
Synthesis and microwave dielectric properties of boron doped SiC powder by sol-gel method
LI Zhi-min(李智敏), LUO Fa(罗 发), SU Xiao-lei(苏晓磊),
ZHU Dong-mei(朱冬梅), ZHOU Wan-cheng(周万城)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Nano-SiC powders doped by B were synthesized through the carbothermal reduction of xerogels containing the tributyl borate. The results show that the 3C-SiC with minor phase of 6H-SiC is generated at 1 700 ℃, and that there are not the characteristic peaks of any boride in the XRD patterns, which indicates that the boron is available only on the crystallization of 3C-SiC. The Raman spectra of the samples also show the characteristic bands of 3C- and 6H-SiC at 788 and 965 cm-1. But the bands at 1 345 and 1 590 cm-1 are characteristic peaks of amorphous carbon materials. The intensities of peaks at 788 and 965 cm-1 increase with B content in Raman spectra, which also shift to higher wavenumber with the increasing B. The microstructure of SiC powder is composed of agglomerated particles with diameters ranging from 30 to 100 nm. The results of dielectric property show that the sample with 0.005 B has the largest values in ε′ and ε″ among the four samples due to the existence of the intrinsic defects. But the absence of the relaxation polarization leads to low values of all the samples.
Key words:
SiC powder; sol-gel; B dopant; dielectric property; Raman spectroscopy;
1 Introduction
Silicon carbide is often considered the most important carbide, whose application ranges from structural materials to electric devices at elevated temperature[1-4]. The powder of SiC has been developed extensively in recent years, and a great deal of effort has been reported on its synthesis which contains sol-gel and carbothermal reduction, chemical vapor deposition(CVD), laser- or plasma-driven chemical vapor deposition, microwave methods and so on[5-8]. However, little information is available on its dopant for SiC powder and dielectric properties, especially at high frequencies for isolators and electromagnetic wave absorbing materials. It is well-known that doping is an essential method for semiconductor material fabrication to improve the electrical properties. In SiC, both nitrogen and phosphorus can serve as the n-type impurities, while boron and aluminum are the common impurities for p-type doping[9,10]. ZHAO et al[11] have compared the dielectric properties of nano SiC/N composite powder with nano SiC powder, and shown the good microwave absorbing properties of SiC/N composite powder due to the doping of nitrogen. ZHANG et al[12] have also obtained nano-sized solid solution powders of SiC with Al and N by carbothermal reduction process of the xerogels of SiO2-Al2O3.
But till now, little work has been done in the synthesis and dielectric properties of nano SiC powder doped by B. In this study, nano-SiC powders doped by B were synthesized through the carbothermal reduction of xerogels containing the boride, and the phase component, variation and microstructure of which were investigated. Finally, the dielectric properties of the powder as-prepared in the frequency range of 8.2-12.4 GHz were discussed.
2 ExperimentalThe mixture sol was prepared using tetraethoxysilane(TEOS, (C2H5)4SiO4), saccharose (C12H22O11), tributyl borate (C12H27BO3), ethanol and distilled water as starting materials. The TEOS and ethanol, saccharose and water were mixed, respectively, and then mixed together with tributyl borate. During the process of stirring for homogeneity, the pH value of solution was kept at 3-4 with the catalyst of HCl. The sol prepared was placed into drying oven to gel at 60 ℃. Carbothermal reduction of the xerogel was carried out in the vacuum sintering furnace (ZRS-150, Jinzhou China) using the graphite crucible. Namely, dried and ground gels, in which the molar ratio of B to Si is 0.005, 0.01, 0.05 and 0.1, respectively, were fired at 1 700 ℃ with the heating rate of 10 ℃/min in a static argon atmosphere for 1 h.
The crystalline phases of powders as-prepared were identified by X-ray diffraction(XRD, X’Pert PRO MPD, Cu Kα). Raman spectroscopic measurements were performed with a Renishow Invia spectrometer for the change of the crystalline phases, using the 514.5 nm line of an Ar ion laser as the excitation source. The morphology of the powder was investigated by scanning electron microscope(SEM, JEOL JSM-6360LV). The samples for dielectric parameter measurement at room temperature were prepared by blending the produced powders with paraffin in a mass ratio of 1?4, and then being molded into a 10.16 mm×22.83 mm×3 mm brass flange ring. The dielectric parameters were carried out by a vector network analyzer (Angilent Technologies E8362B) in the frequency range of 8.2-12.4 GHz.
3 Results and discussion3.1 Phase structure of as-prepared powder
The XRD patterns of the B-doped powders synthesized at 1 700 ℃ are shown in Fig.1. It can be seen that all the powders are identified by the major phase of cubic 3C-SiC (β-SiC) and the minor phase of hexagonal 6H-SiC (α-SiC), and that there are not any characteristic peaks of the boride. But the peak intensity of 3C-SiC such as the (200) of about 41? becomes greater with the increasing B content, which indicates that the boron in xerogel is helpful to the crystallization of 3C-SiC. Additionally, it is evident from the peak of 6H-SiC that the crystallization of 6H-SiC is restrained as the B content increases.
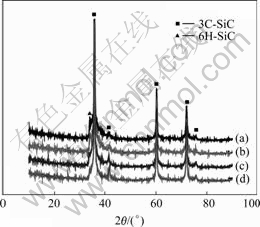
Fig.1 XRD patterns of powders synthesized with different amounts of B: (a) 0.005; (b) 0.01; (c) 0.05; (d) 0.1
The Raman spectra of the samples as-prepared in the 200-2 500 cm-1 region are shown in Fig.2. All of them consist of four peaks. The 3C- and 6H-SiC polytypes are characterized by a transverse optical phonon mode(TO) band at about 788 cm-1 and a longitudinal optical phonon mode(LO) band at 965 cm-1 [13-15]. But the bands at 1 345 cm-1 and 1 590 cm-1 are characteristic peaks of amorphous carbon materials[16], which are not identified in Fig.1. This indicates that the amorphous carbon materials are also generated in the process of carbothermal reduction. Additionally, it can be seen that the intensities of peaks at 788 cm-1 and 965 cm-1 increase with the B content, and that these two peaks shift to higher wavenumber with the increasing B. The band shifts, band broadening and relative intensities of the Raman spectra are closely related to crystal structures of the samples, and especially the LO band will shift to higher wavenumber as the carrier concentration increases[17]. The more the B content, the better the crystallization of SiC in Fig.1, which results in an increase of peak intensity and a peak shift to higher wavenumber. However, it is possible that the band shift at 788 and 965 cm-1 is due to the increase of the hole defect concentration. Namely, B atom can substitute the C atom on the SiC lattice to generate the solid solution of SiC/B in the carbothermal reduction process, but it is much difficult to happen[18].
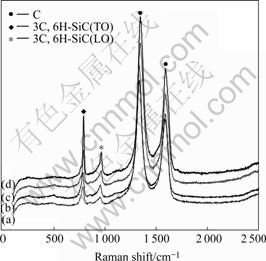
Fig.2 Raman spectra of B-doped SiC powders with different amounts of B: (a) 0.005; (b) 0.01; (c) 0.05; (d) 0.1
3.2 Characterization by SEM
SEM images of the four powders are shown in Fig.3. It can be seen that the powders are composed mostly of agglomerated particles with diameters ranging from 30 to 100 nm. Because of the aid of B on the crystallization, the particles of 0.01 B are more uniform in size than those of 0.005 B. But from the result in Fig.3(d), it can be seen that there exist few particles greater than 100 nm, which are undesired, when the content of B is up to 0.1.
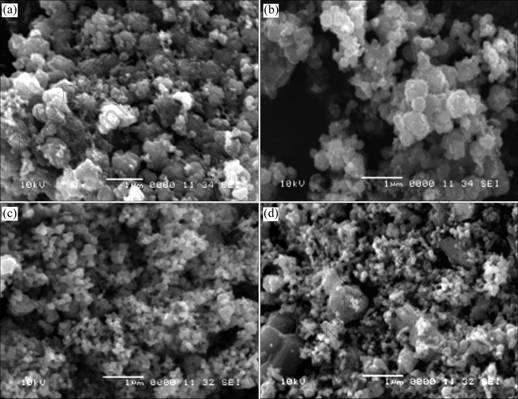
Fig.3 SEM morphologies of B-doped SiC powders with different amounts of B: (a) 0.005; (b) 0.01; (c) 0.05; (d) 0.1
3.3 Dielectric properties
Fig.4 shows the real component of permittivity ε′ of the four samples in the microwave frequency range from 8.2 GHz to 12.4 GHz at room temperature. Though all the samples have lower values of ε′, the sample with 0.005B has the greatest value than the other three samples. This is because that the crystallization of SiC is not perfect under trace or no amount of B and that there is few intrinsic defects in SiC crystal such as VSi and VC which will be polarized at higher frequency to lead to the better value of ε′. However, when the B content is 0.1, the value of ε′ is lower, close to the value of paraffin in sample, which indicates that the SiC crystallization as-prepared is perfect, and proves that there does not exist an electric type relaxation polarization arising from the hole defect caused by the SiC/B solid solution.
The imaginary component of permittivity ε″ as a function of frequency is illustrated in Fig.5. The SiC is a non-magnetic loss material (permeability μ′=1, μ″=0), its power dissipation at high frequency is determined only by dielectric loss, which is characterized by ε″. Having
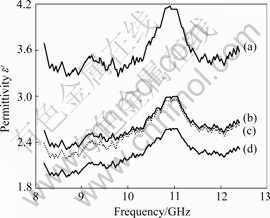
Fig.4 Real components of complex permittivity vs frequency of samples with different B contents: (a) 0.005; (b) 0.01; (c) 0.05; (d) 0.1
the more polarization loss from intrinsic defects, among the four samples the sample with 0.005 B reveals the best value in ε″, but it is only about 0.45. From the results in Fig.5, it is clear that the ε″ decreases heavily with the increasing B content, which depends on the amount of intrinsic defects. In agreement with the results in Fig.4, the lower ε″ for all the samples also indicates that the SiC/B solid solution can not be generated in the carbothermal reduction process, which would result in the relaxation polarization loss.
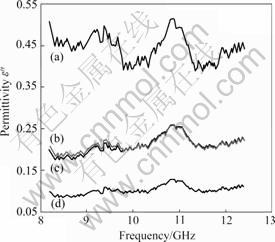
Fig.5 Imaginary components of complex permittivity vs frequency of samples with different B contents: (a) 0.005; (b) 0.01; (c) 0.05; (d) 0.1
4 ConclusionsThe 3C-SiC with the minor phase of 6H-SiC was synthesized at 1 700 ℃ by carbothermal reduction of the xerogel containing tributyl borate, and no boride was identified in the XRD patterns. The boron is available on the crystallization of 3C-SiC and restrained to that of 6H-SiC. The results of the Raman spectra show that the 3C- and 6H-SiC polytypes of the samples are characterized by the bands at 788 cm-1 and 965 cm-1. But the bands at 1 345 and 1 590 cm-1 are characteristic peaks of amorphous carbon materials. Additionally, because of the difference of crystallization, the intensities of sample peaks at 788 cm-1 and 965 cm-1 increase with B content, and these two peaks shift to higher wavenumber with the increasing B content. The microstructure of β-SiC powder is composed of agglomerated particles with diameters ranging from 30 to 100 nm, which indicates that the boron does favor the crystallization of SiC. The real component ε′ and imaginary component ε′′ of permittivity of all the samples show lower values. Because the sample with 0.005B has not as good crystallization as the other three samples and contains few intrinsic defects possibly in crystal, it has the greatest value in ε′ and ε′′ among the four samples. Finally, it is proved that the SiC/B solid solution can not be generated in the carbothermal reduction process.
References[1] LI Zhi-min, DU Hong-liang, LIU Xiao-kui, LUO Fa, ZHOU Wan-cheng. Synthesis and microwave dielectric properties of Si/C/B powders [J]. Trans Nonferrous Met Soc China, 2006, 16: s470-s473.
[2] MARTIN H P, EEKE R, MULLER E. Synthesis of nanocrystalline silicon carbide powder by carbothermal reduction [J]. Journal of European Ceramic Society, 1998, 18: 1737-1742.
[3] KLEIN S, WINTERER M, HAHN H. Reduced-pressure chemical vapor synthesis of nanocrystalline silicon carbide powders [J]. Chem Vap Deposition, 1998, 4: 143-149.
[4] MENG G W, CUI Z, ZHANG L D. Growth and characterization of nanostructured β-SiC via carbothermal reduction of SiO2 xerogels containing carbon nanoparticles [J]. Journal of Crystal Growth, 2000, 209: 801-806.
[5] WANG Xiang-dong, QIAO Guan-jun, JIN Zhi-hao. Preparation of SiC/BN nanocomposite powders by chemical processing[J]. Materials Letters, 2004, 58: 1419-1423.
[6] XU H W, TANIA B, SWARNIMA A, NITIN P P, ANGEL L O, FRANCISCO L. Microstructural evolution in liquid-phase-sintered SiC (Part I): Effect of starting powder [J]. J Am Ceram Soc, 2001, 84(7): 1578-84.
[7] TIAN Jie-mo, LI Jin-wang, DONG Li-Min. Synthesis of SiC precursor by sol-gel process [J]. Journal of Inorganic Materials, 1999, 14(2): 297-301. (in Chinese)
[8] DAI Xue-gang, ZHEN Guo-liang, RONG Jing-long, WANG Jian-guo, ZHANG Qi-xiong. Preparation of SiC ultrafine powder by plasma technique [J]. Engineering Chemistry & Metallurgy, 1996, 17(4): 310-315. (in Chinese)
[9] TIAN Z, SALAMA I A, QUICK N R, KARA A. Effects of different laser sources and doping methods used to dope silicon carbide [J]. Acta Materialia, 2005, 53: 2835-2844.
[10] KOJIMA K, KURODA S, OKUMURA H. Influence of lattice polarity of nitrogen and aluminum doping on 4H-SiC epitaxial layer [J]. Microelectronic Engineering, 2006, 83: 79-81.
[11] ZHAO Dong-lin, ZHAO Hong-sheng, ZHOU Wan-cheng. Dielectric properties of nano Si/C/N composite powder and nano SiC powder at high frequencies [J]. Physica E, 2001, 9: 679-685.
[12] ZHANG Bo, LI Jin-bao, SUN Jing-ing. Solid solution of Al and N in nano-sized a-SiC powder by carbothermal reduction of the xerogels of SiO2-Al2O3 [J]. Materials Letters, 2001, 51: 219-224.
[13] WARD Y, YOUNG R J. Application of Raman microscopy to the analysis of silicon carbide monofilaments [J]. Journal of Materials Science, 2004, 39: 6781-6790.
[14] MAHER S A, LAVANYA D, MOSTAFA M. Raman mapping of local phases and local stress fields in silicon-silicon carbide composites [J]. Materials Chemistry and Physics, 2006, 98: 410-414.
[15] MARTIN H P, MILLER E, IRMER G, BABONNEAU F. Crystallisation behaviour and polytype transformation of polymer-derived silicon carbide [J]. Journal of the European Ceramic Society, 1997, 17: 659-666.
[16] VERES M, KOOS M, TOTH S, FULE M, POCSIK I, TOTH A. Characteriastion of α-C?H and oxygen-containing Si?C?H films by Raman spectroscopy and XPS [J]. Diamond & Related Materials, 2005, 14: 1051-1056.
[17] NAKASHIMA S, MITANI T, SENZAKI J, OKUMURA H. Deep ultraviolet Raman scattering characterization of ion-implanted SiC crystals [J]. Journal of Applied Physics, 2005, 97: 123507-7.
[18] PARFENOVA I I, RESHANOV S A, RASTEGAEV V P. Solubility of impurities in silicon carbide during vapor growth [J]. Inorganic Materials, 2002, 35: 476-481.
Foundation item: Project(50572090) supported by the National Natural Science Foundation of China
Corresponding author: LI Zhi-min; Tel: +86-29-88488007; Fax: +86-29-88494574; E-mail: lizhmin@163.com


