献报道。
TANGE等[10]对该工艺进行改进后获得了较理性的孔洞形貌、连通性及孔隙率,但其抗压强度与弹性模量只有1.84~7.97 MPa和0.15~0.55 GPa,力学性能较差。WANG等[11]进行的工艺改进是将纯钛粉与聚乙烯醇(PVA)配成液浆,经浸浆烧结所得多孔钛的抗压强度有较大提高,约为8.9~83.6 MPa。LIU等[12]将该法制备的Nb-Ti-Ta多孔合金植入兔子体内,术后检测显示该移植体自有的100~600 μm连通孔完全满足新骨组织的长入,且组织细胞在多孔合金表面有良好的粘附与增生能力,且该移植材料的自身强度与模量(抗压强度17.45~121.67 MPa,弹性模量0.11~2.08 GPa)与生物骨的力学匹配性也表现良好。
有机海绵复制法制备的多孔钛,连通性与孔隙率都比较高(见图2(a)),但其孔结构、大小和分布取决于海绵结构;有机泡沫骨架在浸渍阶段被浆料完全包裹,经高温挥发后造成孔壁内部中空,同时在孔壁上形成6~45 μm的微孔,导致孔壁的有效厚度和强度下降,最终影响泡沫产品的力学性能[9]。
1.2.2 浆料发泡法
浆料发泡法是将金属粉末、稳定剂、发泡剂与水混合制成浆料,在潮湿环境下充分发泡,然后低温干燥、高温烧结得到多孔钛。
KATO等[13]采用该法制得孔隙率在17%~80%,抗压强度与弹性模量分别为150~240 MPa和11~12 GPa的多孔钛。KAPAT等[14]改用鸡蛋清作发泡剂、一水合柠檬酸作稳定剂,与Ti6Al4V粉末混合制成浆料,经低温干燥与1400 ℃烧结所得多孔钛的孔隙率较前者有很大提升,约为89.3%~65.2%,孔径范围约为44.6~653.8 μm,抗压强度与弹性模量分别是2.46~65.5 MPa和7.3~98.5 GPa。
该法所得多孔钛制品的孔隙率通常都比较高,并且连通窗口的尺寸也比较大,组织能够长入,但发泡形成的胞洞大小及分布的均匀性很难控制(见图2(b)),而且浆料中的有机物会引入C、O元素易在晶界富集,其占比可达2.38%~2.49%和1.37%~1.49%,对泡沫产品的力学性能影响较大[14]。
1.2.3 凝胶注模法
凝胶注模法最早是由美国橡树岭实验室开发的一种制备陶瓷的工艺方法。该法制备多孔钛是将有机单体、交联剂、去离子水与Ti粉末混合制成液浆,随后加入引发剂、催化剂并快速搅拌,然后将混合液注入模具中密封,在室温下搅拌使混合液充分发泡后浇注成型,最后干燥、烧结得到泡沫钛。
ERK等[15]采用此法制备的泡沫钛孔隙率为4%~44%,屈服强度最高可达200 MPa。SINGH等[4]用该法制得多孔试样孔隙率虽大幅度提高到72%~88%,孔径分布为269~688 μm,但孔隙率的提高却导致屈服强度与弹性模量显著下降。BIASETTO等[16]用蛋清作为发泡剂,甲基纤维素水溶液作稳定剂与Ti6Al4V粉末配浆,80 ℃下蛋清中的氨基酸受热发泡产生孔洞,再经干燥后烧结得到的多孔钛,孔隙率及孔径分布均与前者相近,抗压强度保持在24.4~79.1 MPa。
该法可对产品结构进行设计,所得产品的开孔率很高且连通性良好,但在制备过程中会因原料中含有有机物而引入杂质元素,产生TiyOx与TiC等各化合物,最终对材料的使用强度及模量造成危害,这一问题与前述浆料发泡法存在的问题类似,其形貌见图2(c)。
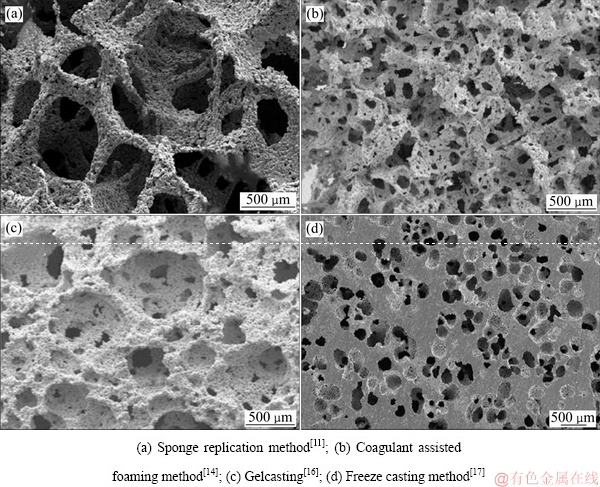
图2 发泡法制备的多孔钛SEM像
Fig. 2 SEM images of porous titanium prepared by foaming method
1.2.4 冷冻铸造法
冷冻铸造法是将冷冻剂(常用水或芡烯)、分散剂、黏合剂与钛粉末进行混合球磨,在真空干燥器中搅拌脱气直至将球磨阶段产生的气泡完全脱除,然后浇注到模具中冷冻使冷冻剂充分冻结干燥,脱模后真空烧结成型。
JUNG等[17-18]以芡烯作为冷冻剂,低聚聚酯作为分散剂,与钛粉末混合,在55 ℃下球磨30 min后注入特制的铝制圆柱形模具中,浇注是在44 ℃模具内保持30 r/min转速下完成,之后模具继续保持旋转防止颗粒沉淀,期间芡烯发生形核长大使浆料固化成型,脱模后在200 MPa下冷等静压使混合物完全致密化,冷冻干燥后再经1300 ℃真空烧结2 h,获得孔隙率为52%~71%、孔径为95~362 μm、抗压强度和弹性模量分别为57~183 MPa和1.3~5.0 GPa的多孔钛。WANG等[19]将该工艺制备的多孔钛经表面氧化后植入兔股骨进行活体实验,证明了氧化处理所得纳米活性层有利于骨组织在移植体表面进行活性分化,最终形成移植体-生物骨的嵌合体。
冷冻铸造法可预先设计产品的尺寸与形状,有利于待修复部位与移植体之间的匹配;但是芡烯晶体在冻干阶段被移除后易导致生坯结构的强度下降而产生坍塌[17]。如图2(d)所示,冷冻铸造法可制备胞洞尺寸较均匀的产品,但胞洞的分布均匀性较差,导致产品的开孔率降低。另外,芡烯自身具有毒性,今后宜考虑无毒冷冻剂开展工艺研究。
1.3 固相成孔法
1.3.1 放电等离子烧结法
放电等离子烧结(SPS)是一种粉末冶金烧结技术,该方法制备多孔钛是将钛粉与造孔剂及黏合剂均匀混合后装入石墨模具中,烧结时模具两端施加脉冲电压,模具内的粉末因尖端放电产生等离子体使粉末快速升温,烧结同时施加一定压力使粉体致密,是一种高效的粉末烧结制备技术。
QUAN等[20]将Ti6Al4V粉末、造孔剂及乙烯醇混合装入圆柱形石墨模具,700 ℃下加压50 MPa烧结8 min得到密实坯体,在水中溶解去除NaCl造孔剂后,再经干燥、无压烧结所得的多孔合金孔隙率为44.7%~70.0%,孔径分布125~250 μm,屈服强度与弹性模量分别为43.0~110.2 MPa和9.5~33.0 GPa,体外细胞培养实验显示类骨细胞可在其表面存活。YAMANOGLU等[21]则是直接将Ti5Al2.5Fe合金球加压烧结得到孔隙率为28.4%~29.1%的多孔合金,其抗压强度最高达300 MPa,在其表面制备的HA涂层经过验证具备良好的生物相容性。
SPS在粉末烧结方面有着升温快、效率高的巨大优势,通过造孔剂可预先控制孔洞大小进而控制了孔隙率,且孔洞之间的连通以较大尺寸窗口为主[20](见图3(a));但采用合金球直接加压烧结成型获得多孔材料,整体孔隙率过低,不利于组织的长入。
1.3.2 添加造孔剂法
添加造孔剂法是将金属粉末与造孔剂混合后压缩成型获得密实化生坯体,去除造孔剂后高温烧结成型。该方法工艺较为简单,很早就被研究者应用在多孔钛的制备上。
WEN等[22]以200~600 μm碳酸氢铵作造孔剂与纯钛粉均匀混合,100 MPa冷压成型,在200 ℃下保温5 h使造孔剂挥发,然后在1200 ℃烧结2 h获得孔隙率为78%,抗压强度与弹性模量分别是35 MPa和5.3 GPa的多孔材料;IMWINKELRIED[23]则将碳酸氢铵开发的60%~65%孔隙率多孔钛应用到了类脊椎移植体的制备上,所得产品的抗压强度最高可达到200 MPa。HSU等[24-25]利用相同的方法制得Ti-7.5Mo多孔合金,并对表面仿生处理后获得了具有良好生物相容性的类骨磷灰石涂层。LUPPO等[26]将碳酸氢铵做造孔剂制备的Zr-Ti-Nb多孔合金进行表面改性后,进行了鼠胫骨移植实验,术后骨组织与移植体的长合表现良好。DUNAND等[27]以NaCl为造孔剂与纯钛粉混合制得孔隙率42%~51%的多孔钛,弹性模量达29~39 GPa。LI等[28]在这类多孔钛表面进行了载银涂层的制备,并经过细胞培养实验证实了载银涂层具有良好的灭菌与生物相容性。PRADO等[29]用尿素开发多孔钛并进行表面仿生处理,细胞培养实验表明该材料有良好的生物活性。其他还有以糖球做造孔剂开发多孔钛的研究[30],但所得结果并不理想。
造孔剂法制备方便,孔洞形貌、孔径尺寸及孔隙率都可通过造孔剂的尺寸、形状及加入量进行调节,制备过程易于控制,可获得所需性能的多孔钛,是当前多孔钛制备应用非常广泛的一种方法,产品形貌见图3(b);但该方法在制备较大尺寸及复杂形状产品时不占优势,而且将造孔剂与纯钛粉直接混合制得的多孔材料,闭孔率相对增大[27]。因此,如何提高空隙排布的整体均匀性及有效减少局部区域闭孔,也是该方法需要解决重要问题。
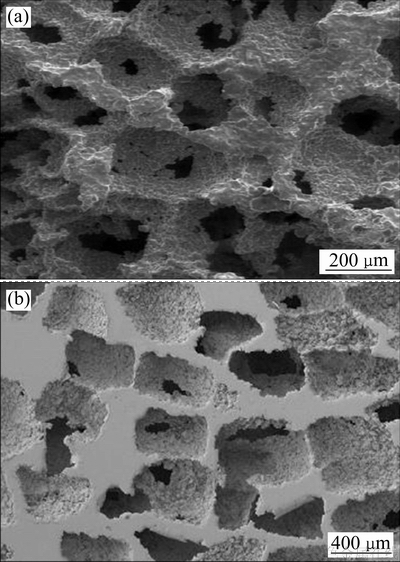
图3 SPS烧结Ti6Al4V泡沫合金SEM像[20]和造孔剂法制备的泡沫钛SEM像[27]
Fig. 3 SEM images of Ti6Al4V foams of the spark plasma sintered[20](a) and titanium foams created space holder method[27](b)
1.4 无介质成孔法
1.4.1 增材制造法
3D打印增材制造法,包括选择性激光烧结(SLS)、选择性激光熔化(SLM)、激光工程化净成形(LENS)、电子束选区熔化(EBM)、3D纤维沉积等不同工艺途径,主要步骤为CAD模型设计、片层离散处理、逐层打印及后处理。其中SLS、SLM、EBM技术在多孔钛合金的制备上应用最多。
选择性激光烧结(SLS)是以激光束为能量源,通过预先设计模型和程序控制对固体粉末进行有选择性的逐层烧结成型,由于功率较小,所得产品带有微孔[31]。SHISHKOVSKY[32]很早便利用SLS技术试制出应用在口腔修复领域的多孔钛合金。而SLS制备的孔隙率72%的多孔钛经表面沉积HA涂层后[33],在山羊骨节移植修复实验中的表现出令人满意的结果(见图4(a)和(b))。
选择性激光熔化技术(SLM)与选择性激光烧结技术(SLS)原理类似,但SLM功率大、效率高,产品质量较SLS也有所提高[31]。AMIN等[34]用SLM制备的Ti6Al4V多孔合金,其孔隙率为68%~84%,孔径分布480~600 μm[35],用阳极氧化法在材料表面制取的TiO2活性层,也已被很多研究人员证实具有很好的生物活性,细胞在其表面的增殖分化效果大大提升。JAN等[36]将SLM制备的柱状Ti6Al4V多孔合金用在山羊腿骨缺陷移植修复实验中(见图4(c)和(d)),术后监测显示移植体与骨组织长合良好,而且生长时间越长,山羊腿骨新生嵌合体的模量与强度还会有所降低。PEI等[37]在狗股骨移植实验中也证实了骨细胞在该多孔移植体上具有很好的增殖分化效果。
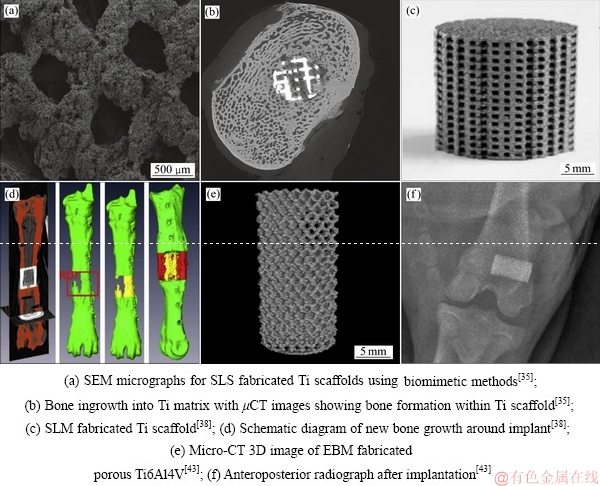
图4 3D打印多孔钛结构及其移植应用
Fig. 4 3D printing porous titanium and its transplantation application
电子束选区熔化(EBM)是利用高能量密度及高利用率的电子束对钛粉末进行扫描熔化、逐层制造获得三维多孔钛材料[38]。MURR等[39]用EBM技术制备出孔隙率55%~89%的多孔钛合金。KNORRA等[40]对EBM制备的Ti6Al4V多孔合金通过化学气相沉积(CVD)对其表面成功制备了均匀的碳化硅涂层,且基体材料经热沉积后力学性能并未降低。用这种多孔合金[41]进行山羊体骨移植实验(见图4(e)和(f)),发现它对新骨组织的形成及骨整合都有很大提高;通过沉积不同表面活性层可进一步增加细胞在移植材料表面附着、增殖、分化,从而降低细胞凋亡率[42-43]。HARA等[44]将EBM制备的不同孔径多孔合金在兔股骨移植实验中验证了800 μm孔径的多孔植入体对骨组织的长入有着很好的促进作用。
增材制造技术在制备复杂结构方面具有极大地优势,可对骨缺陷患者的实际受损部位进行个性化设计,包括孔洞形状与尺寸分布,孔洞空间分布形式,孔隙率,孔壁厚度等,使产品更好的与待修复部位进行匹配。SLS与SLM制备工艺类似,后者比前者功率更大效率更高,但两者在制备过程中易使C、H、O元素在晶界处偏聚,所制备产品的孔壁上容易产生微孔隙,降低了孔壁的有效厚度和力学性能[31]。就增材制造制备多孔钛而言,设备、材料及工艺规范等需要进一步研究和发展[45-46]。
1.4.2 纤维合成法
纤维合成法是将钛丝经缠绕形成立体网状,再经过高温烧结得到网状多孔钛。贺国等[47]将直径为0.08~0.15 mm的纯钛丝缠结成立体网状,经20~120 MPa压缩成型,1200 ℃烧结得到孔隙分布50~200 μm,孔隙率为48%~82%,屈服强度12.6~180.4 MPa,弹性模量0.33~1.05 GPa的多孔钛网;并对该结构注入具有生物相容的明胶和抗菌性庆大霉素混合溶液,合成明胶-多孔钛混合体,合成材料的药物释放能力在模拟体液中表现良好[48]。LI等[49]将钛纤维在850℃经真空扩散制成网状立体结构的多孔钛,其孔隙率为30%~ 70%,孔径100~650 μm,弹性模量及屈服强度分别为1.5~7.0 GPa和10~110 MPa,兔骨关节的移植修复实验被证明这种材料具有良好的缺陷骨修复整合能力[50]。
纤维烧结制备的多孔钛强度适中且弹性模量较低,制备过程简单、成本低,但缠绕烧结法得到的不规则多孔钛网(见图5(c)),内部纤维排序紊乱,整体结构较为松散,导致其模量偏低,抗变形能力较差[47]。图5(b)所示为100~200 μm直径钛纤维进行规则编织,再经真空扩散连接获得的尺寸稳定、连通性良好,整体结构为全开孔的三维网状多孔钛,且各项性能都较纤维缠绕烧结的多孔钛有所提高。但对于承重骨来讲, 其力学性能仍然难以满足需求,因此较多应用在对强度及弹性模量要求较低的修复部位,例如眼眶的修复。
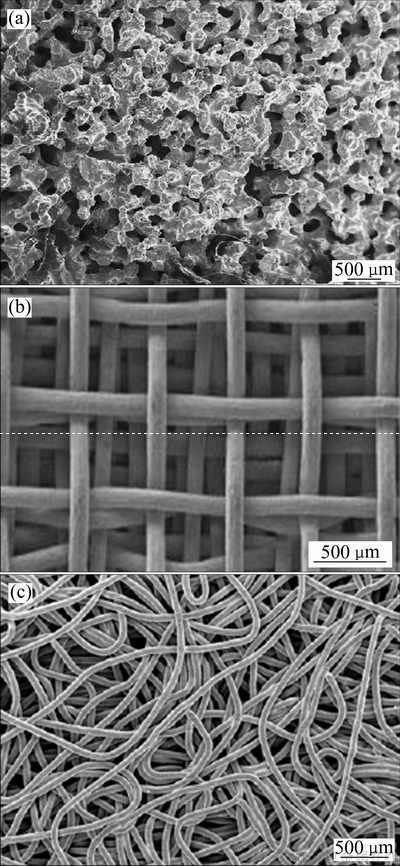
图5 SHS制备的Ni-Ti泡沫合金[51]、纤维编织制备的多孔网状[50]和纤维缠绕制备多孔网状的SEM像[47]
Fig. 5 SEM images of nickel-titanium foam prepared by SHS[51](a), mesh porous titanium prepared by fiber weaving[50](b) and mesh porous titanium prepared by fiber tangled[47](c)
1.4.3 自蔓延高温合成法
自蔓延高温合成法(SHS)是依靠反应系统内的自维持反应制备多孔材料。ARCINIEGAS等[51]用该法制备出孔隙率65%~70%的NiTi医用泡沫合金,孔径分布为370~440 μm,最高屈服强度可达(142.5±29.3) MPa,弹性模量(1.21±0.31) GPa,疲劳循环次数可达1×108。ARAKAWA等[52]将Al、Ti作为基体元素,B4C作发热剂并在400 ℃引燃,合成的Al-Ti泡沫合金孔隙率为60%~70%。
自蔓延法制备的泡沫合金具有较高孔隙率和较好连通性,力学性能也满足移植需求,其形貌如图5(a)所示。自蔓延燃烧产生的瞬间高温可使金属粉末熔化,容易产生偏析和各类缺陷,制备过程较难控制,因此,该法在制备医用泡沫钛合金方面报道较少。
1.5 改良造孔剂法制备多孔钛
针对多孔钛空隙形状不规则、分布不均及容易形成闭孔等问题,本文作者利用改进的造孔剂法制备了孔隙分布均匀、形状规则的多孔多孔钛。
以NaCl为原料,淀粉和蒸馏水混合物为粘合剂,通过油浴加热和机械搅拌制备NaCl球丸,经740 ℃烧结后获得盐球状造孔剂。如图6所示,经高温烧结后NaCl微粒之间具有十几微米的微孔,这些微孔有助于造孔剂从坯体中快速溶解去除。
筛选1000~2360 μm的盐球作为造孔剂与金属粉末分批装入预制模具,利用AUTOTAP振实密度仪将钛粉振动填充到预先铺设好的“盐床”缝隙中(工艺过程见图7),通过粉末下降的高度判断填充是否完成。混合物经冷压成型获得“造孔剂-粉末”生坯体,用自来水溶解去除盐球得到三维连通的多孔钛素坯体,经1400 ℃烧结2 h获得孔径均匀可控的多孔钛。
相比于直接混料法所得多孔材料,改进工艺制备的多孔钛胞洞复制于预先排列整齐的盐球,胞洞之间平均有4~6个连通窗口,造孔剂溶解去除彻底,闭孔少。且结构均匀可控、重现性好,所得多孔钛的孔隙率为70%~80%(见图8)。
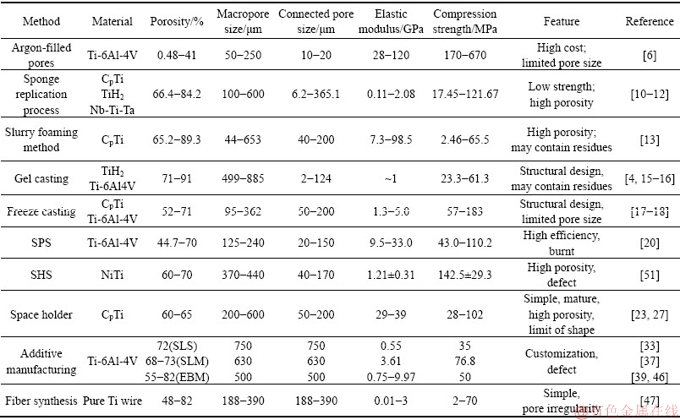
多孔钛制备方法及性能、特征列于表1。其中,惰性气体成泡法、SHS法、SPS法由于工艺本身及成本原因导致近年来报道很少。浆料发泡法与凝胶注模法还有研究者在尝试改进。海绵复制法、冷冻铸造法、造孔剂法、增材制造法和纤维合成法等工艺自开发出来就一直被各研究单位不断向前推进,部分工艺材料已进入活体实验或临床应用阶段。
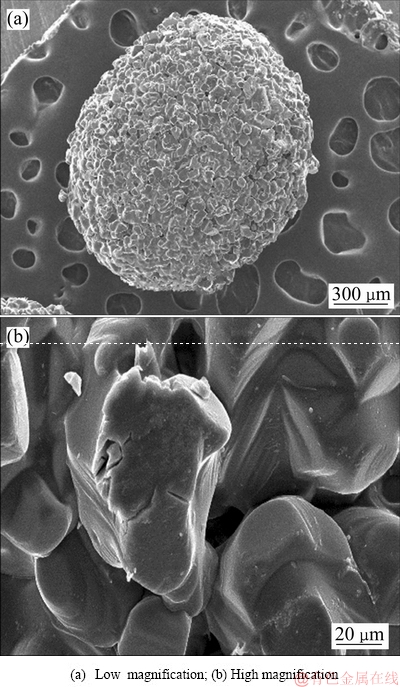
图6 烧结后NaCl盐球的SEM像
Fig. 6 SEM images of sintered NaCl Beads
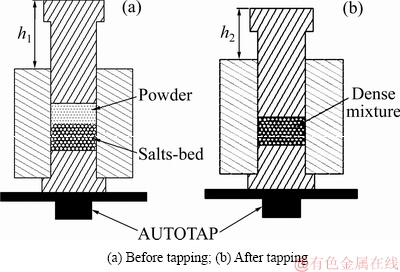
图7 多孔结构构筑过程示意图
Fig. 7 Schematic process of porous structure construction
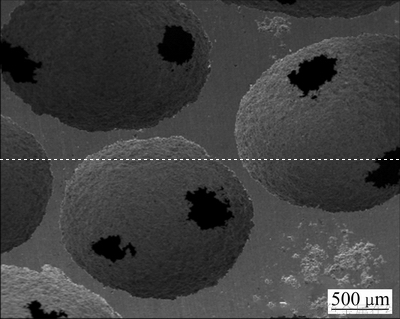
图8 烧结后多孔钛的SEM像
Fig. 8 SEM image of sintered porous titanium
表1 多孔钛及其合金制备工艺与性能概要
Table 1 Summary of preparation process and properties of porous titanium and its alloys
2 多孔钛的应用概况
当前,多孔钛已用于临床修复人体颅面骨,颌骨,膝关节,髋关节等遭受病变或损伤的骨组织部位。
PENG等[53]利用聚乙烯涂层改性的多孔钛网材料对机械性创伤导致眼眶骨折的患者进行修复治疗,术后对患者的恢复情况跟踪监测,发现这种钛网修复材料对眼眶的重建具有长期的机械稳定性,能够将眼球内陷,复视,手术并发症等风险降到最低。
WINTHER等[54]利用高孔隙率多孔钛对患者实施全膝关节置换术(TKA),术后定期对移植修复部位的位移情况进行检测,结果显示重生体的迁移速率非常低,下沉量也非常小。HARWIN等[55]也在219名患者的全膝关节置换(TKA)治疗中也证实了这种高孔隙多孔钛的机械稳定性与修复能力。QASSEMYAR等[56]利用3D打印的多孔钛下颌骨假体对下颚脱落的患者进行颚骨重建手术,使患者面部的完整性及功能恢复正常化,术后经过一段时期的护理,手术处无明显疤痕,表明这种假体有很好的组织整合能力,在人体颌面修复上表现出良好的应用前景。
DELANOIS等[57]利用高孔隙多孔钛材料对35名高龄患者的髋关节实施置换手术,经过5年以上的追踪监测,仅有一名患者出现病菌感染,两名患者出现败血现象,其余恢复情况均表现良好,证明这种多孔钛臼杯对髋关节具有很好的修复能力。国内的北京爱康医疗公司在2016年推出3D打印的多孔钛人工椎体和脊柱融合器(国械注准20163460859)并获得食药监局认证,成功进入脊柱置换植入物人工器官市场,该产品拥有良好的生物相容性和骨整合能力,与松质骨的弹性模量有更好的力学匹配性,目前使用性能尚为稳定。
3 结语
多孔钛作为人体骨移植材料,其自身具有良好生物惰性,可用来修复或替换损伤的骨组织;对其表面进行仿生处理,可提高移植材料生物活性,改善细胞的附着、增殖和分化能力,加快组织在多孔结构中的生长速率,使移植体与原生组织更好地长合,以达到修复缺陷的目的。
当前,在动物体移植实验和临床应用研究较多的主要有3D打印法、造孔剂法、冷冻铸造法、纤维烧结等方法制备的多孔钛。不同制备方法具有各自优点,但普遍存在制备成本较高或性能偏低等问题;另外,针对不同患者的实际骨质情况,往往需要不同力学性能及孔隙结构的骨移植材料,因此,在较大范围内对多孔钛骨移植材料结构和弹性模量进行调节仍然需继续研究,以更好地解决移植体与待修复部位的力学匹配问题。
多孔钛临床应用虽然已有一段时期,但这种人体骨替代材料的耐久性、适用性、相容性仍然有待后续的长期检验。
REFERENCES
[1] 刘喜春. 骨植入材料的应用及发展前景[J]. 中国医药技术经济与管理, 2007, 1(5): 30-33.
LIU Xi-chun. Application and development prospect of bone implant materials[J]. Chinese Medical Technology Economy and Management, 2007, 1(5): 30-33.
[2] LEWIS G. Properties of open-cell porous metals and alloys for orthopaedic applications[J]. Journal of Materials Science Materials in Medicine, 2013, 24 (10): 2293-2325.
[3] AGUILAR MAYA A E, GRANA D R, HAZARABEDIAN A, KOKUBU G A, LUPPO M I, VIGNA G. Zr-Ti-Nb porous alloys for biomedical application[J]. Materials Science & Engineering C, 2012, 32(2): 321-329.
[4] SINGH R, LEE P D, JONES J R, POOLOGASUNDARAMPILLAI G, POST T, LINDLEY T C, DASHWOOD R J. Hierarchically structured titanium foams for tissue scaffold applications[J]. Acta Biomaterialia, 2010, 6(12): 4596-4604.
[5] ASHBY M F, EVANS A G, FLECK N A, GIBSON L J, HUTCHINSON J W, WADLEY H N G. Metal foams: A design guide[J]. Applied Mechanics Reviews, 2001, 23(6): 119.
[6] OPPENHEIMER S, DUNAND D C. Solid-state foaming of Ti-6Al-4V by creep or superplastic expansion of argon-filled pores[J]. Acta Materialia, 2010, 58 (13): 4387-4397.
[7] CACHINHO S C, CORREIA R N. Titanium scaffolds for osteointegration: Mechanical, in vitro and corrosion behaviour[J]. Journal of Materials Science Materials in Medicine, 2008, 19(1): 451-457.
[8] LEE J H, KIM H E, KOH Y H. Highly porous titanium (Ti) scaffolds with bioactive microporous hydroxyapatite/TiO2, hybrid coating layer[J]. Materials Letters, 2009, 63(23): 1995-1998.
[9] GAO Y, XU X X, YANG Z M. Novel TiC/Ti open cellular foams prepared by a modified sponge-coating method using high frequency induction heating process[J]. Journal of Materials Science & Technology, 2013, 29(4): 339-343.
[10] TANGE M, MANONUKUL A, SRIKUDVIEN P. The effects of organic template and thickening agent on structure and mechanical properties of titanium foam fabricated by replica impregnation method[J]. Materials Science & Engineering A, 2015, 641: 54- 61.
[11] WANG C L, CHEN H J, ZHU X D, XIAO Z W, ZHANG K, ZHANG X D. An improved polymeric sponge replication method for biomedical porous titanium scaffolds[J]. Materials Science & Engineering C, 2017, 70(2): 1192-1199.
[12] LIU J, RUAN J M, CHANG L, YANG H L, RUAN W. Porous Nb-Ti-Ta alloy scaffolds for bone tissue engineering: Fabrication, mechanical properties and in vitro/vivo biocompatibility[J]. Materials Science & Engineering C, 2017, 78: 503-512.
[13] KATO K, OCHIAI S, YAMAMOTO A, DAIGO Y, HONMA K, MATANO S, OMORI K. Novel multilayer Ti foam with cortical bone strength and cytocompatibility[J]. Acta Biomaterialia, 2013, 9(3): 5802-5809.
[14] KAPAT K, SRIVAS P K, DHARA S. Coagulant assisted foaming—A method for cellular Ti6Al4V: Influence of microstructure on mechanical properties[J]. Materials Science & Engineering A, 2017, 689: 63-71.
[15] ERK K A, DUNAND D C, SHULL K R. Titanium with controllable pore fractions by thermoreversible gelcasting of TiH2[J]. Acta Materialia, 2008, 56(18): 5147-5157.
[16] BIASETTO L, MORAES E G, COLOMBO P, BONOLLO F. Ovalbumin as foaming agent for Ti6Al4V foams produced by gelcasting[J]. Journal of Alloys & Compounds, 2016, 687: 839-844.
[17] JUNG H D, YOOK S W, JANG T S, LI Y L, KIM H E, KOH Y H. Dynamic freeze casting for the production of porous titanium (Ti) scaffolds[J]. Materials Science & Engineering C, 2013, 33(1): 59-63.
[18] LEE H, JANG T S, SONG J, KIM H E, JUNG H D. Multi- scale porous Ti6Al4V scaffolds with enhanced strength and biocompatibility formed via dynamic freeze-casting coupled with micro-arc oxidation[J]. Materials Letters, 2016, 185: 21-24.
[19] WANG G H, FU H, ZHAO Y Z, ZHOU K C, ZHU S H. Bone integration properties of antibacterial biomimetic porous titanium implants[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(9): 2007-2014.
[20] QUAN Y J, ZHANG F M, REBL H, NEBE B, KEβLER O, BURKEL E. Ti6Al4V foams fabricated by spark plasma sintering with post-heat treatment[J]. Materials Science & Engineering A, 2013, 565 (10):118-125.
[21] YAMANOGLU R, GULSOY N, OLEVSKY E A, GULSOY H O. Production of porous Ti5Al2.5Fe alloy via pressure-less spark plasma sintering[J]. Journal of Alloys & Compounds, 2016, 680: 654-658.
[22] WEN C E,MABUCHI M, YAMADA Y, SHIMOJIMA K, CHINO Y, ASAHINA T. Processing of biocompatible porous Ti and Mg[J]. Scripta Materialia, 2001, 45(10): 1147-1153.
[23] IMWINKELRIED T. Mechanical properties of open-pore titanium foam[J]. Journal of Biomedical Materials Research Part A, 2007, 81(4): 964-970.
[24] HSU H C, WU S C, HSU S K, TSAI M S, CHANG T Y, HO W F. Processing and mechanical properties of porous Ti-7.5Mo alloy[J]. Materials & Design, 2013, 47(9): 21-26.
[25] HSU H C, WU S C, HSU S K, CHANG T Y, HO W F. Effect of ball milling on properties of porous Ti-7.5Mo alloy for biomedical applications[J]. Journal of Alloys & Compounds, 2014, 582(2): 793-801.
[26] AGUILAR MAYA A E, GRANAD R, HAZARABEDIAN A, KOKUBU G A, LUPPO M I, VIGNA G. Zr-Ti-Nb porous alloys for biomedical application[J]. Materials Science & Engineering C, 2012, 32(2): 321-329.
[27] YE B, DUNAND D C. Titanium foams produced by solid-state replication of NaCl powders[J]. Materials Science & Engineering A, 2010, 528(2): 691-697.
[28] LI M T, WANG Y, GAO L L, SUN Y H, WANG J X, QU S X, DUAN K, WENG J, FENG B. Porous titanium scaffold surfaces modified with silver loaded gelatin microspheres and their antibacterial behavior[J]. Surface & Coatings Technology, 2016, 286: 140-147.
[29] PRADO R F, OLIVEIRA F S, NASCIMENTO R D, VASCONCELLOS L M R, CARVALHO Y R, CAIRO C A A. Osteoblast response to porous titanium and biomimetic surface: In vitro analysis[J]. Materials Science & Engineering C, 2015, 52: 194-203.
[30] CHEN Y H, KENT D, BERMINGHAM M, MANSHADI A D, DARGUSCH M. Manufacturing of biocompatible porous titanium scaffolds using a novel spherical sugar pellet space holder[J]. Materials Letters, 2017, 195(92): 92-95.
[31] 颉芳霞, 何雪明, 吕彦明, 武美萍, 何新波, 曲选辉. 生物医用多孔钛及钛合金激光快速成形研究进展[J]. 材料导报, 2016, 30(7): 109-114.
XIE Fang-xia, HE Xue-ming, Lü Yan-ming, WU Mei-ping, HE Xin-bo, QU Xuan-hui. Research progress in laser rapid forming of porous titanium and its alloys for biomedical applications[J]. Materials Review, 2016, 30(7): 109-114.
[32]
[33] TAMADDON M, SAMIZADEH S, WANG L, BLUNN G, LIU C Z. Intrinsic osteoinductivity of porous titanium scaffold for bone tissue engineering[J]. International Journal of Biomaterials, 2017, 2017: 1-11.
[34] AMIN YAVARI S, CHAI Y C, BOTTGER A J, WAUTHLE R, SCHROOTEN J, WEINANS H, ZADPOOR A A. Effects of anodizing parameters and heat treatment on nano-topographical features, bioactivity, and cell culture response of additively manufactured porous titanium[J]. Materials Science & Engineering C, 2015, 51: 132-138.
[35] HEDAYATI R, AMIN Y S, ZADPOOR A A. Fatigue crack propagation in additively manufactured porous biomaterials[J]. Materials Science & Engineering C, 2017, 76: 457-463.
[36] JAN W D, LINDNER T, BERGSCHMIDT P, BADER R. Bio-mechanical stability of novel mechanically adapted open-porous titanium scaffolds in metatarsal bone defects of sheep[J]. Biomaterials, 2015, 46: 35-47.
[37] PEI X, ZHANG B Q, FAN Y G, ZHU X D, SUN Y, WANG Q G, ZHANG X D, ZHOU C C. Bionic mechanical design of titanium bone tissue implants and 3D printing manufacture[J]. Materials Letters, 2017, 208(128): 133-137.
[38] CANSIZOGLU O, HARRYSSON O, CORMIER D, WEST H, MAHALE T. Properties of Ti-6Al-4V non-stochastic lattice structures fabricated via electron beam melting[J]. Materials Science & Engineering A, 2008, 492(1/2): 468-474.
[39] MURR L E, GAYTAN S M, MEDINA F, MARTINEZ E, MARTINEZ J L, HERNANDEZ D H, MACHADO B I, RAMIREZ D A, WICKER R B. Characterization of Ti-6Al-4V open cellular foams fabricated by additive manufacturing using electron beam melting[J]. Materials Science & Engineering A, 2010, 527(7/8): 1861-1868.
[40] KNORR T, HEINL P, SCHWERDTFEGER J, KORNER C, SINGER R F, ETZOLD B J M. Process specific catalyst supports Selective electron beam melted cellular metal structures coated with microporous carbon[J]. Chemical Engineering Journal, 2012, 181/182(1): 725-733.
[41] HUANG H, LAN P H, ZHANG Y Q, LI X K, ZHANG X, YUAN C F, ZHENG X B, GUO Z. Surface characterization and in vivo performance of plasma-sprayed hydroxylapatite coated porous Ti6Al4V implants generated by electron beam melting[J]. Surface &Coatings Technology, 2015, 283: 80-88.
[42] LI X, MA X Y, FENG Y F, MA Z S, WANG J, MA T C, QI W, LEI W, WANG L. Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions[J]. Biomaterials, 2015, 36: 44-54.
[43] WANG L, HU X F, MA X Y, MA Z S, ZHANG Y, LUA Y Z, LI X, LEI W, FENG Y F. Promotion of osteointegration under diabetic conditions by tantalum coating-based surface modification on 3-dimensional printed porous titanium implants[J]. Colloids & Surfaces B Biointerfaces, 2016, 148: 440-452.
[44] HARA D, NAKASHIMA Y, SATO Y, HIRATA M, KANAZAWA M, KOHNO Y, YOSHIMOTO K, YOSHIHARA Y, NAKAMURA A, NAKAO Y, IWAMOTO Y. Bone bonding strength of diamond-structured porous titanium alloy implants manufactured using the electron beam melting technique[J]. Materials Science & Engineering C, 2016, 59: 1047-1052.
[45] 肖 健, 邱贵宝. 泡沫或多孔钛的制备方法研究进展[J]. 稀有金属材料与工程, 2017(6): 1734-1748.
XIAO Jian, QIU Gui-bao. Research progress in preparation methods of titanium foams or porous titanium[J]. Rare Metal Materials and Engineering, 2017(6): 1734-1748.
[46] LIU Y J, LI S J, WANG H L, HOU W T, HAO Y L, YANG R, SERCOMBE T B, ZHANG L C. Microstructure, defects and mechanical behavior of beta-type titanium porous structures manufactured by electron beam melting and selective laser melting[J]. Acta Materialia, 2016, 113: 56-67.
[47] HE G, LIU P, TAN Q. Porous titanium materials with entangled wire structure for load-bearing biomedical applications[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2012, 5(1): 16-31.
[48] LI Q, HE G. Gelatin-enhanced porous titanium loaded with gentamicin sulphate and in vitro release behavior[J]. Materials & Design, 2016, 99: 459-466.
[49] LI F P, LI J S, XU G S, LIU G J, KOU H C, ZHOU L. Fabrication, pore structure and compressive of behavior anisotropic porous titanium for human trabecular bone implant applications[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2015, 46: 104-114.
[50] CHANG B, SONG W, HAN T X, YAN J, LI F P, ZHAO L Z, KOU H C, ZHANG Y M. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth[J]. Acta Biomaterialia, 2016, 33: 311-321.
[51] ARCINIEGAS M, APARICIO C, MANERO J M, GIL F J. Low elastic modulus metals for joint prosthesis: Tantalum and nickel-titanium foams[J]. Journal of the European Ceramic Society, 2007, 27(11): 3391-3398.
[52] ARAKAWA Y, KOBASHI M, KANETAKE N. Foaming behavior of long-scale Al-Ti intermetallic foam by SHS mode combustion reaction[J]. Intermetallics, 2013, 41(10): 22-27.
[53] PENG M Y, MERBS S L, GRANT M P, MAHONEY N R. Orbital fracture repair outcomes with preformed titanium mesh implants and comparison to porous polyethylene coated titanium sheets[J]. Journal of Cranio-maxillo-facial Surgery, 2016, 45(2): 271-274.
[54] WINTHER N S, JENSEN C L, JENSEN C M, LIND T, SCHRODER H M, FLIVIK G, PETERSEN M M. Comparison of a novel porous titanium construct (Regenerex ) to a well proven porous coated tibial surface in cementless total knee arthroplasty—A prospective randomized RSA study with two-year follow-up[J]. The Knee, 2016, 23(6): 1002-1011.
) to a well proven porous coated tibial surface in cementless total knee arthroplasty—A prospective randomized RSA study with two-year follow-up[J]. The Knee, 2016, 23(6): 1002-1011.
[55] HARWIN S F, PATEL N K, FRCS, CHUGHTAI M, KHLOPAS A, RAMKUMAR P N, ROCHE M, MONT M A. Outcomes of newer generation cementless total knee arthroplasty: Beaded periapatite-coated vs highly porous titanium-coated implants[J]. The Journal of Arthroplasty, 2017, 32: 2156-2160.
[56] QASSEMYAR Q, ASSOULY N, TEMAM S, KOLB F. Use of a three-dimensional custom-made porous titanium prosthesis for mandibular body reconstruction[J]. International Journal of Oral & Maxillofacial Surgery, 2017, 46(10): 1248-1251.
[57] DELANOIS R E, GWAM C U, MOHAMED N, KHLOPAS A, CHUGHTAI M, MALKANI A L, MONT M A. Midterm outcomes of revision total hip arthroplasty with the use of a multihole highly-porous titanium shell[J]. The Journal of Arthroplasty, 2017, 32: 2806-2809.
Preparation and development of porous titanium material for bone transplanting
JIA Jian-gang, JING Yong-zhi, GAO Chang-qi, LIU Chang, JI Gen-shun, GUO Tie-ming
(Lanzhou University of Technology, State Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals, Lanzhou 730050, China)
Abstract: The porous titanium is a porous metal material with a three-dimensional open interconnected pore structure. Compared with dense biometal materials, its elastic modulus is greatly reduced due to its openability and connectivity, and thus it can be well matched to human bones, at the same time, free circulation of body fluids, combined with good biocompatibility of titanium, thus the porous titanium is used as orthopedic transplant material. How to control the porous structure and obtain the porous titanium matrix with excellent performance is the premise and foundation for carry out related research and application. The porous titanium alloy have been developed, and the preparation methods conclude gas foaming method, organic sponge replication method, slurry foaming method, gel column molding method, frozen casting method, self-propagating high-temperature synthesis method, and spark plasma sintering method, added pore-forming agent method, additive manufacturing method, fiber synthesis method, etc. Different preparation methods have their own advantages, but most of them suffer problems, such as high preparation cost or low performance. The orthopedic transplant material with different mechanical properties and pore structure are required for the actual bone condition of different patients. Therefore, adjusting the structure and modulus of porous titanium for bone-transplanting in a large range still will be studied. The porous titanium bone graft materials have achieved clinical application, the porous titanium vertebral implant materials are rolled out by domestic institutions. Porous titanium as a bone graft material, there is still much room for research and clinical applications in the future.
Key words: porous titanium; foams metal; bone graft material; porous preparation; biocompatibility
Foundation item: Project(51461029) supported by the National Natural Science Foundation of China; Project (1602GKDD012) supported Major Special of Science & Technology of Gansu Province, China
Received date: 2018-06-12; Accepted date: 2019-03-06
Corresponding author: JIA Jian-gang; Tel: +86-13919809312; E-mail: lzhjiajiangang@163.com
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51461029);甘肃省科技重大专项计划项目(1602GKDD012)
收稿日期:2018-06-12;修订日期:2019-03-06
通信作者:贾建刚,副教授,博士;电话:13919809312;E-mail:lzhjiajiangang@163.com
摘 要:多孔钛是一种具有三维连通孔结构的金属材料,与致密生物金属材料相比,由于自身的开孔性和连通性使其弹性模量大幅度降低,从而能够与人体骨模量较好匹配,同时使体液自由流通,加之钛具有良好的生物相容性,因而被用作骨科移植材料。而如何控制多孔结构,获得性能优异的多孔钛基体是开展相关研究和应用的前提与基础。已开发出的多孔钛及其合金的制备方法有气体成泡法、有机海绵复制法、浆料发泡法、凝胶柱模法、冷冻铸造法、自蔓延高温合成法、放电等离子烧结法、添加造孔剂法、增材制造法、纤维合成法等。不同制备方法具有各自优点,但普遍存在制备成本较高或性能偏低等问题;另外,针对不同患者的实际骨质情况,需要不同力学性能及孔隙结构的骨移植材料。因此,在较大范围内对多孔钛骨移植材料结构和模量进行调节仍然需继续研究。目前,多孔钛骨移植材料已实现临床应用,国内机构也已经推出多孔钛椎体植入材料用于临床实践。未来,多孔钛作为骨移植材料的研究和临床应用仍有很大空间。


