
Interface microstructure and diffusion of vacuum annealed ceramic coating on superalloy GH202
GU Yi(古 一), XIA Chang-qing(夏长清), QIU Guan-zhou(邱冠周), WANG Zhi-fa(王志法)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
In order to improve the wear resistance and high-temperature oxidation resistance of superalloy GH202, the ultrafine-grain ceramic coating containing nano-size nickel particles was obtained by flow coat method on the surface of GH202. The interface microstructure between ceramic coating and superalloy GH202 substrate during vacuum diffusion was studied. It is shown that Al2O3 oxide layer and (Ti, N) compound exist in the alloy substrate close to interface. During long time diffusion, nano-size nickel powders gradually congregate and grow up, and the confluent interface appears, which shows that the nano-size nickel powders have the effect of restraining the coating from failure.
Key words:
GH202 superalloy; nano-size nickel powder; ceramic coating; vacuum annealing; microstructure;
1 Introduction
For applications in the first generation turbine blades, nickel-based superalloys in various wrought and cast forms, augmented by coatings since 1960s, have been successful material systems[1-4]. Protective coatings are applied for prolonging component life[5-9]. Ceramics, as coating materials, in contrast to metals, are often more resistant to oxidation, corrosion and wear, as well as have better thermal stability. During the past decade, extensive research efforts have been devoted to the development and manufacture of thermal barrier coatings(TBCs) on turbine part[10-14]. However, the inherent low ductility of the ceramic coatings and the lack of stability at high temperature are great concerns in restricting their applications.
Nano-composition coatings have wide application prospects in modifying the surface of turbine materials for their characteristics of high strength, ductility and hardness. POMEROY[15] has reported the Pt-modified aluminide coatings on single-crystalline CMSX-4 and CMSX-10 superalloy substrates, showing that Al diffuses into the bulk of the alloy via γ matrix channels and Ni diffuses outward along the same route. These inter- diffusional processes influence the long term stability of both coating and substrate. KITAYAMA and PASK[16] studied the agglomeration phenomenon of the high purity nano-size alumina powder and the results showed that it has positive effect on dispersion of the nano ceramic slurry.
In this work, a ceramic coating was developed by adding nano-size nickel particles into ultra-fine ceramic slurry, which combined both the ductility of the metallic elements and the stability of the ceramics at high temperature. Furthermore, as the failure of the coatings is mainly influenced by the interface microstructure between the coating and substrate, the interfacial inter-diffusion behavior of as-produced ceramic coating on superalloy GH202 after vacuum annealing was also investigated.
2 Experimental
The ultra-fine ceramic coating used in this experiment was prepared using the mixture of oxide powders according to optimized proportion (SiO2 52.6%, CaO 17.0%, MgO 1.8%, Al2O3 9.3%, TiO2 3.5%, BaO 8.8%, and B2O3 7.0%, mass fraction) followed by sintering, water quenching and grinding.
The aqueous ceramic slurry containing 50% (mass fraction) solids was prepared, of which more than 80% particles were less than 1.0 μm. The average particle diameter of nano-size nickel particles was about 80 nm (see Fig.1). The viscosity of the coating was adjusted using distilled and deionized water. In order to enhance
the toughness and wear-resistance, the coatings were prepared by adding 10% (mass fraction) nano-size nickel particles followed by mixing and dispersion[17-19]. The substrate used was Ni-based alloy GH202, which had been ground by a 400-grit diamond sand paper. Then, the ground surface of the GH202 was treated by acid and alkali in turn. Flow coating method was applied to preparing the specimens, which were dried in air, kept in thermo-stated container at 50 ℃ for 1 h prior to further treatment. All the samples were sintered at 1 050 ℃ for 5 min in a high-temperature furnace. The interface reaction was investigated by means of interface diffusion annealing experiments in vacuum. Coated specimens were used for diffusion annealing experiments in a high temperature vacuum diffusing furnace. The diffusion temperature was 900 ℃ and holding time was 0, 50, 100, 200, 300 and 500 h, respectively. The composition and microstructure of the specimens were analyzed by scanning electron microscope (SEM, JSM-5600LV) equipped with energy-dispersion analytical X-ray spectroscope (EDS).
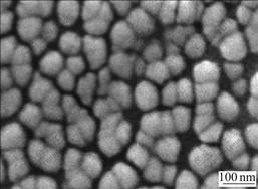
Fig.1 SEM image of nano-size nickel powders
3 Results and discussion
The interface microstructures of the coated specimens after diffusion annealing are shown in Fig.2, and the annealing time is 0, 50, 100, 200, 300 and 500 h in turn from Fig.2(a) to Fig.2(f).
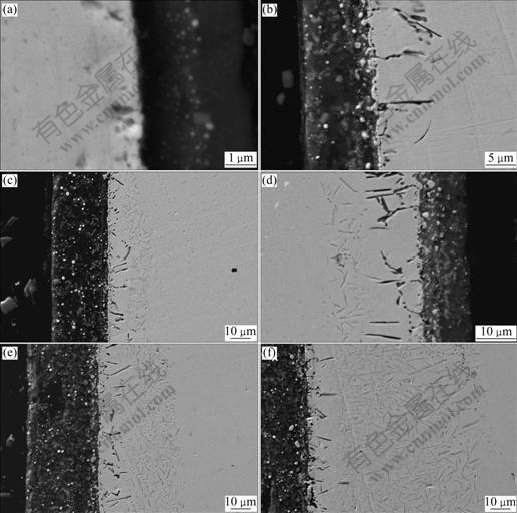
Fig.2 SEM images of interface between coating and substrate of sample annealed at 900 ℃ in vacuum for different time (without erosion): (a) 0 h; (b) 50 h; (c)100 h; (d)200 h; (e) 300 h; (f) 500 h
Fig.2 shows that nickel particles are dispersed homogeneously in the coating with no apparent aggregation. The nano-size nickel particles are mainly distributed along the coating/substrate interface as a “transition layer” (see Fig.2(a)) before annealing. However, this distribution has changed after diffusion annealing at 900 ℃ for 500 h. There are some white particles in the coating with the size of about 1 μm, and the result of linear scanning of element Ni in the coating shows that the white particles are nickel particles formatting through the aggregation of nano-size nickel particles during annealing (see Fig.3). The amorphous phases in the coating crystallize and network mica is precipitated with the increase of diffusion time (see Figs.2(b)-(f)).
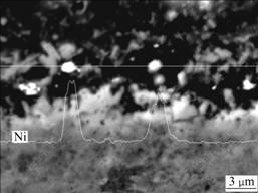
Fig.3 Linear scanning result of element Ni in coating
The enlarged cross-sectional SEM image of the specimens after vacuum annealing at 900 ℃ for 500 h is shown in Fig.4. It can be seen that the nano-size nickel particles diffuse not only to the interior of the coating but also to the substrate during the vacuum annealing, and even fuse with the substrate at the interface, which cause the interface to be metallurgically bonded. It is also shown that the nano-size nickel particles are advantage to increasing the bonding strength of the coating and the substrates.
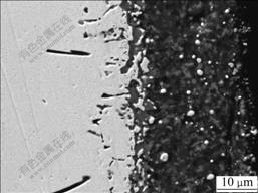
Fig.4 Metallurgical bonding of interface between coating and substrate
From Fig.2 it can also be seen that some black phases are found to extend to the substrate near the interface in the coated sample after vacuum annealing at 900 ℃. The element linear scanning of the black phases indicates that they are mainly Al2O3 intergranular oxides (see Fig.5). The same phenomenon has also been found by CHEN[20]. Furthermore, some needle-shaped precipitates emerge near the Al2O3 intergranular oxides in the substrate, and the educts layer becomes thick with the annealing time prolonging. The educts have length of about 3-10 μm and width of about 300 nm. Because the educts are fine and their quantity is small, they can hardly be identified by XRD technique. So, the planar distribution of main elements was used to qualitatively analyze the educts, and the results of the planar distributions of main elements are shown in Fig.6.
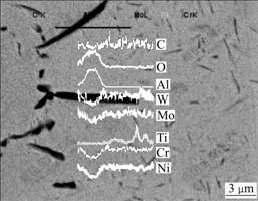
Fig.5 SEM images and element linear scanning result of black phases appearing after vacuum annealing at 900 ℃
Fig.6(a) shows the needle-shaped precipitates in the substrate. Figs.6(b)-(e) show the planar distributions of element Ti, N, O and Ni, respectively. It is observed that the elements of Ti and N are rich but O and Ni are absent in the educts, which can predicate that the educts may be (Ti, N) compounds.
All the analyses show that the Al2O3 intergranular oxides layer and (Ti, N) educts layer form in the substrate near the interface during vacuum annealing at 900 ℃. The potential reasons for this phenomenon may be as follows. Elements N and O diffuse from coating to substrate during annealing, and because the activity of Al is higher than that of element Ti, then element Al in the alloy reacts with O and N to form Al2O3 and AlN. Then, the AlN is further oxidized and element N is released. Furthermore, the released N diffuses to the inner of the alloy and reacts with Ti to form (Ti, N) compounds, so the Al2O3 layer and (Ti, N) compound layer emerge in the substrate close to the interface.
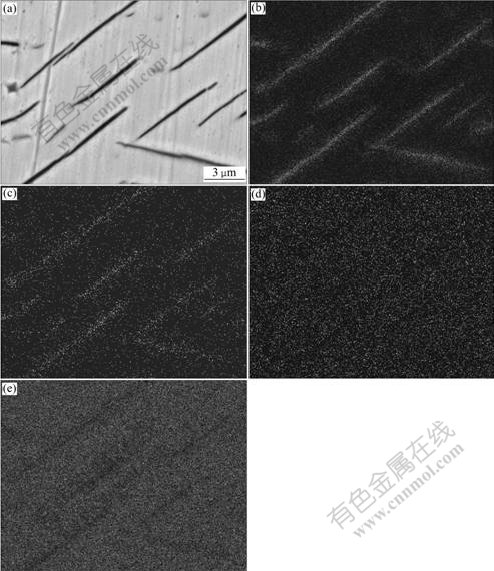
Fig.6 SEM images of needle precipitates near interface (a) and their element distributions of Ti (b), N (c), O (d) and Ni (e)
4 Conclusions
1) The nano-size nickel particles diffuse not only into the interior of the coating but also into the substrate during the vacuum annealing, and even fuse with the substrate at the interface, which causes the interface to be metallurgically bonded.
2) Al2O3 layer and (Ti, N) compound layer are formed in the substrate, and all the reaction layers become thicker and thicker with prolonging the annealing time.
References
[1] LI Min-wei, ZHU Jing-chuan, XIANG Xing-hua, YIN Zhong-da. Progress in study on ceramic/metal high-temperature thermal barrier coatings [J]. Materials Review, 2000, 14(8): 19-21. (in Chinese)
[2] GOWARD G W. Progress in coatings for gas turbine airfoils [J]. Surface and Coatings Technology, 1998, 108/109(1/3): 73-79.
[3] LEEU K N. Current status of environmental barrier coatings for Si-based ceramics [J]. Surface and Coatings Technology, 2000, 133/134: 1-7.
[4] LI M H, ZHANG Z Y, SUN X F, GUAN H R, HU W Y, HU Z Q. Oxidation and degradation of EB-PVD thermal-barrier coatings [J]. Oxidation of Metals, 2002, 58(5/6): 499-512.
[5] LI M H, ZHANG Z Y, SUN X F, LI J G, YIN F S, HU W Y, GUAN H R, HU Z Q. Oxidation behavior of sputter-deposited NiCrAlY coating [J]. Surface and Coatings Technology, 2003, 165(3): 241-247.
[6] GADOW R, LISCHKA M. Lanthanum hexaaluminate—novel thermal barrier coatings for gas turbine applications—materials and process development [J]. Surface and Coatings Technology, 2002, 151(2): 392-399.
[7] ITOH Y, SAITOH M, ISHIWATA Y. Influence of high-temperature protective coatings on the mechanical properties of nickel-based superalloys [J]. Journal of Materials Science, 1999, 34(16): 3957-3966.
[8] LI M H, SUN X F, LI J G. Oxidation behavior of a single-crystal Ni-base superalloy in air at 800 and 900 ℃ [J]. Oxidation of Metals, 2003, 59(5/6): 591-605.
[9] CAO X Q, VASSEN R, STOEVER D. Ceramic materials for thermal barrier coatings [J]. Journal of the European Ceramic Society, 2004, 24(1): 1-10.
[10] CERNUSCHI F, BIANCHI P, LEONI M, SCARDI P. Thermal diffusivity/microstructure relationship in Y-PSZ thermal barrier coatings [J]. Journal of Thermal Spray Technology 1999, 8(1): 102-109.
[11] DEMASI M T, JEANINE K. Protective coatings in the gas turbine engine [J]. Surface and Coatings Technology, 1994, 68: 1-9.
[12] WIGREN J, PEIRYD L. Thermal spray meeting the challenges of the 21st Century [C]// CODDET C. Proceedings of the 15th International Thermal Spray Conference. ASM International. OH, USA: Materials Park, 1998: 1531.
[13] MILLER R A. Thermal barrier coatings for aircraft engines: History and directions [J]. Journal of Thermal Spray Technology, 1997, 6(1): 35-42.
[14] WANG Xin, SUN Kang-ning, YIN Yan-sheng, HOU Yao-yu, ZHOU Yu. Progress of study on ceramic matrix nanocomposites [J]. Acta Materiae Compositae Sinica, 1999, 16(1): 105-110. (in Chinese)
[15] POMEROY M J. Coatings for gas turbine materials and long term stability issues [J]. Materials and Design, 2005, 26: 223-231.
[16] KITAYAMA M, PASK J A. Formation and control of agglomerates in alumina powder [J]. Journal of the American Ceramic Society, 1996, 79: 2003-2011.
[17] XIA Chang-qing, GU Yi, ZENG Fan-hao. Stability of aqueous nano-ceramic coatings with two different dispersants [J]. J Cent South Univ Technol, 2003, 10(2): 87-90.
[18] GU Yi, XIA Chang-qing, ZENG Fan-hao. Stabilized dispersion of nano-ceramic coating [J]. Trans Nonferrous Met Soc China, 2003, 13(4): 890-892.
[19] GU Yi, WANG Zhi-fa, XIA Chang-qing. The study of the crystallization behavior of nano nickel poeder/ultra-fine ceramic coating containing nano nickel powders [J]. Mining and Metallurgical Engineering, 2007, 27(3): 96-98. (in Chinese)
[20] CHEN He-xing. Study on the diffusion and reaction at the interface of thermal barrier coatings on superalloy substrate [D]. Changsha: Central South University, 2004. (in Chinese)
(Edited by YANG Bing)
Foundation item: Project supported by the Postdoctoral Science Foundation of Central South University, China
Corresponding author: GU Yi; Tel: +86-731-88877736; E-mail: guyi@mail.csu.edu.cn


