DOI: 10.11817/j.ysxb.1004.0609.2021-40052
LiSrBO3:Bi3+/RE3+(RE=Sm, Eu, Tb)荧光粉的合成与发光性能
鲁 雅1,张敏芝2,梁刚锋1,李春霞2,赵国良1, 2
(1. 浙江师范大学 行知学院,金华 321004;
2. 浙江师范大学 化学与生命科学学院,金华 321004)
摘 要:
以Li2CO3、SrCO3、H3BO3、Sm2(CO3)3、Eu2(CO3)3、Tb2(CO3)3、(BiO)2CO3为原料,用高温固相法合成了稀土激活离子RE3+与敏化离子Bi3+共掺杂的LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb)三个系列荧光粉。合成分两步进行,首先制备稀土单掺杂的荧光粉LiSr1-xBO3:xRE3+(RE=Sm, Eu, Tb),以确定发光强度最大的样品所对应的稀土掺杂量x;其次在RE3+最佳掺杂量下,通过改变Bi3+的掺杂量来制备LiSr1-x-yBO3: yBi3+/xRE3+(RE=Sm, Eu, Tb)共掺杂荧光粉,从而研究敏化离子对激活离子的敏化情况及其中的能量转移机理。产物用X射线粉末衍射(XRD)与扫描电镜(SEM)测试其物相结构和形貌特征,研究离子共掺杂对荧光粉发光性能的影响,同时分析了荧光粉中掺杂离子之间的能量传递现象以及能量传递机制。结果表明,铋离子的掺入均能敏化基质中稀土离子的发光,在实验的掺杂量范围内呈现先增强而后减弱的变化趋势。根据Dexter能量转移公式并利用发光强度进行计算,可知偶极-偶极相互作用是Bi3+到RE3+能量传递的主要方式。
关键词:
文章编号:1004-0609(2021)-07-1879-12 中图分类号:O611.4;O641;O482.31 文献标志码:A
引文格式:鲁 雅, 张敏芝, 梁刚锋, 等. LiSrBO3:Bi3+/RE3+(RE=Sm, Eu, Tb)荧光粉的合成与发光性能[J]. 中国有色金属学报, 2021, 31(7): 1879-1890. DOI: 10.11817/j.ysxb.1004.0609.2021-40052
LU Ya, ZHANG Min-zhi, LIANG Gang-feng, et al. Synthesis and luminescent property of LiSr1-x-yBO3: yBi3+/xRE3+ (RE=Sm, Eu, Tb), phosphors[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(7): 1879-1890. DOI: 10.11817/j.ysxb.1004.0609.2021-40052
近几十年来,对LED(Light emitting diode)的研究和应用一直如火如荼,而荧光粉作为LED的重要组成部分,被认为是显示和照明功能成功与否的先决条件[1]。迄今为止,人们已广泛开发出各类型基质的LED荧光粉,主要包括硅酸盐、铝酸盐、硼酸盐、钨/钼酸盐、硫化物及氮(氧)化物等[2-13]。硅酸盐基荧光粉的发射峰都比较窄,封装LED后白光的显色效果不佳,稳定性较差;铝酸盐基荧光粉则有煅烧温度高、抗湿性不好、抗氧化性差以及单相性的基质制备困难等诸多缺点;硫化物荧光粉稳定性欠佳、价格较高;氮(氧)化物荧光粉的合成成本高、且制备工艺复杂、难以实现工业产业化。硼酸盐基荧光粉由于拥有较高的光学损伤阈值和荧光效率、较宽的透光区域,并且制备工艺简单、合成温度低、热稳定性好,因而可作为掺杂稀土元素的一种优良发光基质材料[14-19]。由于碱金属和碱土金属的配位方式差异很大,在硼酸盐基质材料中引入碱金属和碱土金属很容易与硼酸形成各种晶体结构,这为寻找新的具有不同发光性能的材料提供了广泛的机会[20]。从文献报道来看,国内外学者研究了一些碱金属和碱土金属硼酸盐为基质的稀土单掺杂或双掺杂的荧光粉,其中LiSrBO3是比较有代表性的基质之一。文献[21-28]叙述了LiSrBO3基质中掺入Sm3+、Eu3+、Tb3+等单一稀土离子或双稀土离子作为激活离子时荧光粉的合成、结构表征、发光性能与发光机理,取得了一些成果,但往此体系中掺入敏化剂Bi3+来研究其对基质中激活离子发光性能的改善则鲜有报道。本文首先制备了三种稀土离子单掺杂的荧光粉LiSr1-xBO3:xRE3+(RE = Sm, Eu, Tb),找出发光强度最大相对应的最佳掺杂量x,再据此工艺参数来制备Bi3+/RE3+共掺杂的荧光粉LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb),并对产物进行XRD、SEM表征,通过对各系列产物发射光谱的测定来考察敏化离子(Bi3+)掺杂量对基质中稀土激活离子RE3+(RE=Sm, Eu, Tb)发光强度的影响、探讨了它们的发光性能和发光机理,以期为此类发光材料的深入研究和应用开发提供实验依据。
1 实验
1.1 试剂
Li2CO3、SrCO3、H3BO3、Sm2(CO3)3、Eu2(CO3)3、Tb2(CO3)3、(BiO)2CO3均为分析纯,且直接使用。
1.2 测试方法
用德国布鲁克公司的D8 Advance型X射线衍射仪测试产物的物相组成与晶体结构。仪器的测试条件为:采用Cu靶Kα辐射(波长λ=1.542  ),扫描的角度范围为2θ=20°~80°,管电压为40 kV,电流40 mA,狭缝宽为6.6 nm;用日本Hitachi公司的S-4800型扫描电子显微镜进行形貌测试;用英国爱丁堡公司的FLS980荧光光谱仪测定产物的激发光谱和发射光谱,数据的处理与拟合是通过Origin9软件完成的。
),扫描的角度范围为2θ=20°~80°,管电压为40 kV,电流40 mA,狭缝宽为6.6 nm;用日本Hitachi公司的S-4800型扫描电子显微镜进行形貌测试;用英国爱丁堡公司的FLS980荧光光谱仪测定产物的激发光谱和发射光谱,数据的处理与拟合是通过Origin9软件完成的。
1.3 荧光粉的制备
1.3.1 LiSr1-xBO3:xRE3+(RE=Sm, Eu, Tb)单掺杂荧光粉的制备
按化学计量比分别精确称取碳酸锂、碳酸锶、硼酸和相应的稀土碳酸盐,经研磨混合均匀后转移到刚玉坩埚内,在高温电阻炉中700 ℃煅烧3 h,再次研磨得LiSr1-xBO3:xRE3+粉体。
1.3.2 LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb)共掺杂荧光粉的制备
在确定掺杂量x的情况下,按化学计量比分别精确称取上述原料,在此增加(BiO)2CO3,研磨至少0.5 h,使原料混合均匀;在高温电阻炉内500 ℃预煅烧2 h,这可使反应更充分,以促进固相反应的进行。待冷却至室温后,再次研磨均匀,放入高温箱式电阻炉内于700 ℃下煅烧3 h,自然冷却至室温,将其研磨均匀即可得到产物。
2 结果和讨论
2.1 单掺杂LiSr1-xBO3:xEu3+(RE=Sm, Eu, Tb)荧光粉最佳掺杂量x的确定
把掺杂量x的数值(摩尔分数)选定在0.01~0.07之间,制备相应的三种稀土离子单掺杂的LiSr1-xBO3:xEu3+( RE=Sm, Eu, Tb)荧光粉,通过荧光光谱测定,将发光强度最大的样品的对应x值确定为最佳稀土掺杂量。结果表明LiSr0.97BO3:0.03Sm3+、LiSr0.95BO3:0.05Eu3+、LiSr0.94BO3:0.06Tb3+在各自的系列中发光强度最大,由此来制备稀土与铋共掺杂的荧光粉LiSr1-x-yBO3:yBi3+/xRE3+(RE=Eu, Sm, Tb)。
2.2 产物的物相表征
2.2.1 单掺杂LiSr1-xBO3:xEu3+(RE=Sm, Eu, Tb)荧光粉的物相表征
图1所示为700 ℃煅烧3 h制备出的LiSr0.97BO3: 0.03Sm3+、LiSr0.95BO3:0.05Eu3+、LiSr0.94BO3:0.06Tb3+荧光粉的XRD谱。单掺杂样品的衍射峰与LiSrBO3晶体的XRD谱(PDF 55-0935)是相吻合的,与文献[20, 22, 24, 26, 29]报道的相似,属于单斜晶系,空间群为P21/n,样品物相纯度较高,且析晶良好,表明三种稀土离子RE3+成功地进入主晶格,占据并替代了Sr2+原本在晶格中的位置,且未对主晶相的晶格结构造成破坏。
2.2.2 稀土/铋共掺杂LiSr1-x-yBO3:yBi3+/xRE3+(RE= Sm, Eu, Tb)荧光粉的物相表征
图2(a)~(c)所示分别为LiSr0.97-yBO3:0.03Sm3+/ yBi3+(y=0.000,0.005)、LiSr0.95-yBO3:0.05Eu3+/yBi3+ (y=0.02,0.03,0.04)、LiSr0.94-yBO3:0.06Tb3+/yBi3+ (y=0.03, 0.04, 0.05)荧光粉样品的XRD谱。由图2可知,三个系列样品的衍射峰与LiSrBO3晶体的XRD谱(PDF 55-0935)相吻合,也与文献[20, 22, 24, 26, 28-30]报道的相似,表明制备的荧光粉样品的物相是纯相。Sm3+(1.13  )、Eu3+(0.95
)、Eu3+(0.95  )、Tb3+(0.923
)、Tb3+(0.923  )和Bi3+(1.03
)和Bi3+(1.03  )的离子半径与Sr2+(1.21
)的离子半径与Sr2+(1.21  )较为接近,说明RE3+和Bi3+成功地进入到主晶格,可替代原先Sr2+在晶格的位置,且对主晶格的结构未造成明显破坏。
)较为接近,说明RE3+和Bi3+成功地进入到主晶格,可替代原先Sr2+在晶格的位置,且对主晶格的结构未造成明显破坏。
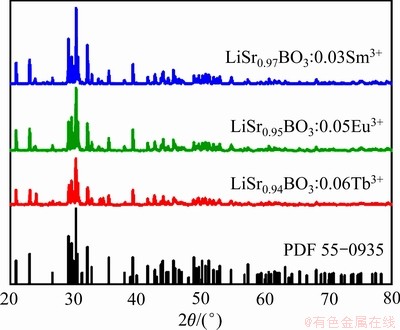
图1 LiSr1-xBO3:xEu3+(RE=Sm, Eu, Tb)荧光粉样品的XRD谱
Fig. 1 XRD patterns of LiSr1-xBO3:xEu3+(RE=Sm, Eu, Tb) phosphor samples
2.3 产物的形貌
图3(a)~(c)所示分别为在放大5000倍的情况下LiSr0.96BO3:0.03Sm3+/0.01Bi3+、LiSr0.94BO3:0.05Eu3+/ 0.01Bi3+、LiSr0.91BO3:0.06Tb3+/0.03Bi3+荧光粉的SEM像。从图3中可观察到,样品主要由不规则的块状团聚物组成,这些团聚物的尺寸有几个微米至十几微米大小,而微米尺寸的荧光粉有利于提高发光亮度。
2.4 激发和发射光谱
2.4.1 LiSr0.97BO3:0.03Sm3+荧光粉的激发和发射光谱

图2 共掺杂荧光粉样品的XRD谱
Fig. 2 XRD patterns of co-doped phosphor samples
LiSr0.97BO3:0.03Sm3+样品的激发和发射光谱如图4所示,在300~500 nm的范围内,346 nm、367 nm、377 nm、406 nm以及431 nm处均有较强的激发峰,对应于Sm3+的f→f跃迁。其中,最强的激发峰在波长406 nm,对应于Sm3+的6H5/2→4F7/2跃迁。以406 nm作为激发波长,得到样品的发射光谱,可看到在红光区域出现了三组Sm3+的特征发射峰:563 nm(4G5/2→6H5/2)、600 nm(4G5/2→6H7/2)和647 nm(4G5/2→6H9/2);最强的发射峰在波长600 nm处。同时,还观察到三组发射峰均发生了不同程度的分裂,4G5/2→6H5/2跃迁分裂为563 nm和569 nm的二重峰,4G5/2→6H7/2跃迁分裂为600 nm和607 nm的二重峰,4G5/2→6H9/2跃迁分裂为647 nm和651 nm的二重峰。Sm3+能级分裂引起了这些发射峰发生分裂,而晶体场效应是造成能级分裂的主要原因,且发生分裂的程度与Sm3+在基质中所处的格位以及主晶体的结晶程度是相关的[31]。
2.4.2 LiSr0.97-yBO3:0.03Sm3+/yBi3+荧光粉的发光性能分析
通过改变敏化离子Bi3+的掺杂量,来研究共掺杂敏化离子Bi3+与激活离子Sm3+对LiSr0.97-yBO3: 0.03Sm3+/yBi3+荧光粉的发光性能影响。图5(a)和(b)所示分别为LiSr0.97-yBO3:0.03Sm3+/yBi3+(y=0.001, 0.003, 0.005, 0.01, 0.015, 0.02)荧光粉的发射光谱以及在600 nm处发光强度随Bi3+掺杂量变化的折线图。从图5中观察到,掺杂量的改变并未改变荧光粉发射峰的位置及形状,所有荧光粉样品的发射光谱均包括563 nm、600 nm和647 nm处Sm3+的三个特征发射峰,并且均出现明显的发射峰分裂现象。Bi3+掺杂量的改变对荧光粉样品的发射光强度有着明显的影响,由图5可知,当y从0.001增加到0.003时,LiSr0.97-yBO3:0.03Sm3+/yBi3+荧光粉的发光强度随之增强;当y为0.003时,发光强度达到最强。这可能是随着Bi3+掺杂量的渐渐增加,存在更多能量转移到Sm3+的,从Bi3+到Sm3+的有效能量转移趋势也随之增大,使得发光强度有所增强。当继续增大掺入量时,样品的发光强度反而逐渐减弱,这可能源于过量的Bi3+存在聚集效应,使得离子之间距离变小而产生浓度猝灭现象。

图3 荧光粉样品的SEM像
Fig. 3 SEM images of phosphor samples
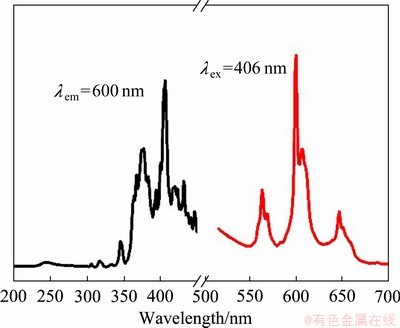
图4 LiSr0.97BO3:0.03Sm3+荧光粉的激发和发射光谱
Fig. 4 Excitation and emission spectra of LiSr0.97BO3: 0.03Sm3+ phosphor
2.4.3 LiSr0.95BO3:0.05Eu3+荧光粉的激发和发射光谱
LiSr0.95BO3:0.05Eu3+荧光粉样品的激发光谱和发射光谱如图6所示。在613 nm的监测波长下,样品的激发光谱在200~500 nm范围内均有激发峰出现。其中230~300 nm范围内的宽带激发峰归属于电子从O2-离子的已被填满的2p轨道转移到Eu3+离子未被填满的4f轨道时吸收能量而形成的电荷迁移带[32]。而从310到500 nm之间的尖峰则是对应于Eu3+离子固有的4f→4f特征跃迁,其中363nm处的激发峰对应于7F0→5D4跃迁,383 nm处的激发峰为7F0→5L7跃迁,395 nm处为Eu3+离子的7F0→5L6跃迁,412 nm以及465 nm处的激发峰则分别为7F0→5D3跃迁和7F0→5D2跃迁。其中,268 nm、395 nm处的激发峰相对较强,而395 nm处为最强激发峰,且能够与LED近紫外芯片的光谱范围相匹配。当发射光谱的激发波长为395 nm时,Eu3+的特征发射峰位于580~715 nm之间,主要的发射峰位于595 nm、613 nm、654 nm和705 nm处,分别对应于Eu3+的5D0→7F1、5D0→7F2、5D0→7F3、5D0→7F4特征跃迁。其中,最强发射峰位于613nm处,呈红光发射。

图5 LiSr0.97-yBO3:0.03Sm3+/yBi3+样品的发射光谱及其在600 nm处的发光强度
Fig. 5 Emission spectra(a) of LiSr0.97-yBO3:0.03Sm3+/ yBi3+ sample and their relative emission intensities(b) as function of Bi3+ concentration for 600 nm peak
在Eu3+的特征跃迁中,5D0→7F1为磁偶极跃迁,而5D0→7F2为电偶极跃迁。根据选择规则,当Eu3+在晶格中位于反演对称中心时,对应的5D0→7F1跃迁是被允许的,而电偶极跃迁(5D0→7F2)却是禁阻的,因而其发射光谱应以位于595 nm附近的发射峰为主。然而,当Eu3+逐渐偏离反演对称中心时,选择规则会因相反的宇称组态的混入而放宽,从而使得原先禁阻的5D0→7F2的电偶极跃迁的发生成为可能。当其处于完全非反演对称中心时,发射光谱中5D0→7F2电偶极跃迁的发射峰会成为最强发射 峰[31]。从图4-2中可清楚地观察到样品中的发射光谱中613 nm(5D0→7F2)处的发射峰强度明显强于595 nm(5D0→7F1)处的发射峰,说明了LiSr1-xBO3:Eu3+荧光粉中的Eu3+位于非反演对称中心的格位。

图6 LiSr0.95BO3:0.05Eu3+荧光粉的激发和发射光谱
Fig. 6 Excitation and emission spectra of LiSr0.95BO3: 0.05Eu3+ phosphor
2.4.4 LiSr0.95-yBO3:0.05Eu3+/yBi3+荧光粉的发光性能分析
通过对LiSr0.95-yBO3:0.05Eu3+/yBi3+(x=0.01, 0.02, 0.03, 0.04, 0.05, 0.06)荧光粉样品进行荧光测试,并与单掺杂激活离子Eu3+的样品进行比较,发现前者的发光强度好于后者,这说明了Bi3+的掺入增强了激活离子Eu3+的发光强度,但样品的发射峰的位置与形状几乎没有发生变化,只是强度有差异(见图7)。从图7中观察到,所有荧光粉样品的发射光谱均包含595 nm、613 nm、654 nm和703 nm处Eu3+的四个特征发射峰,且发射峰的位置及形状并没有因掺杂量的改变而变化。而Bi3+的掺杂量的改变对荧光粉样品的发射光强度有着明显的影响,当y从0.01增加到0.03时,LiSr0.95-yBO3:0.05Eu3+/yBi3+荧光粉的发光强度随之增强;当y=0.03时,发光强度达到最强。TANG等[33]对此做出了解释,可能是由于太少的Bi3+对发光强度的提高幅度有限,而稍高的Bi3+的掺入导致有更多的能量转移到Eu3+,自然提高了发光强度。其后,随着掺杂量y从0.03增加到0.06时,样品的发光强度逐渐减弱,这可能是由于过量的Bi3+之间中存在聚集效应,使得离子之间距离变小而产生浓度猝灭现象。上述结果也说明了Bi3+对激活离子Eu3+的敏化作用取决于Bi3+的浓度(掺杂量)。

图7 LiSr0.95-yBO3:0.05Eu3+/yBi3+的发射光谱及其在613 nm处的发光强度
Fig. 7 Emission spectra(a) of LiSr0.95-yBO3:0.05Eu3+/yBi3+ and their relative emission intensities(b) as function of Bi3+ concentration for 613 nm peak
2.5 LiSr0.94BO3:0.06Tb3+荧光粉的发光性能分析
图8所示为LiSr0.94BO3:0.06Tb3+荧光粉的激发光谱与发射光谱,通过监测544 nm的发射波长,荧光粉样品的激发光谱在230~400 nm范围内均有较强的吸收峰。其中,最强的激发峰在375 nm处,属于Tb3+的4f→4f跃迁;251 nm处的强激发峰则属于Tb3+的4f8→4f75d1跃迁。因此,选定激发波长为375 nm,样品主要的发射峰位于487 nm、544 nm、589 nm和621 nm,分别对应于Tb3+的5D4→7FJ(J=6,5,4,3)特征跃迁。其中,最强的发射峰位于544 nm(5D4→7F5),且强度远大于其他发射峰。同时,以251 nm作为激发波长时,发现其发射光谱的发射峰位置与峰型并无明显差别,但发射峰的强度不如375 nm波长激发。这可能是由于晶体场的影响,导致高能级跃迁(d-f)受到了一定程度的抑制,而低能级跃迁(f-f)的几率反而增大了。
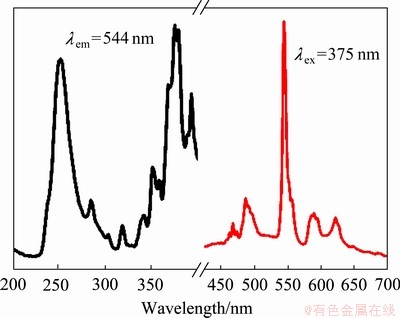
图8 LiSr0.94BO3:0.06Tb3+荧光粉的激发光谱和发射光谱
Fig. 8 Excitation and emission spectra of LiSr0.94BO3: 0.06Tb3+ phosphor
2.6 LiSr0.94-yBO3:0.06Tb3+/yBi3+荧光粉的发光性能分析
研究了不同掺杂量的Bi3+与Tb3+共掺杂对LiSrBO3基荧光粉发光性能的影响,图9(a)所示为LiSr0.94-yBO3:0.06Tb3+/yBi3+(y=0.01~0.06)荧光粉样品的发射光谱,图9(b)所示为该荧光粉在544 nm处发光强度随Bi3+掺杂量变化的折线图。从图9中可观察到,Bi3+掺杂量的改变并未改变样品发射峰的位置及形状,所有荧光粉样品的发射光谱均包含487 nm、544 nm、589 nm和621 nm的四个主要发射峰。但是随着Bi3+的掺杂量的改变,发射光的强度变化较大。起初,当y=0.01~0.03时,LiSr0.94-xBO3: 0.06Tb3+/yBi3+荧光粉的发光强度随Bi3+的掺杂量增加而增强;当y=0.03时,发光强度达到最大,这主要是稍高的Bi3+掺杂量有转移更多能量到Tb3+的趋势,自然提高了发光强度。此后,当Bi3+掺杂量继续增加,发光强度减弱。这说明了Bi3+对激活离子Tb3+的敏化作用取决于Bi3+的掺杂浓度。
2.7 敏化离子Bi3+与激活离子RE3+间的能量转移机制
Bi3+与RE3+之间的临界距离可由BLASSE[34]提出的敏化离子与激活离子之间临界距离公式进行计算:
 (1)
(1)
式中:V代表晶胞体积;N为单位晶胞中所含的分子数;Xc表示发生浓度猝灭时掺杂离子的浓度。基质LiSrBO3的晶胞体积V=312.89  3,单位晶胞中所含的分子数N=4,对LiSr0.97-yBO3:0.03Sm3+/yBi3+荧光粉而言,Bi3+与Sm3+的临界浓度的总和为X=0.033,计算得到的临界距离Rc为16.5
3,单位晶胞中所含的分子数N=4,对LiSr0.97-yBO3:0.03Sm3+/yBi3+荧光粉而言,Bi3+与Sm3+的临界浓度的总和为X=0.033,计算得到的临界距离Rc为16.5  。对于LiSr0.95-yBO3:0.05Eu3+/yBi3+荧光粉,Bi3+与Eu3+的临界浓度的总和X=0.08,计算得到的临界距离Rc为12.3
。对于LiSr0.95-yBO3:0.05Eu3+/yBi3+荧光粉,Bi3+与Eu3+的临界浓度的总和X=0.08,计算得到的临界距离Rc为12.3  。对于LiSr0.94-yBO3:0.06Tb3+/yBi3+荧光粉而言,Bi3+与Tb3+的临界浓度的总和为X=0.09,计算得到的临界距离Rc为11.8
。对于LiSr0.94-yBO3:0.06Tb3+/yBi3+荧光粉而言,Bi3+与Tb3+的临界浓度的总和为X=0.09,计算得到的临界距离Rc为11.8  。三个系列的Rc数值均大于5
。三个系列的Rc数值均大于5  ,这说明在LiSrBO3中,Bi3+与RE3+(Sm3+, Eu3+, Tb3+)离子间的能量传递是多极相互作用占主导。
,这说明在LiSrBO3中,Bi3+与RE3+(Sm3+, Eu3+, Tb3+)离子间的能量传递是多极相互作用占主导。

图9 LiSr0.94-yBO3:0.06Tb3+/yBi3+的发射光谱及其在544 nm处的发光强度
Fig. 9 Emission spectra(a) of LiSr0.94-yBO3:0.06Tb3+/yBi3+ and their relative emission intensities(b) as function of Bi3+ concentration for 544 nm peak
为进一步研究LiSr1-x-yBO3:xRE3+/yBi3+荧光粉中敏化离子Bi3+与激活离子RE3+之间的多极-多极相互作用,可根据DEXTER等[35]的能量转移公式进行计算推断:
 (2)
(2)
式中:y为敏化离子Bi3+的浓度(掺杂量);I为各y值对应的荧光粉的发光强度;c为常数;θ为6、8和10,分别对应于偶极-偶极、偶极-四极、四极-四极相互作用。利用各系列中的有关数据进行计算和拟合,得到三个线性方程分别为:lg(I/y)= -1.44653lgy+4.14245,lg(I/y) =-1.95250lgy+ 3.95911,lg(I/y) =-1.76707lg y + 4.02777;所得lg(I/y)与lg y的关系如图10(a)~(c)所示,由线性拟合所得到的θ值分别为4.33、5.86、5.30,最接近于6,因而在共掺杂荧光粉中,偶极-偶极相互作用是Bi3+到RE3+(Sm3+, Eu3+, Tb3+)能量传递的主要方式。
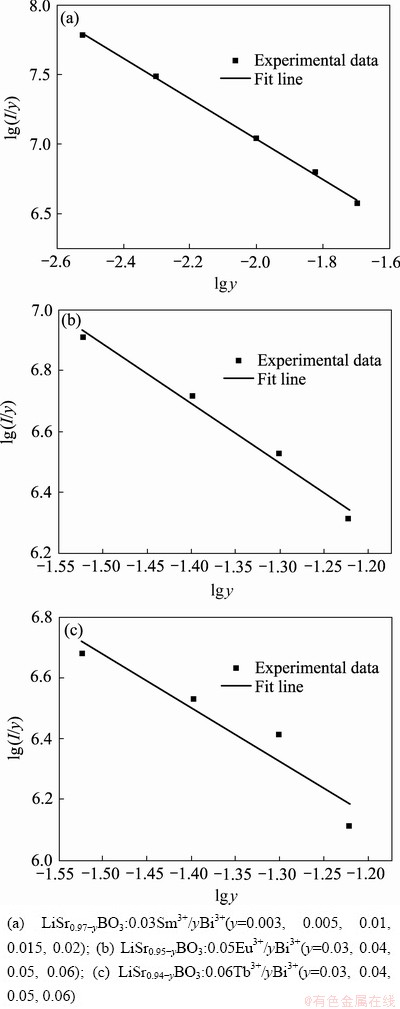
图10 荧光粉中lg(I/y)与lg y的关系
Fig. 10 Relationship between lg(I/y) and lg y of phosphors
上述三个系列产物的能量传递示意图如图11所示(以铕系列荧光粉为例)。

图11 LiSr0.95-yBO3:0.05Eu3+/yBi3+中Bi3+至Eu3+的能量传递示意图
Fig. 11 Schematic diagram of energy transfer from Bi3+ to Eu3+of LiSr0.95-yBO3:0.05Eu3+/yBi3+
2.8 色坐标
利用发射光谱,得到LiSr1-x-yBO3: yBi3+/xRE3+ (RE=Sm, Eu, Tb)三个系列荧光粉的CIE色度坐标(见表1~3),相应的坐标图见图12(a)~(c)。对于钐系列LiSr0.97-yBO3:0.03Sm3+/yBi3+来说,样品的色坐标位置均位于橙色光区域的边缘,发光颜色饱和度较高。同时,可观察到,随着Bi3+掺杂量改变,所制备的荧光粉样品的色坐标变化小,说明样品发光性能比较稳定。对于LiSr0.95-yBO3:0.05Eu3+/yBi3+系列荧光粉,将荧光粉样品的色坐标与NTSC规定的理想红光色坐标(0.670,0.330)进行了比较,样品的色坐标与其较为接近,其位置均靠近红光区域的边缘,发光颜色饱和度较高。与单掺杂Eu3+荧光粉样品的色坐标(0.608,0.372)相比,随着Bi3+掺杂量改变,所制备的LiSr0.95-yBO3:0.05Eu3+/yBi3+荧光粉样品的色坐标变化小,说明样品发光性能比较稳定。对于LiSr0.94-yBO3:0.06Tb3+/yBi3+系列荧光粉来说,所有样品的色度坐标几乎都位于绿光区域,且随着Bi3+掺杂量改变,制备的荧光粉样品颜色可由浅黄绿色变化到绿色,说明不同Bi3+掺杂量使得荧光粉的发光颜色可调。
表1 LiSr0.97-yBO3:0.03Sm3+/ yBi3+荧光粉在406 nm处的色度坐标
Table 1 Chromaticity coordinates of LiSr0.97-yBO3:0.03Sm3+/ yBi3+ phosphors excited at 406 nm

表2 LiSr0.95-yBO3:0.05Eu3+/ yBi3+荧光粉在395 nm处的色度坐标
Table 2 Chromaticity coordinates of LiSr0.95-yBO3:0.05Eu3+/ yBi3+ phosphors excited at 395 nm

表3 LiBa0.94-yBO3:0.06Tb3+/ yBi3+荧光粉在369 nm处的色度坐标
Table 3 Chromaticity coordinates of LiBa0.94-yBO3:0.06Tb3+/ yBi3+ phosphors excited at 369 nm


图12 LiSr1-x-yBO3:xRE3+/yBi3+样品的CIE坐标图
Fig. 12 CIE chromaticity diagram of LiSr1-x-yBO3
3 结论
1) 本文用固相反应法制备了三个系列的LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb)荧光粉,通过XRD、SEM对产物的物相结构和形貌进行了表征。
2) X射线衍射实验结果表明,无论是稀土离子RE3+单掺杂还是RE3+/Bi3+共掺杂,基质LiSrBO3的晶相结构未被破坏。荧光光谱测定结果表明,共掺杂荧光粉的发光强度优于单掺杂荧光粉的发光强度,说明敏化剂Bi3+对稀土激活离子RE3+有敏化作用,具体表现为随着Bi3+掺杂量的增加,发光强度先行增加然后又降低,中间出现极大值。通过分析RE3+的发光强度与Bi3+掺杂量y之间的关系发现,存在Bi3+到RE3+能量转移,且偶极-偶极相互作用是主要的能量传递方式。
3) CIE色坐标研究结果表明LiSr0.97-yBO3: 0.03Sm3+/yBi3+系列荧光粉的发光颜色为橙红色,LiSr0.95-yBO3:0.05Eu3+/yBi3+系列荧光粉为红色,LiSr0.94-yBO3:0.06Tb3+/yBi3+系列荧光粉为绿色,它们的共同点是色饱和度均较高且颜色较为稳定,具有潜在的应用前景。
4) 本实验反应温度相对较低、原料易得、操作简便、无损耗、无三废,这可为此类荧光粉的生产提供借鉴。
REFERENCES
[1] FELDMANN C, JüSTEL T, RONDA C R, et al Inorganic luminescent materials: 100 years of research and application[J]. Advanced Functional Materials, 2003, 13(7): 511-516.
[2] QIAN Xing-lu, ZOU Jun, SHI Ming-ming, et al. Development of optical-thermal coupled model for phosphor-converted LEDs[J]. Frontiers of Optoelectronics, 2019, 12: 249-267.
[3] ZHANG Xiang-ting, SHEN Tian-ze, KAN Dong-xiao, et al. Study on the local structure and luminescence properties of a Y2Mg2Al2Si2O12:Eu3+ red phosphor for white-light-emitting diodes[J]. Inorganic Chemistry, 2020, 59(14): 9927-9937.
[4] CAO Ren-ping, Lü Xin-yan, JIAO Yuming, et al. Ca3La6Si6O24:Eu3+ orange-red-emitting phosphor: Synthesis, structure and luminescence properties[J]. Materials Research Bulletin, 2020, 122: 110651.
[5] 卫 琪. 近紫外激发白光LED用硅酸盐发光材料的制备及性能研究[D]. 兰州: 兰州大学, 2020.
WEI Qi. Preparation and properties of silicate luminescent materials for n-UV excited white LED[D]. Lanzhou: Lanzhou University, 2020.
[6] 王 倩, 刘全生, 藏茂荣, 等. 铝硅酸盐基白光LED荧光粉的研究现状[J]. 应用技术学报, 2017, 17(3): 213-219.
WANG Qian, LIU QUAN-sheng, ZANG Mao-rong, et al. Research status of aluminosilicate phosphor for white LED[J]. Journal of Technology, 2017, 17(3): 213-219.
[7] 王连军, 周蓓莹, 顾士甲, 等. 硅基氧化物发光玻璃及其制备技术研究进展[J]. 无机材料学报, 2016, 31(10): 1013-1022.
WANG Lian-Jun, ZHOU Bei-Ying, GU Shi-Jia, et al. Research progress on silicon-based luminescent glass and its preparation techniques[J]. Journal of Inorganic Materials, 2016, 31(10): 1013-1022.
[8] 赵春雷, 胡运生, 陈 凯, 等. 白光LED用硅基氮(氧)化物荧光粉的研究进展[J]. 稀有金属, 2017, 41(12): 1374-1385.
ZHAO Chun-lei, HU Yun-sheng, CHEN Kai, et al. Research progress in silicon-based (oxy) nitride phosphors for phosphor-converted white LED[J]. Chinese Journal of Rare Mentals, 2017, 41(12): 1374-1385.
[9] HOERDER G J, SEIBALD M, BAUMANN D, et al. Sr[Li2Al2O2N2]:Eu2+-A high performance red phosphor to brighten the future[J]. Nature Communications, 2019, 10: 1284.
[10] 王萧慧, 李桂芳, 卫云鸽, 等. 形貌可控的NaCaGd(WO4)3:Eu3+ 红色荧光粉的制备及发光性能[J]. 无机化学学报, 2020, 36(2): 276-282.
WANG Xiao-hui, LI Gui-fang, WEI Yun-ge, et al. Morphology-controlled synthesis and luminescence properties of red-emitting NaCaGd(WO4)3:Eu3+ phosphors[J]. Chinese Journal of Inorganic Chemistry, 2020, 36(2): 276-282.
[11] 李慧玲, 李蓝苹, 饶啟亮, 等. Er3+/Yb3+共掺杂YPO4上转换发光材料的制备与性能[J]. 中国有色金属学报, 2020, 30(6): 1349-1357.
LI Hui-ling, LI Lan-ping, RAO Qi-liang, et al. Synthesis ang properties of Er3+/Yb3+ co-doped YPO4 up-conversion luminescence materials[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(6): 1349-1357.
[12] 欧 娅, 付 兵, 朱莉萍, 等. 共沉淀法制备Eu3+掺杂La3PO7荧光材料及其性能[J]. 中国有色金属学报, 2017, 27(5): 974-980.
OU Ya, FU Bing, ZHU Li-ping, et al. Co-precipitation synthesis and optical properties of Eu3+ doped La3PO7 phosphors[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(5): 974-980.
[13] NAN Shang-rui, HONG Feng, XU Hai-ping, et al. Luminescence properties and energy transfer of Tb3+, Eu3+ co-doped YTaO4 phosphors obtained via sol-gel combustion process[J]. Journal of Materials Science: Materials in Electronics, 2020, 31(16): 13688-13695.
[14] DAI W B, YE S, LI E L, et al. High quality LED lamps using color-tunable Ce3+-activated yellow-green oxyfluoride solid-solution and Eu3+-doped red borate phosphors[J]. Journal of Materials Chemistry C, 2015, 3(31): 8132-8141.
[15] YAO Wen-jiao, XU Tao, JIANG Xing-xing, et al. Ca3Be6B5O16F: the first alkaline-earth beryllium borate with fluorine anions[J]. Dalton Transactions, 2014, 43(26): 9998-10004.
[16] MüLLER M, FISCHER S, JüSTEL T. Luminescence and energy transfer of co-doped Sr5MgLa2(BO3)6:Ce3+, Mn2+[J]. RSC Advances, 2015, 5(83): 67979-67987.
[17] ZHAO Dan, MA Fa-xue, ZHANG Rui-juan, et al. A new rare-earth borate K3LuB6O12: crystal and electronic structure, and luminescent properties activated by Eu3+[J]. Journal of Materials Science: Materials in Electronics, 2017, 28(1): 129-136.
[18] ZHENG Jiang-hui, CHENG Qi-jin, WU Sun-qing, et al. Structure, electronic properties, luminescence and chromaticity investigations of rare earth doped KMgBO3 phosphors[J]. Materials Chemistry and Physics, 2015, 165: 168-176.
[19] FAWAD U, KIM H J, KHAN S, et al. Photoluminescent properties of white-light-emitting Li6Y(BO3)3:Dy3+ phosphor[J]. Solid State Sciences, 2016, 62: 1-5.
[20] CAI G M, YANG N, LIU H X, et al. Single-phased and color tunable LiSrBO3:Dy3+, Tm3+, Eu3+ phosphors for white-light- emitting application[J]. Journal of Luminescence, 2017, 187: 211-220.
[21] HERRMANN A, FRIEDRICH D, ZSCHECKEL T, et al. Luminescence properties of Sm3+ doped alkali/earth alkali orthoborates of the type XZBO(3) with X=Li, Na, Cs and Z=Ca, Sr, Ba[J]. Journal of Luminescence, 2019, 214: 116550.
[22] PITALE S S, NAGPURE I M, KUMAR V, et al. Investigations on the low voltage cathodoluminescence stability and surface chemical behaviour using Auger and X-ray photoelectron spectroscopy on LiSrBO3:Sm3+ phosphor[J]. Materials Research Bulletin, 2011, 46(7): 987-994.
[23] 刘红利, 郝玉英, 许并社. 白光发光二级管用红色荧光粉LiSrBO3:Eu3+的制备与发光性能研究[J]. 物理学报,2013, 62(10): 108504.
LIU Hong-li, HAO Yu-Ying, XU Bing-she. Preparation and photoluminescence of LiSrBO3:Eu3+ red-emitting phosphors for white leds[J]. Acta Physica Sinica, 2013, 62(10): 108054.
[24] LI Pan-lai, WANG Zhi-jun, YANG Zhi-ping, et al. Luminescent characteristics ofLiSrBO3:Eu3+ phosphor for white light emitting diode[J]. Chinese Physics Letters, 2009, 26(4): 047801.
[25] JIANG L H, ZHANG Y L, LI C Y, HAO J Q, et al. Thermoluminescence studies of LiSrBO3:RE3+ (RE=Dy, Tb, Tm and Ce)[J]. Applied Radiation and Isotopes, 2010, 68(1): 196-200.
[26] LI Pan-lai, WANG Zhi-jun, YANG Zhi-ping, et al. Luminescent characteristics of LiSrBO3:M (M=Eu3+, Sm3+, Tb3+, Ce3+, Dy3+) phosphor for white light-emitting diode[J]. Materials Research Bulletin, 2011, 44(11): 2068-2071.
[27] WANG Zhi-jun, LI Pan-lai, YANG Zhi-ping, et al. Spectra Characteristics of LiM(M=Ca, Sr, Ba)BO3:Tb3+ Phosphor[J]. Spectroscopy and Spectral Analysis, 2009, 29(11): 2914-2916.
[28] LI Pan-lai, WANG Zhi-jun, YANG Zhi-ping, et al. Spectra Characteristics of LiSrBO3:Tb3+ phosphor[J]. Chemical Research in Chinese Universities, 2009, 25(5): 610-613.
[29] CHENG W D, ZHANG H, LIN Q S, et al. Syntheses,crystal and electronic structures,and linear optics of LiMBO3(M=Sr,Ba) orthoborates[J]. Chemistry of Materials, 2001, 13(5): 1841-1847.
[30] ZHANG Zhi-wei, Lü Rui-jiao, ZHU Xiao-yan, et al. Investigation of luminescence properties and the energy transfer mechanism of LiSrBO3:Ce3+, Tb3+ phosphors[J]. Journal of Materials Science: Materials in Electronics, 2016, 27(7): 6925-6931.
[31] 胡 巍. 稀土离子激活的碱金属、碱土金属硼酸盐荧光粉的合成、发光及在LED的应用[D]. 中山大学, 2010.
HU Wei. Synthesis and luminescence of rare-earth-doped alkali and alkaline-earth borate phosphors and their application in LED[D]. Sun Yat-sen University, 2010.
[32] PIRES A M, DAVOLOS M R, MALTA O L. Eu3+-O2- associates luminescence in Ba2SiO4[J]. Journal of Luminescence, 1997, 72: 244-246.
[33] TANG An, ZHANG Ding-fei, YANG Liu, et al. Luminescent properties of a new red-emitting phosphor based on LaInO3 for LED[J]. Optoelectronics and Advanced Materials, 2011, 5(10): 1031-1034.
[34] BLASS G. Energy transfer in oxidicphosphors[J]. Philips Research Reports, 1968, 28(6): 444-445.
[35] DEXTER D L, SCHULMAN J H. Theory of concentration quenching in inorganic phosphors[J]. Journal of Chemical Physics, 1954, 22(6):1063-1070.
Synthesis and luminescent property of LiSr1-x-yBO3: yBi3+/xRE3+(RE=Sm, Eu, Tb) phosphors
LU Ya1, ZHANG Min-zhi2, LIANG Gang-feng1, LI Chun-xia2, ZHAO Guo-liang1, 2
(1. Xingzhi College, Zhejiang Normal University, Jinhua 321004, China;
2. College of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, China)
Abstract: Three series of phosphors LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb) co-doped rare earth activating ions RE3+ and sensitizing ion Bi3+ were synthesized by high temperature solid-state reaction method from Li2CO3, SrCO3, H3BO3, Sm2(CO3)3, Eu2(CO3)3, Tb2(CO3)3, (BiO)2CO3. The synthesis was carried out in two steps. First, the single-doped rare earth RE3+phosphors LiSr1-xBO3:xRE3+(RE=Sm, Eu, Tb) were prepared so as to determine the amount of rare earth doping x corresponding to the sample with the highest luminous intensity. Secondly, under the optimal doping concentration of RE3+, LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb) co-doped phosphors were prepared by changing the doping amount of Bi3+ to study the sensitization effect of sensitized ion on activated ions and the energy transfer mechanism in it. The products were tested by X-ray powder diffractometry (XRD) and scanning electron microscopy (SEM) to test its phase structure and morphological characteristics. The effect of ion co-doping on the luminescence performance of the phosphor was studied. Meanwhile, the energy transfer phenomenon and energy transfer mechanism between co-doped ions in the phosphors were analyzed. The results show that the doping of bismuth ions can all sensitize the luminescence of rare earth ions in the matrix, and within the range of the experimental doping amount, it shows a trend of first enhancing and then weakening. According to Dexter’s energy transfer formula and calculation using luminous intensity, it can be seen that dipole-dipole interaction is the main way of energy transfer from Bi3+ to RE3+.
Key words: LiSrBO3; phosphor; rare earth; co-doped; luminescent property
Foundation item: Project(51872263) supported by the National Natural Science Foundation of China
Received date: 2020-08-31; Accepted date: 2020-12-18
Corresponding author: ZHAO Guo-liang; Tel: +86-13857990881; E-mail: sky53@zjnu.cn
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51872263)
收稿日期:2020-08-31;修订日期:2020-12-18
通信作者:赵国良,教授,硕士;电话:13857990881;E-mail:sky53@zjnu.cn
摘 要:以Li2CO3、SrCO3、H3BO3、Sm2(CO3)3、Eu2(CO3)3、Tb2(CO3)3、(BiO)2CO3为原料,用高温固相法合成了稀土激活离子RE3+与敏化离子Bi3+共掺杂的LiSr1-x-yBO3:yBi3+/xRE3+(RE=Sm, Eu, Tb)三个系列荧光粉。合成分两步进行,首先制备稀土单掺杂的荧光粉LiSr1-xBO3:xRE3+(RE=Sm, Eu, Tb),以确定发光强度最大的样品所对应的稀土掺杂量x;其次在RE3+最佳掺杂量下,通过改变Bi3+的掺杂量来制备LiSr1-x-yBO3: yBi3+/xRE3+(RE=Sm, Eu, Tb)共掺杂荧光粉,从而研究敏化离子对激活离子的敏化情况及其中的能量转移机理。产物用X射线粉末衍射(XRD)与扫描电镜(SEM)测试其物相结构和形貌特征,研究离子共掺杂对荧光粉发光性能的影响,同时分析了荧光粉中掺杂离子之间的能量传递现象以及能量传递机制。结果表明,铋离子的掺入均能敏化基质中稀土离子的发光,在实验的掺杂量范围内呈现先增强而后减弱的变化趋势。根据Dexter能量转移公式并利用发光强度进行计算,可知偶极-偶极相互作用是Bi3+到RE3+能量传递的主要方式。
[5] 卫 琪. 近紫外激发白光LED用硅酸盐发光材料的制备及性能研究[D]. 兰州: 兰州大学, 2020.
[6] 王 倩, 刘全生, 藏茂荣, 等. 铝硅酸盐基白光LED荧光粉的研究现状[J]. 应用技术学报, 2017, 17(3): 213-219.
[7] 王连军, 周蓓莹, 顾士甲, 等. 硅基氧化物发光玻璃及其制备技术研究进展[J]. 无机材料学报, 2016, 31(10): 1013-1022.
[8] 赵春雷, 胡运生, 陈 凯, 等. 白光LED用硅基氮(氧)化物荧光粉的研究进展[J]. 稀有金属, 2017, 41(12): 1374-1385.
[10] 王萧慧, 李桂芳, 卫云鸽, 等. 形貌可控的NaCaGd(WO4)3:Eu3+ 红色荧光粉的制备及发光性能[J]. 无机化学学报, 2020, 36(2): 276-282.
[11] 李慧玲, 李蓝苹, 饶啟亮, 等. Er3+/Yb3+共掺杂YPO4上转换发光材料的制备与性能[J]. 中国有色金属学报, 2020, 30(6): 1349-1357.
[12] 欧 娅, 付 兵, 朱莉萍, 等. 共沉淀法制备Eu3+掺杂La3PO7荧光材料及其性能[J]. 中国有色金属学报, 2017, 27(5): 974-980.
[23] 刘红利, 郝玉英, 许并社. 白光发光二级管用红色荧光粉LiSrBO3:Eu3+的制备与发光性能研究[J]. 物理学报,2013, 62(10): 108504.
[31] 胡 巍. 稀土离子激活的碱金属、碱土金属硼酸盐荧光粉的合成、发光及在LED的应用[D]. 中山大学, 2010.
[34] BLASS G. Energy transfer in oxidicphosphors[J]. Philips Research Reports, 1968, 28(6): 444-445.


