Trans. Nonferrous Met. Soc. China 24(2014) 1086-1093
Molecular dynamics simulation of relationship between local structure and dynamics during glass transition of Mg7Zn3 alloy
Zhao-yang HOU1, Rang-su LIU2, Chun-long XU1, Xue-min SHUAI1, Yu SHU1
1. Department of Applied Physics, Chang’an University, Xi’an 710064, China;
2. School of Physics and Microelectronics Science, Hunan University, Changsha 410082, China
Received 17 April 2013; accepted 5 July 2013
Abstract:
The rapid solidification process of Mg7Zn3 alloy was simulated by the molecular dynamics method. The relationship between the local structure and the dynamics during the liquid-glass transition was deeply investigated. It was found that the Mg-centered FK polyhedron and the Zn-centered icosahedron play a critical role in the formation of Mg7Zn3 metallic glass. The self-diffusion coefficients of Mg and Zn atoms deviate from the Arrhenius law near the melting temperature and then satisfy the power law. According to the time correlation functions of mean-square displacement, incoherent intermediate scattering function and non-Gaussian parameter, it was found that the β-relaxation in Mg7Zn3 supercooled liquid becomes more and more evident with decreasing temperature, and the α-relaxation time rapidly increases in the VFT law. Moreover, the smaller Zn atom has a faster relaxation behavior than the Mg atom. Some local atomic structures with short-range order have lower mobility, and they play a critical role in the appearance of cage effect in the β-relaxation regime. The dynamics deviates from the Arrhenius law just at the temperature as the number of local atomic structures begins to rapidly increase. The dynamic glass transition temperature (Tc) is close to the glass transition point in structure (T gStr).
Key words:
Mg7Zn3 alloy; glass transition; dynamics; structural relaxation; molecular dynamics simulation;
1 Introduction
Liquid-glass transition has been extensively studied by experiments and theories [1], since the first metallic glass Au75Si25was obtained by rapidly quenching the liquid [2]. During the liquid-glass transition, the structural and thermodynamic quantities show a very gentle temperature dependence. While the dynamic quantities, such as the self-diffusion coefficient and relaxation time, usually have a much more pronounced temperature dependence changing by as much as 13 orders of magnitude [3]. Therefore, the studies on the dynamic essence of the liquid-glass transition have always been the focus in the metallic glasses researches.
Since the dynamic quantities change sharply during the liquid-glass transition and the supercooled liquid is metastable, it is difficult to accurately acquire the dynamic quantities in experiments. And many reported experimental results are obtained by the direct observations of colloidal and polymer systems [4,5]. To understand the dynamic mechanism of glass transition, some theoretical models such as the free volume theory, configuration entropy theory, and mode-coupling theory have been developed [6]. Thereinto, the mode-coupling theory (MCT) [7] makes very detailed descriptions on the time and temperature dependences of the dynamics in the supercooled liquid, based on the nonlinear coupling of density fluctuation. It predicts a dynamical singularity temperature Tc above which the temperature dependence of diffusivity satisfies the power law. Then a transition from ergodic to nonergodic occurs since most atoms are localized within the cages formed by their neighbors. Since many of the predictions of MCT have been confirmed in experiments and computer simulations, it is currently regarded as one successful theory on the dynamics of glass transition [3].
The dynamic liquid-glass transition usually accompanies the change of local atomic structures. DZUGUTOV et al [8] found that the dynamic heterogeneity of supercooled Lennard-Jones liquid was associated with the icosahedral order. The researches by LI et al [9] indicated that the dynamic heterogeneities in both liquid and glass states aroused from the cooperative motion of immobile atoms. However, the studies by KAWASAKI and TANAKA [10] showed that the structural origin of dynamic heterogeneity in the colloidal glass was the medium-range crystalline bond orientational order. All of these previous studies indicated that the knowledge about the relationship between the local structure and the dynamics during the liquid-glass transition is still rather limited, and it is important to deeply understand the nature of liquid-glass transition.
Mg7Zn3 metallic glass is one of few laboratory glass alloy composed of only two simple metallic elements, and it has attracted many researches [11-15]. SUCK et al [12] measured its dynamical structure factor and vibrational density of states using neutron inelastic scattering techniques. ALTOUNIAN et al [13] investigated its crystallization characteristics through differential scanning calorimetry, X-ray photography and measurement of electrical resistance and magnetic susceptibility. Recently, the medium-range order in Mg7Zn3 metallic glass has been deeply investigated [14]. On the basis of the pervious works [14-17], in this work the rapid solidification process of Mg7Zn3 alloy will be simulated, and the relationship between the local structure and the dynamics during the liquid-glass transition will be further studied.
2 Simulation methods
The rapid solidification process of Mg7Zn3 alloy is simulated by the constant-pressure molecular dynamics (MD) techniques. The MD simulations are performed for a system containing 10000 atoms (n(Mg):n(Zn)=7:3) in a cubic box with the periodic boundary condition. The interatomic potential is the effective pair potential derived from the generalized non-local model pseudopotential (GNMP), which is based upon the first-principle interaction force in the second order perturbation theory [18,19]. For simple metals and their alloys, the accuracy and reliability of this potential have been extensively demonstrated by its computations of structural, dynamic, and thermodynamics properties [16-19]. The potential is cut off at 20 atomic unit (a.u.). The motion equations are integrated through the leap-frog algorithm with a time step of 2.5 fs.
The simulation calculations start at 973 K (the melting point Tm of Mg7Zn3 alloy is about 610 K). First of all, let the system run 50000 time steps at 973 K to obtain an equilibrium liquid determined by the energy change of system. Then, the Gaussian thermostat is adopted to decrease the system temperature to 273 K at a cooling rate of 1×1012 K/s. In the cooling process, the system totally runs 280000 time steps. At the selected temperatures: 973 K, 923 K, …, 273 K (every 50 K from 973 K to 273 K), the systems are isothermally relaxed 500 ps respectively. Finally, the structural quantities of pair distribution function (PDF) and local atomic clusters, the dynamic quantities of mean-square displacement (MSD), incoherent intermediate scattering function Fs(q,t), and non-Gaussian parameter (NGP) are detected.
3 Results and discussion
3.1 Local atomic structures
Since the PDF is a Fourier transformation of the structure factor obtained from diffraction experiments, it is widely used to detect the structural characteristics of liquids and glass solids. Figure 1 shows the temperature dependence of the total PDF during the solidification process of Mg7Zn3alloy, together with the experimental data at the liquid (673 K) and glass solid (293 K) temperature measured by the neutron diffraction [20]. With the decrease of temperature, the increase in height and the decrease in width of the first peak indicate the enhancement of short-rang order (SRO) during the rapid quenching process. The second peak gradually splits into two sub-peaks and the first sub-peak grows higher than the second one, which indicates the formation of Mg7Zn3metallic glass. The glass transition temperature TgWA is about 430 K obtained from the temperature dependence of Wendt-Abraham ratio R=gmin/gmax (gmin and gmax are the minimum and the first maximum of RDF, respectively) [21], as shown in Fig. 2. Furthermore, the simulated PDFs for the liquid and glass structures both agree well with the experimental results (see Fig. 1). This indicates that the present simulation is rather successful in presenting the solidification process of Mg7Zn3 alloy.
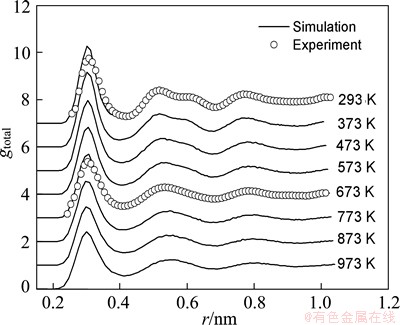
Fig. 1 Temperature dependence of total pair distribution function during solidification process of Mg7Zn3alloy
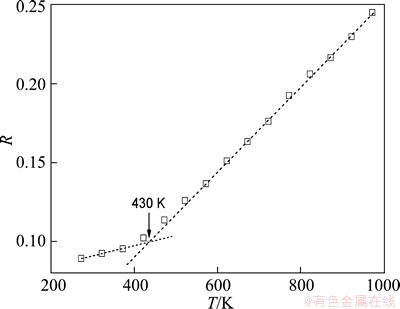
Fig. 2 Temperature dependence of Wendt-Abraham ratio R during glass formation process of Mg7Zn3alloy
The RDF reflects the atomic distribution in one- dimension statistical average and is incapable to describe the local atomic structures. Based on the work of QI and WANG [22], a new cluster structure description method of cluster-type index method (CTIM) has been proposed [14-17]. And some cluster structures have been confirmed in the experiment of HIRATA et al [23]. The CTIM adopts four indices (N, n1, n2, n3) to designate different types of basic clusters, where N is the number of the nearest-neighbor atoms, namely the coordination number (CN), and n1, n2, n3 are the numbers of 1441, 1551 and 1661 bond-types formed by the surrounding atoms with the central atom in the basic cluster, respectively. For example, the (9 3 6 0) and (10 2 8 0) stand for the Bernal polyhedrons of tri-capped trigonal prism and bi-capped square Archimedean antiprism, respectively; the icosahedron (ICO) can be expressed as (12 0 12 0); the CN14, CN15, and CN16 Frank-Kasper (FK) polyhedrons can be expressed as (14 0 12 2), (15 0 12 3), and (16 0 12 4), respectively. The schematic configurations of these basic clusters are shown in Ref. [14]. According to the CTIM, the main basic clusters in Mg7Zn3 metallic glass (273K) and their changes with temperature can be obtained, as shown in Fig. 3. From Fig. 3(a), it can be clearly seen that the Mg-centered basic clusters are mostly FK polyhedrons and their distorted cases of (14 1 10 3), (14 2 8 4), and (15 1 10 4) which possess larger CN; while the Zn-centered basic clusters are mostly icosahedron and their distorted cases (11 2 8 0), (12 2 8 2), and (12 3 6 3) which possess smaller CN. From Fig. 3(b), it can be found that the number of atomic clusters is very few at high temperature, and they begin to grow rapidly near Tm. But the growth slows down around 423 K which is near the glass transition temperature TgWA(≈430 K) obtained by the Wendt-Abraham ratio. According to the analysis above, it can be found that the Mg-centered FK polyhedron and the Zn-centered icosahedron play a critical role in the formation of Mg7Zn3 metallic glass. The glass transition temperature determined by the evolution of microstructures T gStris about 430 K.
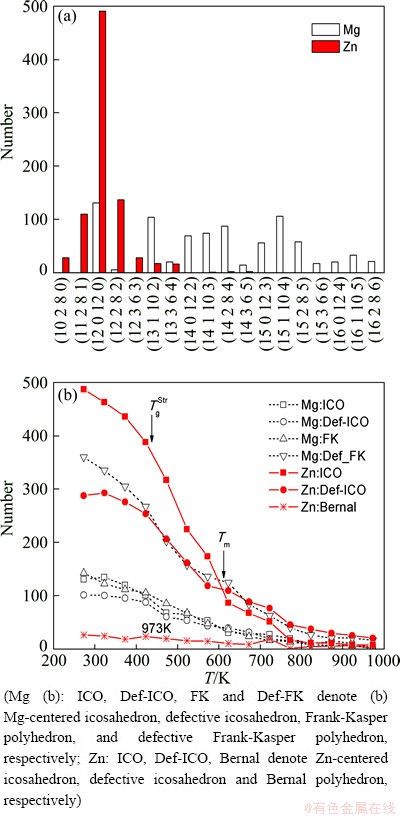
Fig. 3 Number distribution of main basic clusters in Mg7Zn3 metallic glass (273 K) (a) and their changes with temperature
3.2 Dynamics
The MSD is usually used to study the dynamic motion of atoms, which is defined as
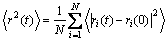 (1)
(1)
where N is the number of atoms in a system; ri(0) and ri(t) are the position vectors of the ith atom at the initial moment and the time t, respectively. Since the time dependences of MSD for Mg and Zn atoms are very similar, for clarity, only the MSD for Zn is displayed at different temperatures in Fig. 4. At a short time (t<0.2 ps), an increment in MSD proportional to t2 is observed which is due to the ballistic of atoms. For a longer time (0.2 ps<t<20 ps) a plateau appears and becomes pronounced when the temperature is lower than 573 K. The occurrence of this plateau results from the so-called “cage effect” of tagged atom. The tagged atom needs some time to escape from the cage formed by its neighboring atoms, and the cage becomes more and more rigid with decreasing temperature. So the time needed to escape from the cage increases correspondingly. In the MCT, the time window correlated with the cage effect is usually called the β-relaxation regime and the following time window is the α-relaxation regime [7].
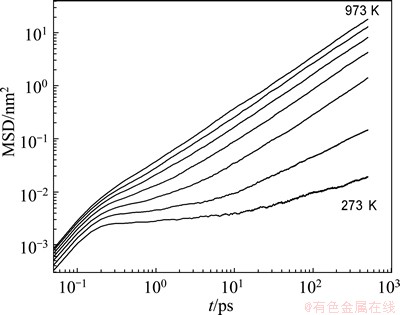
Fig. 4 Time dependence of MSD for Zn in Mg7Zn3alloy on adouble logarithmic scale at different temperatures which vary from 973 K (top) to 273 K (bottom) at interval of 100 K
The MSD can be used to calculate the self-diffusion coefficient D according to the Einstein relation as follows:
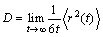 (2)
(2)
The temperature dependences of D for Mg and Zn atoms are shown in Fig. 5, together with the fits by the Arrhenius law (Eq. (3)) and MCT power law (Eq. (4)), respectively, in different temperature regions.
 (3)
(3)
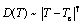 (4)
(4)
The fitting parameters in Eqs. (3) and (4) are listed in Table 1. In the high-temperature liquid, the dependence of self-diffusion coefficient follows the Arrhenius law (Eq. (3)). The activation energy of Zn atom is smaller than that of Mg atom, which indicates that the smaller Zn atom diffuses faster than the bigger Mg. The size dependence of the activation energy is believed to reflect essential features of the actual diffusion mechanism. Upon cooling from Tm, D drops faster deviating from the Arrhenius law while it is well fitted by the MCT power law (Eq. (4)) till down to 523 K. It is found that for Mg, TcMg= 449 K, γMg=1.83, and for Zn, TcZn=421 K and γZn=1.85. The critical temperature TcZn is smaller than that of Mg, indicating that smaller Zn atom has a faster relaxation. This will be further confirmed in the following calculation of relaxation time. The Tc for the entire system can be evaluated according to the expression as follows:
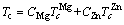 (5)
(5)
where CMgand CZnare the concentration of Mg and Zn atoms, respectively. It is Tc=440.6 K, being close to TgStr≈430 K.
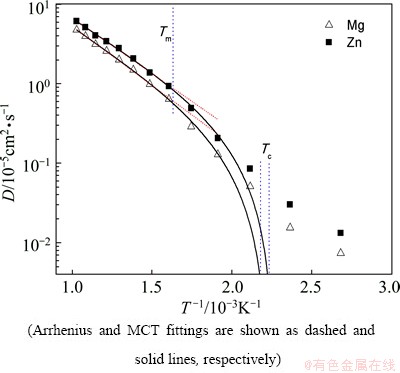
Fig. 5 Temperature dependence of diffusion coefficient D for Mg and Zn atoms during glass formation process of Mg7Zn3alloy
Table 1 Fitting parameters for diffusion coefficients
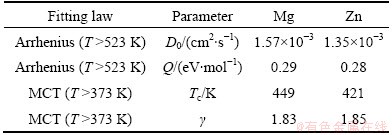
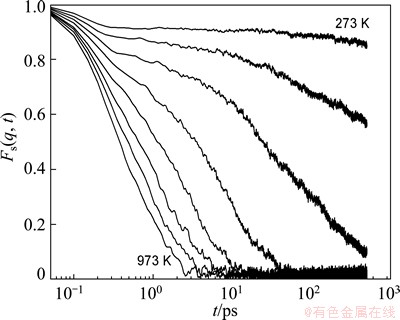
Fig. 6 Time dependence of incoherent intermediate scattering function Fs(q,t) for Zn in Mg7Zn3alloy at different temperatures which vary from 973 K (bottom) to 273 K (top) at an interval of 100 K
The incoherent intermediate scattering function is the Fourier transform of the self-part of the van Hove function [3] as follows:
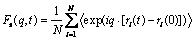 (6)
(6)
The wave vector q is normally selected to be the location of the first maximum in the static structure factor. This function is usually used to descript the relaxation dynamics of supercooled liquids and glasses. Since the time dependences of Fs(q,t) for Mg and Zn atoms are very similar, for clarity, only the correlation function Fs(q,t) for Zn is displayed at different temperatures in Fig. 6. At high temperatures, the Fs(q,t) shows the relaxation behavior of simple liquid, i.e., a quadratic dependence on short time due to the ballistic motion of atoms, and an exponential decay to zero in the following stage. At low temperatures below 573 K, the Fs(q,t) shows different relaxation behavior. A plateau with slow decay rate appears in the intermediate time and its length increases rapidly with decreasing temperature. The time windows of the plateau and the following exponential decay are consistent with that of β-relaxation and α-relaxation, respectively, comparing with the time dependence of MSD curves in Fig. 4. The reason for the existence of this plateau is the same as the cage effect. The α-relaxation time (τα) is defined as the time when the correlation function Fs(q,t) decays to e-1[3]. Its temperature dependence usually can be described well by the empirical Vogel-Fulcher-Tammann (VFT) law as follows [24-26]:
τα(T)=τα0exp[B/(T-T0)] (7)
Figure 7 shows the time dependence of α-relaxation time for Mg and Zn atoms during the glass formation process, together with the VFT fitting. The fitting parameters are listed in Table 2. With the decrease of temperature, the cage effect enhances and the time of cage-breaking rearrangement increases. Thus the α-relaxation time increases rapidly in the VFT exponential law. At 373 K, the α-relaxation time for Mg and Zn atoms are ταMg=3.11×105ps and ταZn=3.04×105ps, respectively, which are far beyond the computing power. Furthermore, it can be found that the α-relaxation time for Zn atom is shorter than that for Mg atom at the same temperature, indicating that the smaller Zn atom has a faster relaxation.
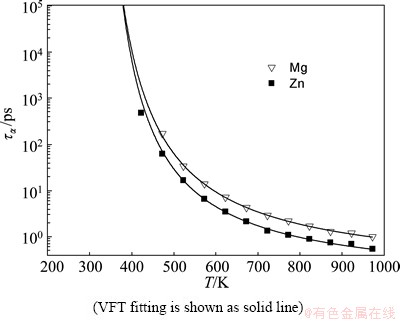
Fig. 7 Temperature dependence of α-relaxation time (τα) for Mg and Zn atoms during glass formation process of Mg7Zn3alloy
Table 2 Fitting parameters of VFT law for α-relaxation time

The non-Gaussian parameter (NGP) [27] is frequently used to quantify the dynamic heterogeneity of supercooled liquid, and is defined as
 (8)
(8)
where <>4(t)> is the mean quartic displacement. The time dependences of NGP for Zn in the Mg7Zn3alloy at different temperatures are shown in Fig. 8. For short time (t <0.2 ps), all NGPs at different temperatures are zero, which indicates that the vibration of atoms follows the Gaussian distribution. During the β-relaxation regime, all the NGP curves rise monotonically to a maximum due to the cooperative motion of cage atoms. The positions of maximum NGP shift towards longer time with the decrease of temperature. This suggests that the dynamic heterogeneity increases with the enhancement of cage effect. During the α-relaxation regime, the NGP curves drop down with the cage-breaking rearrangement, and their descending rates vary with the temperature. For the supercooled liquid above Tm, the NGP curves finally drop to zero due to the diffusion motion of atoms. Below Tc, the glassy solid is incapable of relaxing because the relaxation times are much longer than the scale of simulation time, thus the motion of atoms presents non-ergodic and the NGP curves cannot drop to zero.
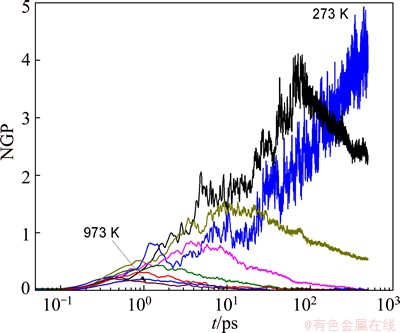
Fig. 8 Time dependence of non-Gaussian parameter (NGP) for Zn in Mg7Zn3alloy at different temperatures which vary from 973 K (bottom) to 273 K (top) at an interval of 100 K
3.3 Relationship between local atomic clusters and dynamics
According to the analyses above, it can be found that the dynamics gradually deviates from the Arrhenius law with the abundant emergence of local atomic clusters, and the glass transition temperatures determined by the change of microstructures TgStris close to the dynamic transition temperature Tc. To further elucidate the relationship between the local atomic structures and dynamics during the glass transition, Fig. 9 shows the time dependence of MSD for several main clusters in the supercooled liquid of Mg7Zn3alloy (473 K). It can be seen that these cluster atoms have smaller MSD than the average of Mg or Zn atom. And the plateaus in the MSD curves of these cluster atoms are more evident and their lengths are longer, especially for icosahedron atoms. Furthermore, from the time dependence of NGP for these atomic clusters, as shown in Fig. 10, it can be seen that the NGP curves of these cluster atoms have higher maximum than that of the average for Mg or Zn atom. And their positions all shift towards longer time, especially for icosahedron atoms. This suggests that the Mg-centered FK polyhedron and the Zn-centered icosahedron have lower mobility, and they play a critical role in the appearance of cage effect in the β-relaxation regime.
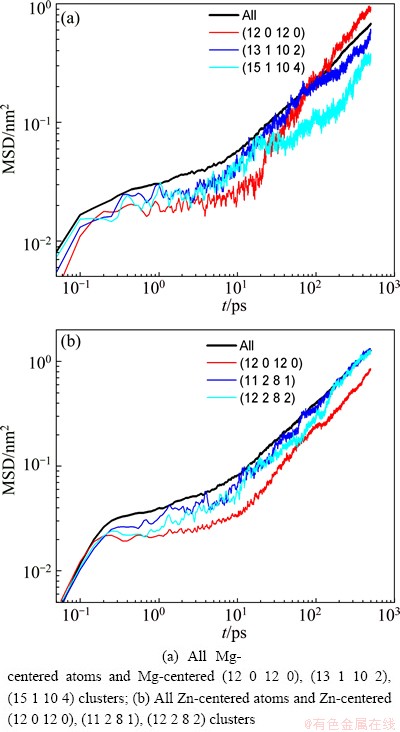
Fig. 9 Time dependence of MSD for several main clusters in supercooled liquid of Mg7Zn3alloy (473 K)
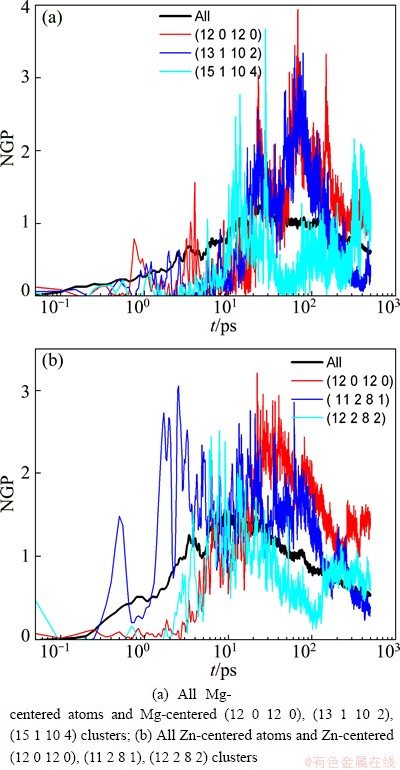
Fig. 10 Time dependence of NGP for several main clusters in supercooled liquid of Mg7Zn3alloy (473 K)
To clearly display the cage effect of local atomic cluster, Fig. 11 gives the behavior of Zn-centered icosahedron atom (12 0 12 0) during the relaxation process (473 K). It can be clearly seen that in the initial relaxation stage (t <300 ps), the atom has been vibrating around their original positions due to the cage effect formed by its neighbors, as shown in the left region of Fig. 11(a). Thus the MSD curve shows a plateau with slight growth on the whole during this relaxation stage. After escaping from the initial cage, the tagged atom is localized within a new cage, as shown in the right region of Fig. 11(a). Correspondingly, the MSD curve increases remarkably in a short period near 300 ps, and then enters into a new plateau.
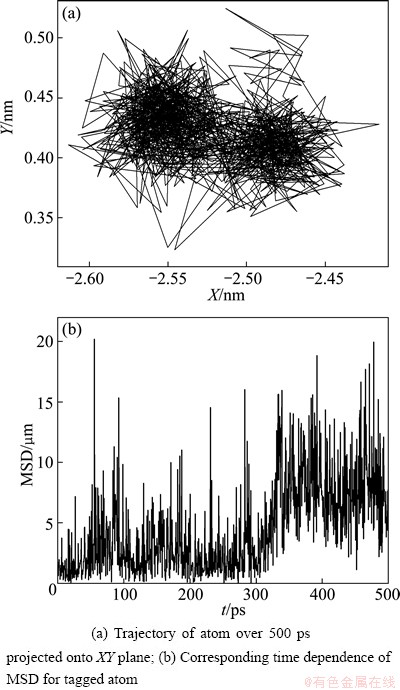
Fig. 11 Behavior of icosahedron atom during relaxation process of Mg7Zn3alloy (473 K)
4 Conclusions
1) The Mg-centered FK polyhedron and the Zn-centered icosahedron increase rapidly during the supercooled region, and play a critical role in the formation of Mg7Zn3 metallic glass. The glass transition temperature (TgStr) determined by the evolution of microstructures is about 430 K.
2) For the high-temperature liquid, the dynamics satisfies the Arrhenius law, and two stages appear during the relaxation process: the vibration motion on short time and the following diffusive motion. Upon cooling from Tm, the dynamics begins to deviate from the Arrhenius law due to the cage effect formed by the neighboring atoms of tagged atoms, and satisfies the MCT power law till down to Tc. During the relaxation process, the β-relaxation regime begins to appear near Tm and becomes more and more evident with the decrease of temperature. Simultaneously, the α-relaxation time rapidly increases in the exponential law.
3) The dynamics deviates from the Arrhenius law just at the temperature as the local atomic structures begin to rapidly increase. The dynamic glass transition temperature (Tc) is close to the glass transition point in structure (T gStr). Moreover, the Mg-centered FK polyhedron and the Zn-centered icosahedron have lower mobility, and they play a critical role in the appearance of cage effect in the β-relaxation regime.
References
[1] MA E, CHENG Y Q. Atomic-level structure and structure–property relationship in metallic glasses [J]. Prog Mater Sci, 2011, 56(4): 379-473.
[2] KLEMENT W, WILLENS R H, DUWEZ P. Non-crystalline structure in solidified gold-silicon alloys [J]. Nature, 1960, 187(4740): 869-870.
[3] KOB W. Computer simulation of supercooled liquids and glasses [J]. J Phys: Condens Matter, 1999, 11(10): R85-R115.
[4] WEEKS E R, CROCKER J C, LEVITT A C, SCHOFIELD A, WEITZ D. A three-dimensional direct imaging of structural relaxation near the colloidal glass transition [J]. Science, 2000, 287(5453): 627-631.
[5] WATANABE K, TANAKA H. Direct observation of medium-range crystalline order in granular liquids near the glass transition [J]. Phys Rev Lett, 2008, 100(15): 158002.
[6] DEBENEDETTI P G, STILLINGER F H. Supercooled liquid and glass transition [J]. Nature, 2001, 410(8): 259-267.
[7]  L. Relaxation processes in supercooled liquids [J]. Rep Prog Phys, 1992, 55(3): 241-376.
L. Relaxation processes in supercooled liquids [J]. Rep Prog Phys, 1992, 55(3): 241-376.
[8] DZUGUTOV M, SIMDYANKIN S I, ZETTERLING F H M. Decoupling of diffusion from structural relaxation and spatial heterogeneity in a supercooled simple liquid [J]. Phys Rev Lett, 2002, 89(19): 195701.
[9] LI M, WANG C Z, MENDELEV M I, HO K M. Molecular dynamics investigation of dynamical heterogeneity and local structure [J]. Phys Rev B, 2008, 77(18): 184202.
[10] KAWASAKI T, TANAKA H. Structural origin of dynamic heterogeneity in three-dimensional colloidal glass formers and its link to crystal nucleation [J]. J Phys: Condens Matter, 2010, 22(23): 232102.
[11] LI J H, DAI Y, CUI Y Y, LIU B X. Atomistic theory for predicting the binary metallic glass formation [J]. Mater Sci Eng R, 2011, 72(1-2): 1-28.
[12] SUCK J B, RUDIN H, GUNTHERODT H J, BECK H J. Dynamical structure factor and vibrational density of states of the metallic glass Mg70Zn30 measured at room temperature [J]. J Phys C: Solid State Phys, 1981, 14(17): 2305-2317.
[13] ALTOUNIAN Z, TU G H, STROM-OLSEN J O. The crystallization characteristics of Mg-Zn metallic glasses from Mg80Zn20 to Mg60Zn40 [J]. J Mater Sci, 1982, 17(11): 3268-3274.
[14] HOU Z Y, LIU R S, TIAN Z A, WANG J G. Icosahedral medium-range order formed in Mg70Zn30 metallic glass a larger-scale molecular dynamics simulation [J]. Chin Phys B, 2011, 20(6): 066102.
[15] YI X H, LIU R S, TIAN Z A, HOU Z Y, LI X Y, ZHOU Q Y. Formation and evolution properties of clusters in liquid metal copper during rapid cooling processes [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(1): 33-39.
[16] HOU Z Y, LIU L X, LIU R S, TIAN Z A, WANG J G. Short-range and medium-range order in Ca7Mg3 metallic glass [J]. J Appl Phys, 2010, 107(8): 083511.
[17] ZHOU L L, LIU R S, TIAN Z A, LIU H R, HOU Z Y, ZHU X M, LIU Q H. Formation and evolution characteristics of bcc phase during isothermal relaxation processes of supercooled liquid and amorphous metal Pb [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 588-597.
[18] WANG S, LAI S K. Structure and electrical resistivities of liquid binary alloys [J]. J Phys F: Met Phys, 1980, 10(12): 2717-2737.
[19] L D H, LI X R, WANG S. Variational calculation of Helmholz free energies with applications to the sp-type liquid metals [J]. J Phys F Met Phys, 1988, 16(3): 309-321.
[20] ANDONOV P, CHIEUX P. Structure study of eutectic Mg0.72Zn0.28 alloy: I Local order in the amorphous and liquid states Comparison with the crystalline phase Mg51Zn20 [J]. J Non-Cryst Solids, 1987, 93(2-3): 331-349.
[21] WENDT H R, ABRAHAM F F. Empirical criterion for the glass transition region based on Monte Carlo simulations [J]. Phys Rev Lett, 1978, 41(18): 1244-1246.
[22] QI D W, WANG S. Icosahedral order and defects in metallic liquids and glasses [J]. Phys Rev B, 1991, 44(2): 4950-4963.
[23] HIRATA A, GUAN P F, FUJITA T, HIROTSU Y, INOUE A, YAVARI A R, SAKURAI T, CHEN M W. Direct observation of local atomic order in a metallic glass [J]. Nat. Mater. 2010, 10(1): 28-33.
[24] VOGEL H. The temperature dependence of the viscosity of liquids act [J]. Phys Zeit, 1921, 22: 645-646.
[25] FULCHER G S. Analysis of recent measurements of the viscosity of glasses [J]. J Am Ceram Soc, 1925, 8(6): 339-355.
[26] TAMMAN G, HESSE W. The dependence of the viscosity on the temperature applied for supercooled liquids [J]. Z Anorg Allg Chem, 1926, 156(1): 245-257.
[27] RAHMAN A. Correlations in the Motion of Atoms in Liquid Argon [J]. Phys Rev A, 1964, 136(19): 405-411.
Mg7Zn3合金玻璃转变过程局域结构与动力学关联的分子动力学模拟
侯兆阳1, 刘让苏2, 徐春龙1, 帅学敏1, 舒 瑜1
1. 长安大学 应用物理系,西安 710064;
2. 湖南大学 物理与微电子科学学院,长沙 410082
摘 要:采用分子动力学方法对Mg7Zn3合金快速凝固过程进行计算机模拟,研究玻璃转变过程局域结构与动力学之间的关联。结果表明:以Mg原子为中心的FK多面体和以Zn原子为中心的二十面体局域结构,对Mg7Zn3金属玻璃的形成起关键性作用。Mg(Zn)原子的扩散系数在熔点附近开始偏离Arrhenius关系,而满足幂指数规律。根据均方位移、非相干中间散射函数和非Gauss函数等时间相关函数,发现:随着温度的降低,β驰豫越来越显著,α弛豫时间以VFT指数规律迅速增加;而且半径较小的Zn原子比Mg原子呈现较快的弛豫动力学行为。另外,部分短程有序局域原子结构具有较慢的动力学行为,对β驰豫中笼子效应起主导作用;并随着其数目的大量出现,体系扩散系数开始偏离Arrhenius关系,玻璃形成过程微观结构转变温度T gStr与动力学转变温度Tc非常接近。
关键词:Mg7Zn3合金;玻璃转变;动力学;结构弛豫;分子动力学模拟
(Edited by Chao WANG)
Foundation item: Project (51101022) supported by the National Natural Science Foundation of China; Project (CHD2012JC096) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Zhao-yang HOU; Tel: +86-29-82334891; E-mail: zhaoyanghou@163.com
DOI: 10.1016/S1003-6326(14)63166-6
Abstract: The rapid solidification process of Mg7Zn3 alloy was simulated by the molecular dynamics method. The relationship between the local structure and the dynamics during the liquid-glass transition was deeply investigated. It was found that the Mg-centered FK polyhedron and the Zn-centered icosahedron play a critical role in the formation of Mg7Zn3 metallic glass. The self-diffusion coefficients of Mg and Zn atoms deviate from the Arrhenius law near the melting temperature and then satisfy the power law. According to the time correlation functions of mean-square displacement, incoherent intermediate scattering function and non-Gaussian parameter, it was found that the β-relaxation in Mg7Zn3 supercooled liquid becomes more and more evident with decreasing temperature, and the α-relaxation time rapidly increases in the VFT law. Moreover, the smaller Zn atom has a faster relaxation behavior than the Mg atom. Some local atomic structures with short-range order have lower mobility, and they play a critical role in the appearance of cage effect in the β-relaxation regime. The dynamics deviates from the Arrhenius law just at the temperature as the number of local atomic structures begins to rapidly increase. The dynamic glass transition temperature (Tc) is close to the glass transition point in structure (T gStr).


