
Microstructures and properties of 1.0%Al2O3/Cu composite treated by rolling
LIU Xiang-bing(刘向兵)1, JIA Cheng-chang(贾成厂)1, CHEN Xiao-hua(陈晓华)1, GAI Guo-sheng(盖国胜)2
1. Powder Metallurgy Institute, University of Science and Technology Beijing, Beijing 100083, China;
2. Department of Materials Science and Engineering, Tsinghua University, Beijing 100084,China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The 1.0%Al2O3/Cu (mass fraction) composite was prepared by hot pressing (HP), then treated by rolling to get a full density. The microstructures and the micro area element distribution of the composite were analyzed by SEM. The density, electric conductivity and tensile strength were also investigated. The experimental results show that the alumina particles are more dispersed and become smaller through a single-pass rolling. The pore existing in the composite is eliminated or closed under the rolling force. The relative density increases from 98.4% to 99.2%. The electric conductivity increases from 88.9%IACS to 91.2%IACS. The tensile strength is increased by 47% from 300 MPa to 440 MPa.
Key words:
Al2O3/Cu composite; hot pressing; rolling; pores;
1 Introduction
Copper has good electric performance[1] and ductility while alumina is very strong and hard. The alumina dispersion strengthened copper composite has combined these advantages to have high strength and large electric conductivity[2-3]. For dispersion strengthening to occur, the dispersed phase must be small enough to provide effective obstacles to dislocation movement and pin the boundary movement of the grain[4-6]. With these good properties the alumina dispersion strengthened copper composite has attracted much attention for the applicability of resistance welding electrode for automobile-industry, integrated circuit lead frame for IC industry and contact material instead of Ag matrix contact[7-15].
Pores existing in the material reduce the effective cross-sectional area and negatively affect strength. In the low porosity region, the relative electric conductivity follows a linear behavior with fractional density[16].
In the present work, the 1.0%Al2O3/Cu (mass fraction) composite was fabricated by hot pressing (HP) firstly. To get the full density (eliminating pores existing in the material), it was treated by rolling. Its microstructures and properties before and after processing were studied to determine the effect of the pore.
2 ExperimentalThe 1.0%Al2O3/Cu (mass fraction) composite was prepared by hot pressing (HP) at 920 ℃ for 30 min at the pressure of 20 MPa with argon gas protection. The microstructures and micro area elemental distribution of the composite were characterized by scanning electron microscopy (SEM).
The mixed powders for fabricating this composite were alumina with a mean particle size of 150 nm and electrolytic copper. Firstly the mixed powders were high energy ball milled for 2 h at the speed of 50 r/min. The ratio of ball to material was 10?1. Secondly the powders were spheroidized by hybridizatinon for 15 min at the speed of 5 000 r/min.
To get full density composite, the 1.0%Al2O3/Cu composite was rolled with 50% decrease in thickness at room temperature.
The density of the composites before and after rolling was tested by the Archimedes technique of water immersion. The electrical conductivity was measured by electrical resistance test instrument Type-2513/A. The curve of stress—strain was obtained from the testing of tensile strength using the machine of REGER3010. The gauge distance of the tensile sheet specimen is 20 mm and the speed of stress loading is 1 mm/s.
3 Results and discussion3.1 Microstructures of 1.0%Al2O3/Cu composite
Fig.1 shows the microstructure of the 1.0%Al2O3/ Cu prepared by HP. The alumina particles (the black spots in the picture) are dispersed in the copper matrix. There are local agglomerations of alumina particles with the size near 1 μm. As shown in Fig.2, SEM micro area element analysis of the big dimension black spots proves that they are alumina particles concentration clusters. Due to the limitation of SEM equipment the small black spots can not be detected accurately. We can only deduce that the dimension of the pore existing in the composite is small and conclude that its mass is also very little.

Fig.1 Surface SEM image of sheet shaped 1.0%Al2O3/Cu fabricated by HP
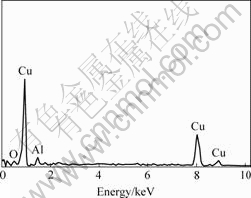
Fig.2 SEM micro area element analysis of black spot in Fig.1
Rolling is an approach to get full density P/M products. The higher the initial material density is and the greater the reduction in thickness is on rolling, the higher the final density is. In other words, more pores will be eliminated or closed through this treatment. Considering these influence factors we select a single-pass rolling operation. The 5 mm sheet composite is rolled to 2.5 mm by one operation. There is some micro cracking on the surface after rolling.
After rolling the alumina particles become more dispersed and more homogeneous, as illustrated in Fig.3. We can find that some agglomerations of the alumina particles have been segregated. There is no obvious shape deformation of the alumina particle on the cross section in length direction(Fig.3(a)) and width direction after rolling(Fig.3(b)). This also proves that the alumina concentration clusters occur to fragmentize. Fig.3(c) shows that the agglomeration degree of the alumina particles in the copper matrix is decreased compared with the pre-rolling composite.
Fig.4 illustrates that the black spots in Fig.3 are mainly Al2O3 particles.
3.2 Properties of 1.0%Al2O3/Cu composite
The relative density is increased from 98.4% to 99.2% through the treatment of the single-pass rolling, which means the mass of the pore has been reduced or the pore size has decreased. The electric conductivity of the composite is increased to 91.2%IACS after rolling.
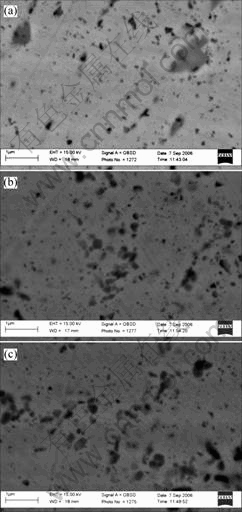
Fig.3 SEM images of 1.0%Al2O3/Cu after rolling: (a) Cross-section morphology in length direction after rolling; (b) Cross-section morphology in width direction after rolling; (c) Lower agglomeration of alumina particles
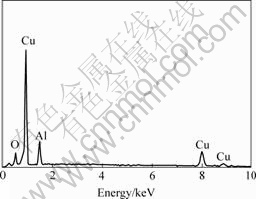
Fig.4 SEM micro area element analysis of black spot in Fig.3
This is due to the decrease or closure of the pore existing in the composite. Pores have negative effect on the electric conductivity. The physical properties of the 1.0%Al2O3/Cu composite are listed in Table 1.
Table 1 Physical properties of 1.0%Al2O3/Cu composite before and after rolling
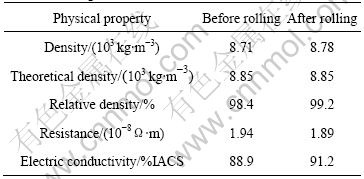
Fig.5 shows the curves of stress—strain relationship. From the curves we can read that the tensile strength of 1.0%Al2O3/Cu before rolling reaches about 300 MPa, while after rolling it is about 440 MPa. There is about 47% increase in tensile strength. This increase is mainly due to two reasons: second phase dispersion strengthening and cold work hardening. After rolling the alumina particles become smaller and more dispersed (shown in Fig.3), which will give the movement of the dislocation more impeding forces. So the strength will increase. But considering the mass of the alumina is very little, this effect on the tensile strength will be small. So the mainly contribution to the increase of the tensile strength is the latter. Cold deformation will increase the density of the dislocation, which makes the dislocation movement difficult.
There is a yield platform in Fig.5(a) and the yield strength is about 250 MPa, while the curve of Fig.5(b) has no yield platform. This proves the composite before rolling possesses better ductility than the composite after rolling.
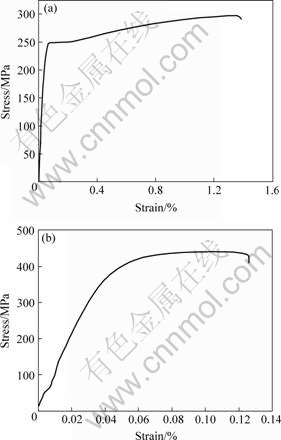
Fig.5 Stress—strain curves of 1.0%Al2O3/Cu composite: (a) Before rolling; (b) After rolling
4 Conclusions1) Rolling can change the distribution and size of the second phase and make the pore eliminate or close. After rolling, the alumina particles are more dispersed and become smaller.
2) The properties of 1.0%Al2O3/Cu composite are improved through rolling. The relative density increases from 98.4% to 99.2%. The electric conductivity increases from 88.9%IACS to 91.2%IACS. The improvement of these physical properties is due to the pore elimination or closure under the rolling force. The tensile strength increases by 47% from 300 MPa to 440 MPa. This is mainly determined by cold work hardening.
References
[1] ZHAN Yong-zhong, ZHANG Guo-ding. The effect of interfacial modifying on the mechanical and wear properties of SiCp/Cu composites[J]. Materials Letters, 2003, 57: 4583-4591.
[2] LING G P, LI Y. Influencing factors on the uniformity of copper coated nano-Al2O3 powders prepared by electroless plating[J]. Materials Letters, 2005, 59: 1610-1613.
[3] LIANG Shu-hua, FAN Zhi-kang, XU Lei, FANG Liang. Kinetic analysis on Al2O3/Cu composite prepared by mechanical activation and internal oxidation[J]. Composites: Part A, 2004, 35: 1441-1446.
[4] SONG Ke-xing, XING Jian-dong, DONG Qi-ming, LIU Ping, TIAN Bao-hong, CAO Xian-jie. Optimization of the processing parameters during internal oxidation of Cu-Al alloy powders using an artificial neural network[J]. Materials and Design, 2005, 26: 337-341.
[5] KIM S H, LEE D N. Recrystallization of alumina dispersion strengthened copper strips[J]. Materials Science and Engineering A, 2001, 313: 24-33.
[6] SHI Zi-yuan, YAN Mao-fang. The preparation of Al2O3-Cu composite by internal oxidation[J]. Applied Surface Science, 1998, 134: 103-106.
[7] CHEN S F, BAO T S, CHIN B A. Braze joints of dispersion strengthened copper[J]. Journal of Nuclear Materials, 1996, 233/237: 902-905.
[8] MOTTA M S, JEAN P K, BROCCHI E A, SOLORZANO I G. Characterization of Cu-Al2O3 nano-scale composites synthesized by in situ reduction[J]. Materials Science and Engineering C, 2001, 15: 175-177
[9] KUDASHOV D V, MARTIN U, HEILMAIER M, OETTEL H. Creep behaviour of ultrafine-grained oxide dispersion strengthened copper prepared by cryomilling[J]. Materials Science and Engineering A, 2004, 387/389: 639-642.
[10] CORREIA J B, CALDAS M P. Dependence of internal oxidation rate of water atomized Cu-Al alloy powders on oxygen partial pressure[J]. Journal of Materials Science Letters, 1996, 15: 465-468.
[11] GUO M X, WANG M P, CAO L F, LEI R S. Work softening characterization of alumina dispersion strengthened copper alloys [J]. Materials Characterization, 2007, 58: 928-935.
[12] SHU K M, TU G C. The microstructure and the thermal expansion characteristics of Cu/SiCp composites[J]. Materials Science and Engineering A, 2003, 349: 236-247.
[13] LI Guo-bin, SUN Ji-bing, GUO Quan-mei, WANG Ru. Fabrication of the nanometer Al2O3/Cu composite by internal oxidation[J]. Journal of Materials Processing Technology, 2005, 170: 336-340.
[14] KANG H K. Microstructure and electrical conductivity of high volume Al2O3-reinforced copper matrix composites produced by plasma spray[J]. Surface and Coatings Technology, 2005, 190: 448-452.
[15] LEE D W, KIM B K. Nanostructured Cu-Al2O3 composite produced by thermochemical process for electrode application[J]. Materials Letters, 2004, 58: 378-383.
[16] GERMAN R M. Powder metallurgy science[M]. 2nd ed. Princeton: Metal Powder Industries Federation, 1997: 380-390.
(Edited by YANG Bing)
Foundation item: Project (50174007) supported by the National Natural Science Foundation of China
Corresponding author: JIA Cheng-chang; Tel: +86-10-62334271; E-mail: jcc@mater.ustb.edu.cn


