文章编号:1004-0609(2011)09-2298-06
草酸钴沉淀过程的溶液体系演变模拟
何显达1, 2, 4, 叶红齐1, 许开华2, 陈永明3, 邓 伟2, 周继锋2, 蒯 勤1, M. HOUNSLOW4
(1. 中南大学 化学化工学院,长沙 410083;2. 深圳市格林美高新技术股份有限公司,深圳 518104;
3. 中南大学 冶金科学与工程学院,长沙 410083;
4. Department of Chemical and Process Engineering, University of Sheffield, Mappin Street S1 3JD, UK)
摘 要:通过pH值在线检测和Co浓度离线分析方法,结合热力学数据建立的平衡模型,对草酸钴沉淀过程的溶液体系进行动态模拟。结果表明,随着沉淀反应的进行,体系pH值和过饱和度不断下降,沉淀率则逐渐升高,沉淀过程依次经历诱导期、成核期和生长期。在较高的反应物浓度下,沉淀反应速度更快,体系pH值和过饱和度下降速度随之增大,而沉淀率也有所增加。SEM和激光粒度分析结果表明,较高的反应物浓度下所得草酸钴粉体的一次晶粒粒径较小,易于团聚形成尺寸较大的刺球状团聚体。通过引入Davies活度系数,pH值在线分析模型和Co浓度离线分析模型吻合度高,模拟结果重现性好。
关键词:
中图分类号: O645;TF123 文献标志码:A
Simulation on evolution of solution system in
precipitation of cobalt oxalate
HE Xian-da1, 2, 4, YE Hong-qi1, XU Kai-hua2, CHEN Yong-ming3,
DENG Wei2, ZHOU Ji-feng2, KUAI Qin2, M. HOUNSLOW4
(1. College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Shenzhen GEM High-Tech Co., Ltd., Shenzhen 518116, China;
3. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
4. Department of Chemical and Process Engineering, University of Sheffield, Mappin Street S1 3JD, UK)
Abstract: Based on online pH value testing and offline Co concentration analysis, a thermodynamic equilibrium model was established for dynamic simulation on precipitation of cobalt oxalate. The obtained results indicate that the precipitation process undergoes induction, nucleation and growth period successively along with decreased pH value and supersaturation, and increases the precipitation ratio. At higher reactant concentration the pH value and supersaturation fall down rapidly as a result of the faster reaction rate, and the precipitation ratio is slightly larger. SEM and laser particle size analysis demonstrate that at higher reactant concentration the primary particles generated with smaller size tend to assemble into spiky agglomerates with larger particle size. By introduction of Davies activity coefficient into the thermodynamic model, the higher consistency is achieved between the online pH based simulation and the offline Co concentration based simulation, and the high repeatability in the simulation is attained.
Key words: cobalt oxalate; precipitation; powder; supersaturation; thermodynamic
液相沉淀是制备粉体材料的重要方法,溶液化学的性质对沉淀粉体的粒径、形貌和组成等特征都有显著影响,因而沉淀体系离子平衡及其分布特征逐渐成为液相制备粉体材料领域的重要研究内容[1-5]。目前,有关离子平衡的研究主要集中在难溶无机盐溶解度的热力学计算,即固-液平衡条件下各相关物种浓度的总和,绘制出的pH—lgc溶解度图可为沉淀粉体制备过程提供理论指导。然而,沉淀过程通常远离固-液平衡状态,在很高的过饱和度下发生,是一个复杂的动态过程,因此pH—lgc溶解度图的应用受到限制。沉淀率和过饱和度是沉淀工艺最重要的两个参数,直接关系到工艺的经济可行性和所制粉体性能[6-7]。目前,通常以离线方式分析溶液中目标物种浓度[8-11],进而计算沉淀率和过饱和度。由于大部分沉淀过程都极其迅速,而离线分析存在时间上的滞后性,因此测量结果很难适时反映溶液中物质浓度的实际变化情况。虽然也可采用离子选择性电极直接在线分析离子浓度[12],但电极的种类十分有限。pH电极是最常用的离子选择性电极,大部分的沉淀过程都伴随着pH值的变化,有可能构建离子平衡的热力学模型定量关联pH值与金属元素的浓度[9-11, 13],进而通过pH电极的实时测量结果计算出沉淀率和过饱和度,最终为沉淀过程的动态变化提供一种便捷有效的在线模拟分析方法。
草酸盐粉体是制备金属氧化物材料的重要前驱 体[14-18]。液相沉淀法作为制备草酸盐粉体的主要手段,已普遍用于草酸钴和草酸稀土等粉体的工业化生产[19-21]。本文作者以草酸钴的间歇式沉淀过程为研究对象,根据在线检测的pH值及离线分析的Co总浓度值,结合热力学平衡计算的模型,详细考察草酸钴沉淀过程中沉淀率和过饱和度的演变规律,探讨采用pH电极实时在线监测研究液相沉淀过程的可行性。
1 热力学平衡模型
草酸钴的沉淀反应体系以Co(NO3)2为金属剂,H2C2O4和K2C2O4的混合溶液为沉淀剂,反应在酸性条件下进行,溶液中存在的离子反应及其平衡常数如表1所列。若不考虑活度效应,以浓度直接替代活度,分别以[Co]T、[Ox]T表示溶液中钴和草酸根的平衡总浓度,[Co]、[Ox]、[H]、[K]、[N]分别为游离态Co2+、C2O42-、H+、K+和NO3-的平衡浓度。根据质量作用定律分别建立钴总浓度和草酸根总浓度的平衡方程式如下:
![]() (1)
(1)
![]() (2)
(2)
基于电中性原理可得:
![]()
![]() (3)
(3)
表1 Co(NO3)2-H2C2O4-K2C2O4体系的化学反应及平衡常 数[22-23]
Table 1 Chemical equations and their equilibrium constants at 25 ℃ in Co(NO3)2-H2C2O4-K2C2O4 system (25 ℃)[22-23]

分别以[Co]0、[Ox]0表示溶液中钴和草酸根的初始总浓度,[C]s表示草酸钴悬浮颗粒的浓度,由物料平衡可得:
![]() (4)
(4)
![]() (5)
(5)
若考虑到活度效应的影响,则必须引入活度系数γi,活度ai为浓度ci与活度系数γi的乘积:
![]() (6)
(6)
用Davies方程计算活度系数γi [24]:
![]() (7)
(7)
式中:I为溶液的离子强度,
![]() (8)
(8)
联立方程式(1)~(5)构建热力学平衡模型,其中共有[H]、[K]、[N]、[Co]0、[Ox]0、[Co]T、[Ox]T、[Co]、[Ox]、[C]s等10个变量,只要任意赋值于其中5个变量,即可求解出其他5个变量的值。
钴的沉淀率定义式如下:
![]() (9)
(9)
将离线测量的钴离子总浓度[Co]T代入式(9)即可计算出钴的沉淀率;也可由在线测量的pH值,加上已知的[K]、[N]、[Co]0、[Ox]0,通过热力学平衡模型求解得[Co]T,进而由式(9)计算出钴的沉淀率Rp。
过饱和度的定义式如下:
![]() (10)
(10)
将离线测量的钴离子总浓度[Co]T和已知的[K]、[N]、[Co]0、[Ox]0一同输入热力学平衡模型,即可求解出[Co]、[Ox]值,进而按式(10)计算出过饱和度;也可将在线测定的pH值连同已知的[K]、[N]、[Co]0、[Ox]0输入热力学平衡模型,求解得出[Co]、[Ox]值,再由式(10)计算出过饱和度S。
2 草酸钴沉淀试验
按体积比1?24的比例配制H2C2O4和K2C2O4的混合溶液,将其与等体积等浓度的Co(NO3)2溶液同时迅速倒入烧杯中,用水浴控制温度在25 ℃左右,搅拌反应1.5 h后进行液固分离,滤饼在110 ℃下真空干燥。分别考察反应物浓度为0.02和0.04 mol/L两种情况下的草酸钴沉淀过程,即混合后初始的钴总浓度[Co]0分别为0.01和0.02 mol/L。沉淀反应过程中,采用PHS-4A型智能酸度计在线监测体系pH值的变化,定时取样,采用原子吸收分光光度计(日本岛津AA-7000)离线测量溶液中钴的总浓度。分别采用扫描电镜(北京中科 KYKY-2800B)和激光粒度仪(珠海欧美克 LS800)对草酸钴粉体的形貌和粒径进行表征。
3 结果与讨论
草酸钴沉淀过程中沉淀率Rp的演变规律如图1所示。由图1可知,pH在线分析模型和钴浓度离线分析模型所揭示的沉淀率演变规律完全一致,即随着沉淀反应的进行,体系pH值不断下降,而草酸钴的沉淀率则不断升高。钴离子的初始浓度对沉淀过程有显著影响。随着钴离子初始浓度的提高,沉淀反应速度加快,体系很快达到平衡,终点pH值随之减小,而沉淀率则有所增加。因此,在草酸钴粉体生产过程中可以适当提高反应物浓度,从而提高生产效率和产品的产率。
由图1还可以看出,在热力学平衡模型中若不考虑活度效应,则所计算出的沉淀率与离线分析的实测值相差较大,且随着反应物浓度的提高,两者差值不断增加。相比之下,在热力学平衡模型中引入Davies活度因子,计算出的沉淀率则与离线分析的实测值非常吻合。
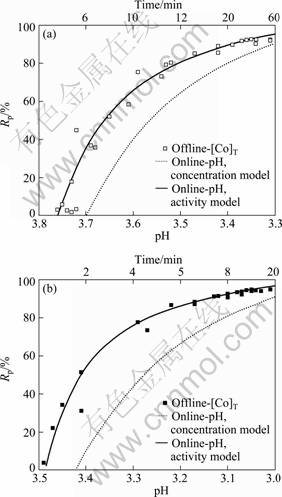
图1 不同钴离子初始浓度时草酸钴沉淀过程中pH值的演变规律
Fig.1 Evolution of pH value during cobalt oxalate precipitation at different initial concentrations of Co2+: (a) 0.01 mol/L; (b) 0.02 mol/L
引入Davies活度因子,对草酸钴沉淀过程中过饱和度的演变规律进行热力学动态模拟,结果如图2所示。随着反应的进行,体系pH值和过饱和度不断下降,沉淀过程依次经历了诱导、成核和晶体生长3个阶段。pH值在线分析模型计算的过饱和度较之于钴浓度离线分析模型结果偏低。反应的前期阶段,两者的一致性较好,但随着反应的进行,两者偏差不断增大。
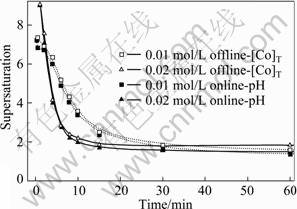
图2 不同反应物浓度时草酸钴沉淀过程中过饱和度的演变规律
Fig.2 Evolution of supersaturation during cobalt oxalate precipitation at different reactant concentrations
基于pH值在线检测结果和钴浓度离线分析模型所得过饱和度及其偏差列于表2。两种分析模型所得过饱和度平均偏差均可控制在10%以内,而在反应中前期,两者偏差均小于5%,但在反应末期,沉淀反应接近化学平衡时,两者偏差达到15%左右。这可能是由于反应末期体系处在较低的pH下,量程靠近pH计的测量下限,因此出现了较大的测量误差。
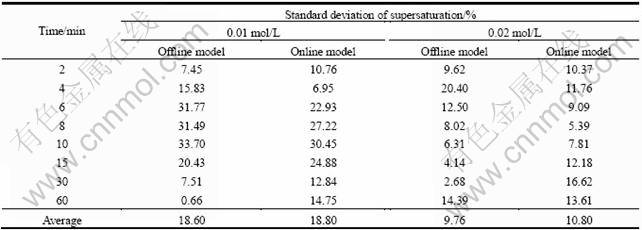
数据的重现性是判定分析方法优劣的重要指标,表3对两种分析模型的模拟结果重现性进行了对比分析。由表3可知,当初始的钴总浓度为0.01 mol/L时,过饱和度的计算值标准差为18%左右,而当初始的钴总浓度为0.02 mol/L时,标准差为10%左右。沉淀过程的不同阶段分析结果的重现性有较大差别。成核阶段反应速度非常快,数据重复性差,晶体生长阶段反应速度较慢,数据波动小,重复性较好。在线分析模型在成核阶段的结果重现性要明显优于离线分析模型,而在晶体生长阶段,离线分析模型的结果重复性更好。
表2 不同反应物浓度时在线分析和离线分析模型所得过饱和度及其偏差
Table 2 Supersaturation and its deviation obtained by online and offline analysis model at different reactant concentrations
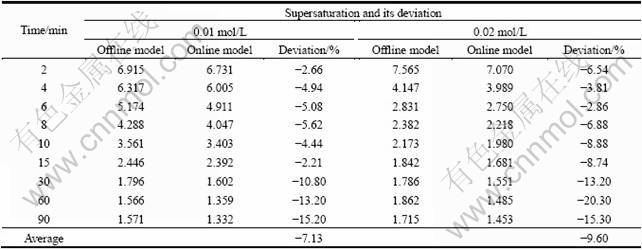
表3 不同反应物浓度时在线分析与离线分析模型所得过饱和度及其标准偏差
Table 3 Standard deviation of supersaturations obtained by online and offline analysis model at different reactant concentrations
不同反应物浓度下所得草酸钴粉体的形貌和粒度分别如图3和4所示。反应物浓度较低时,所得草酸钴晶粒一次粒径较大(见图3(a)),分散性好;随着反应物浓度的提高,生成的草酸钴晶粒一次粒径随之减小。这与图2所示过饱和度模拟分析结果相一致,即在高反应物浓度下,成核期的过饱和度较大,颗粒瞬间爆发成核,生成的晶粒较小,一般具有较高的表面能,易于团聚形成大尺寸的刺球状团聚体。
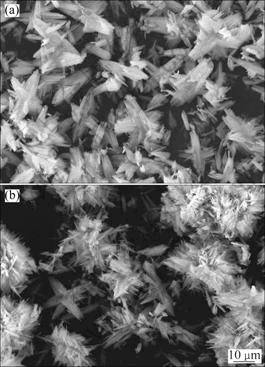
图3 不同反应物浓度条件下所得草酸钴粉体的SEM像
Fig.3 SEM images of cobalt oxalate powders prepared at different reactant concentrations: (a) 0.01 mol/L; (b) 0.02 mol/L
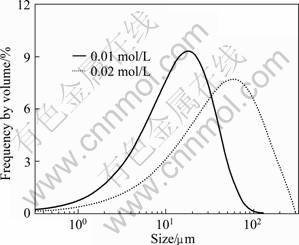
图4 不同反应物浓度条件下所得草酸钴粉体的粒径分布
Fig.4 Particle size distributions of cobalt oxalate powders prepared at different reactant concentrations
4 结论
1) 对溶液体系的热力学模拟结果表明,随着草酸钴沉淀反应的进行,体系pH值和过饱和度不断下降,沉淀率则逐渐升高,沉淀过程依次经历诱导期、成核期和生长期。在较高的反应物浓度下,沉淀反应速度更快,体系pH值和过饱和度下降速度随之增大,而沉淀率也有所增加。
2) 引入Davies活度因子,pH值在线分析模型和Co浓度离线分析模型均能很好地模拟草酸钴沉淀过程的动态演变,两者的模拟结果偏差控制在10%之内,具有较好的吻合性和可靠性。
3) SEM和激光粒度分析结果表明,较高的反应物浓度下所得草酸钴粉体一次晶粒粒径较小,易于团聚形成尺寸较大的刺球状团聚体。
REFERENCES
[1] RUBATTEL S, LEMAITRE J, BOWEN P, RING T A. Aqueous solubility of Y, Ba and Cu oxalates in the system [Y(OH)3, Ba(OH)2, Cu(OH)2]-H2C2O4-[HNO3/NaOH]-H2O[J]. Journal of Crystal Growth, 1994, 135: 135-144.
[2] DENG X, CHEN Z. Preparation of nano-NiO by ammonia precipitation and reaction in solution and competitive balance[J]. Materials Letters, 2004, 58: 276-280.
[3] 彭忠东, 杨建红, 邹 忠, 刘业翔, 李自强. 共沉淀法制备掺杂氧化锌压敏陶瓷粉料热力学分析[J]. 无机材料学报, 1999, 14(5): 733-738.
PENG Zhong-dong, YANG Jian-hong, ZOU Zhong, LIU Ye-xiang, LI Zi-qiang. Thermodynamic analysis on preparing doped zinc oxide varistor ceramic powders by coprecipitation process[J]. Journal of Inorganic Materials, 1999, 14(5): 733-738.
[4] ZHAN Jing, ZHUANG Chuan-fu, LI Tie-jing, WU Jian-hui. Thermodynamic analysis on preparation of fibrous NiO precursor powders with oxalate precipitation process[J]. Transactions of Nonferrous Metals Society of China, 2005, 15(4): 926-930.
[5] FAN You-qi, ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui. Thermodynamic equilibrium calculation on preparation of copper oxalate precursor powder[J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 454-458.
[6] BRAMLEY A S, HOUNSLOW M J, RYALL R L. Aggregation during precipitation from solution. Kinetics for calcium oxalate monohydrate[J]. Chemical Engineeing Science, 1996, 52(5): 747-757.
[7] KUCHER M, BABIC D, KIND M. Precipitation of barium sulfate: Experimental investigation about the influence of supersaturation and free lattice ion ratio on particle formation[J]. Chemical Engineering and Processing, 2006, 45: 900-907.
[8] AOUN-HABBACHE M, GUILLEMET-FRITSCH S, LEMAITRE J, JONES A. Investigation of nucleation and crystal growth kinetics of nickel manganese oxalate[J]. Journal of Crystal Growth, 2005, 279: 531-539.
[9] MOSTAFA M G, MATSUMOTO A, WASE K, KISHI Y. Simulation of chemical phenomena in the Co(NO3)2-Na2CO3-H2O system based on a thermodynamic model[J]. Hydrometallurgy, 2000, 57: 97-108.
[10] SOARE L C, LEMAITRE J, BOWEN P, HOFMANN H. A thermodynamic model for the precipitation of nanostrudtured copper oxalates[J]. Journal of Crystal Growth. 2006, 289: 278-285.
[11] 程文婷, 李志宝, 柯家骏. MgCO3·3H2O 晶体生长及晶形的影响因素[J]. 中国有色金属学报, 2008, 18(专辑1): 230-235.
CHENG Wen-ting, LI Zhi-bao, KE Jia-jun. Effect factors of crystal growth and morphology of MgCO3·3H2O[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(Special 1): 230-235.
[12] ZAUNER R, JONES A G. Determination of nucleation, growth, agglomeration and disruption kinetics from experimental precipitation data: the calcium oxalate system[J]. Chemical Engineering Science, 2000, 55: 4219-4232.
[13] SOARE L C, BOWEN P, LEMAITRE J, HOFMANN H. Precipitation of nanostructured copper oxalate: Substructure and growth mechanism[J]. The Journal of Physical Chemistry B, 2006, 110: 17763-17771.
[14] LIANG Y, YANG S, YI Z, LEI X, SUN J, ZHOU Y. Low temperature synthesis of a stable MoO2 as suitable anode materials for lithium batteries[J]. Materials Science and Engineering B, 2005, 121: 152-155.
[15] SALAVATI-NIASARI M, MOHANDES F, DAVAR F. Preparation of PbO nanocrystals via decomposition of lead oxalate[J]. Polyhedron, 2009, 28: 2263-2267.
[16] AHMAD T, VAIDYA S, SARKAR N, GHOSH S, GANGULI A K. Zinc oxalate nanorods: a convenient precursor to uniform nanoparticles of ZnO[J]. Nanotechnology. 2006, 17: 1236-1240.
[17] 管洪波, 王 培, 王 晖, 赵璧英, 朱月香, 谢有畅. 高比表面纳米MgO的制备及其影响因素研究[J]. 物理化学学报, 2006, 22(7): 804-808.
GUAN Hong-bo, WANG Pei, WANG Hui, ZHAO Bi-ying, ZHU Yue-xiang, XIE You-chang. Preparation of nanometer magnesia with high surface area and study on the influencing factor of the preparation process[J]. Acta Phys -Chim Sin, 2006, 22(7): 804-808.
[18] 廖春发, 梁 勇, 陈辉煌. 由草酸钴热分解制备Co3O4及其物性表征[J]. 中国有色金属学报, 2004, 14(12): 2131-2136.
LIAO Chun-fa, LIANG Yong, CHEN Hui-huang. Preparation and characterization of Co3O4 by thermal decomposition from cobalt oxalate[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(12): 2131-2136.
[19] 袁 平. 草酸钴沉淀工艺对钴粉粒度影响的研究[J]. 硬质合金, 2001, 18(1): 12-15.
YUAN Ping. Study of influence of cobalt oxalate precipitation process on cobalt powder size[J]. Cemented Carbide, 2001, 18(1): 12-15.
[20] 柳召刚, 李 梅, 史振学, 胡艳宏, 王觅堂, 国树山. 草酸盐沉淀法制备超细氧化铈[J]. 中国稀土学报, 2008, 26(5): 666-670.
LIU Zhao-gang, LI Mei, SHI Zhen-xue, HU Yan-hong, WANG Mi-tang, GUO Shu-shan. Synthesis of cerium oxide ultrafine powders by oxalate precipitation[J]. Journal of the Chinese Society of Rave Earths, 2008, 26(5): 666-670.
[21] 高 玮, 古宏晨. 稀土草酸盐沉淀过程中颗粒大小的控制[J]. 稀土, 2000, 21(1): 11-13.
GAO Wei, GU Hong-chen. Control of mean particle size of rare earth oxalate in precipitation process[J]. Chinese Rare Earth, 2000, 21(1): 11-13.
[22] DEAN J. Lange’s handbook of chemistry[M]. 3rd ed. US: McGraw-Hill, Inc, 1999: 846-903.
[23] SOARE L. Precipitation and transformation of nanostructured copper oxalate and copper/cobalt composite precursor synthesis[D]. Switzerland: EPF Lausanne, 2004.
[24] SOHNEL O, GARSIDE J. Precipitation: Basic principles and industrial applications[M]. Manchester, UK: Butterworth-Heinemann Ltd., 1992: 17-18.
(编辑 何学锋)
收稿日期:2010-09-07;修订日期:2011-01-13
通信作者:叶红齐,教授,博士;电话:0731-88876605;E-mail: yeslab@csu.edu.cn


