Trans. Nonferrous Met. Soc. China 23(2013) 2638-2643
Fabrication of β-Sialon/ZrN/ZrON composites using fly ash and zircon
Bei-yue MA, Ming-gang SUN, Yu-shi DING, Chen YAN, Ying LI
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 2 July 2012; accepted 14 September 2012
Abstract:
β-Sialon/ZrN/ZrON composites were successfully fabricated by an in-situ carbothermal reduction-nitridation process with fly ash, zircon and active carbon as raw materials. The effects of raw materials composition and holding time on synthesis process were investigated, and the formation process of the composites was also discussed. The phase composition and microstructure of the composites were characterized by means of XRD and SEM. It was found that increasing carbon content in a sample and holding time could promote the formation of β-Sialon, ZrN and ZrON. The proper processing parameters to synthesize β-Sialon/ZrN/ZrON composites were mass ratio of zircon to fly ash to active carbon of 49:100:100, synthesis temperature of 1550 °C and holding time of 15 h. The average grain size of β-Sialon and ZrN(ZrON) synthesized at 1550 °C for 15 h reached about 2 and 1 μm, respectively. The fabrication process of β-Sialon/ZrN/ZrON composites included the formation of β-Sialon and ZrO2 as well as the conversion of ZrO2 to ZrN and ZrON.
Key words:
Sialon; ZrN; ZrON; in-situ synthesis; carbothermal reduction-nitridation process; fly ash; zircon;
1 Introduction
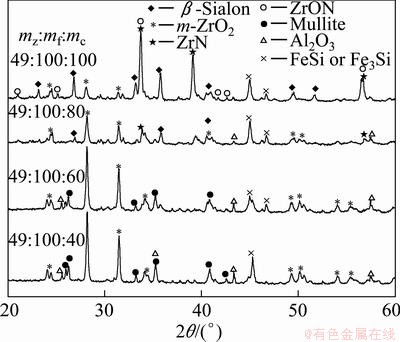
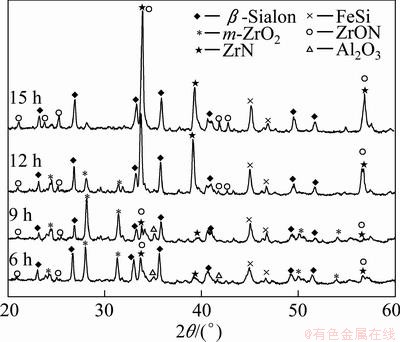
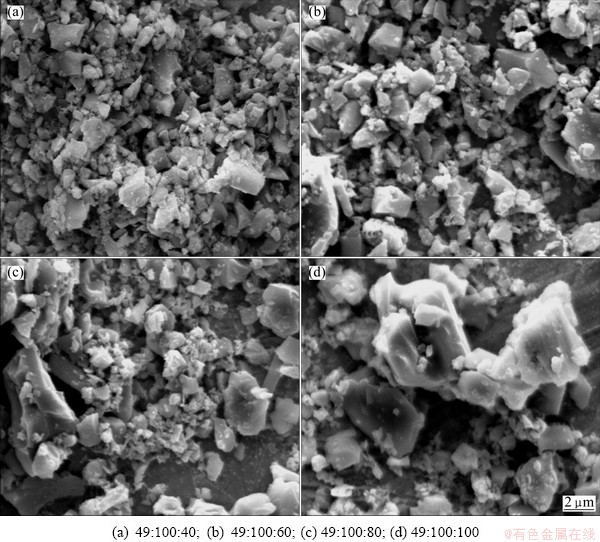
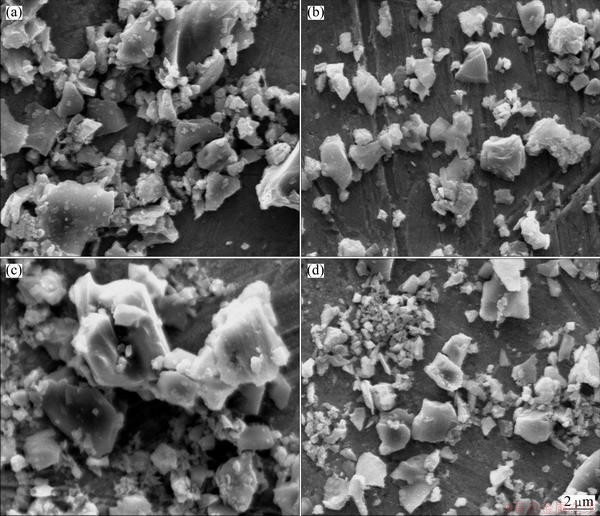
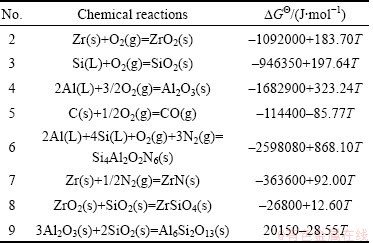
β-Sialon (Si6-zAlzOzN8-z, where 0 β-Sialon has been fabricated successfully via a reaction-bonding method [4], carbothermal reduction- nitridation (CTRN) process [5], combustion synthesis [6], microwave synthesis [7] and sol-gel synthesis [8]. CTRN method has the potential of being an economical synthesis process, utilizing inexpensive and abundant natural minerals and solid wastes containing alumina (Al2O3) and silica (SiO2) such as kaolin [9], pyrophyllite [10], bauxite [11], coal gangue [12] and fly ash [13]. Fly ash emerges as a by-product from the combustion of raw coal in thermal power plants. In recent years, mass waste fly ash has been causing serious environmental problems. So it is necessary to find an effective and comprehensive utilization of fly ash. Zircon (ZrSiO4) consists of zirconia (ZrO2) and SiO2. So far, fly ash and ZrSiO4 have been chosen as the raw materials to synthesize ceramic materials such as β-Sialon [5,13], SiC-AlN [14], SiC-mullite [15], mullite [16], ZrO2- mullite-Al2O3 [17] and ZrO2-SiC [18], etc. In this work, the β-Sialon/ZrN/ZrON composites were fabricated from fly ash and zircon by a CTRN process. The effects of raw materials composition and holding time on the phase composition and microstructure of the products were investigated, and the formation process of the composites was also discussed. 2 Experimental 2.1 Raw materials Fly ash (mesh size ≤74 μm), zircon (mesh size ≤44 μm) and active carbon (analytical reagent) were used as the raw materials. The main crystalline phase of fly ash was mullite (Al6Si2O13). The composition of the raw materials was listed in Table 1. Table 1 Composition of raw materials (mass fraction, %) 2.2 Preparation of samples The overall chemical reaction equation for synthesizing β-Sialon/ZrN/ZrON from fly ash and zircon by a CTRN process can be expressed as follows: 2ZrSiO4(s)+Al6Si2O13(s)+4SiO2(s)+21C(s)+7N2(g)=ZrN(s)+2Si4Al2O2N6(s)+ZrON(s)+Al2O3(s)+21CO(g) (1) According to Eq. (1), the mass ratio of zircon to fly ash to active carbon (mz:mf:mc) was 49:100:34. Over-stoichiometry of active carbon was added to promote the CTRN reaction. Zircon, fly ash and active carbon were weighed and mixed in different mass ratios mz:mf:mc of 49:100:40, 49;100:60, 49:100:80 and 49:100:100, respectively. Mixing was conducted in a ball mill with anhydrous ethanol for 6 h, fully dried at 60 °C, and the samples with 20 mm in diameter and 10 mm in thickness were formed under a pressure of 20 MPa. Then the formed samples were fully dried at 120 °C and put into a graphite crucible. The crucible was placed in an atmosphere-controlled tubular furnace and then heated up to 1550 °C for 6, 9, 12 and 15 h, respectively. During the synthesis process, the N2 flow remained to be 1.0 L/min. After the predetermined temperatures and holding time reached, the system was cooled to room temperature. 2.3 Characterization of samples The samples synthesized at different reaction conditions were oxidized in air at 700 °C for 2 h to remove residual carbon. The phase composition and microstructure of the products were characterized by X-ray diffraction (XRD, Cu Kα radiation, 30 kV and 30 mA) and scanning electronic microscopy (SEM). 3 Results and discussion 3.1 Phase composition Figure 1 shows the XRD patterns of the samples with different raw materials composition synthesized at 1550 °C for 12 h. It can be observed that raw material composition has a great influence on the phase composition of the synthesized samples. The synthesized products with mz:mf:mc values of 49:100:40 and 49:100:60 are mainly composed of mullite, m-ZrO2, Al2O3 and Fe3Si. Sialon and zircon phase are not detected, which indicates that zircon is decomposed completely and forms m-ZrO2 and SiO2. The formed SiO2 can react with C and Fe2O3 to form Fe3Si, where Fe2O3 is an impurity in fly ash and zircon. Increasing carbon content in a sample (mz:mf:mc=49:100:80) causes the phase composition of the products to have a great change. Mullite vanishes completely. β-Sialon and ZrN can be detected. The sample consists of β-Sialon, ZrN, m-ZrO2, Al2O3 and FeSi. By varying the value of mz:mf:mc from 49:100:80 to 49:100:100, the peak intensities of β-Sialon and ZrN strengthen remarkably, however, the peak intensity of m-ZrO2 decreases. Meanwhile, Al2O3 phase vanishes, new phase ZrON forms, and its diffraction intensity is lower. The main crystalline phases of the sample with mz:mf:mc value of 49:100:100 includes β-Sialon and ZrN. Thus, increasing carbon content in a sample is favorable for the CTRN reaction, and the proper raw materials composition (mz:mf:mc) to synthesize β-Sialon/ZrN/ZrON is 49:100:100. Fig. 1 XRD patterns of samples with different mz:mf:mc values synthesized at 1550 °C for 12 h Figure 2 shows the XRD patterns of the samples with mz:mf:mc value of 49:100:100 synthesized at 1550 °C for 6, 9, 12 and 15 h, respectively. It can be seen that holding time also has a great influence on the phase composition of the products. The samples heated at 1550 °C for 6 and 9 h all involve β-Sialon, ZrN, ZrON, m-ZrO2, Al2O3 and FeSi, and their main crystalline phases are β-Sialon and m-ZrO2. When the holding time reaches 12 h, Al2O3 phase vanishes. The peak intensities of β-Sialon, ZrN and ZrON strengthen obviously, however, the diffraction intensity of m-ZrO2 weakens. In the sample fabricated at 1550 °C for 15 h, m-ZrO2 phase vanishes, the peak intensities of β-Sialon, ZrN and ZrON further strengthen, and the main crystalline phases include β-Sialon, ZrN and ZrON. It indicates that increasing holding time is good for the formation of β-Sialon, ZrN and ZrON. Fig. 2 XRD patterns of samples with mz:mf:mc of 49:100:100 synthesized at 1550 °C for 6, 9, 12 and 15 h It can be observed from Figs. 1 and 2 that the proper processing parameters to synthesize β-Sialon/ZrN/ZrON are mz:mf:mc value of 49:100:100, synthesis temperature of 1550 °C and holding time of 15 h. 3.2 Microstructure Figure 3 shows the SEM images of the samples with different raw materials composition (mz:mf:mc) synthesized at 1550 °C for 12 h. There exist many fine particles in the sample with mz:mf:mc values of 49:100:40 and 49:100:60 (Figs. 3(a) and (b)), and their average grain size is about 1 μm. EDS analysis and XRD pattern (Fig. 1) indicate that they are ZrO2. Some big particles with an average grain size of 2-3 μm can also be observed. They consist of Si, Al and O elements. Combining with the XRD pattern as shown in Fig. 1, they are mullite. Increasing carbon content in a sample (Figs. 3(c) and (d)) can promote the CTRN reaction and make the fine particles grow up. Fig. 3 SEM images of samples with different mz:mf:mc values synthesized at 1550 °C for 12 h Fig. 4 SEM images of samples with mz:mf:mc value of 49:100:100 synthesized at 1550 °C for 6 h (a) , 9 h (b), 12 h (c) and 15 h (d) Figure 4 shows the SEM images of the samples with mz:mf:mc value of 49:100:100 synthesized at 1550 °C for 6-15 h. Big flaky and fine particles can be observed in the products synthesized at 1550 °C for 6 h, and their average grain size are 3 and 1 μm, respectively. EDS analysis indicates that the big flaky particles are β-Sialon, and the fine particles are composed of Zr, O and N elements, they are composite body of ZrO2, ZrN and ZrON, which can be confirmed by the XRD pattern (Fig. 2). As increasing the holding time from 6 to 9, 12 and 15 h, respectively (Figs. 4(b), (c) and (d)), fine particles increase gradually, and their average grain size decreases. The reason is that during the CTRN process, ZrO2 in a sample can react with C and N2 to form new fine ZrN and ZrON particles. The average grain size of β-Sialon and ZrN(ZrON) synthesized at 1550 °C for 15 h reaches 2 and 1 μm, respectively. Table 2 Gibbs free energy changes of formation of some compounds in ZrO2-Al2O3-SiO2-C-N2 system 3.3 Analysis of formation process In this work, the fabrication process of β-Sialon/ZrN/ZrON composites is very complex and involves many chemical reactions. During the CTRN process, an important intermediate gas phase is carbon monoxide (CO). As changing the raw materials composition (mz:mf:mc=49:100:40, 49:100:60, 49:100:80 and 49:100:100) and holding time (6, 9, 12 and 15 h), ZrO2, SiO2, Al2O3, β-Sialon (z=2, Si4Al2O2N6), ZrN and ZrON may be formed. Table 2 lists the Gibbs free energy changes of some compounds in ZrO2-Al2O3-SiO2-C-N2 system [19,20]. The relationship between standard Gibbs energy change (△GΘ) and temperature (T) can be calculated according to these thermodynamic data. The chemical reactions are likely to occur during the CTRN process and the relational expression of △GΘ and T (△GΘ–T) are as follows: ZrSiO4(s)=ZrO2(s)+SiO2(s) (10) (1/3)Al2O3(s)+(4/3)SiO2(s)+3C(s)+N2(g)=(1/3)Si4Al2O2N6(s)+3CO(g) (12) 2ZrO2(s)+4C(s)+N2(g)=2ZrN(s)+4CO(g) (13) 3C(s)+N2(g)=(1/3)ZrO2(s)+(1/3)Si4Al2O2N6(s)+(1/6)Al2O3(s)+3CO(g) (14) (2/7)ZrSiO4(s)+(1/7)Al6Si2O13(s)+(4/7)SiO2(s)+(22/7)C(s)+N2(g)=(2/7)ZrN(s)+(2/7)Si4Al2O2N6(s)+ (1/7)Al2O3(s)+(22/7)CO(g) (15) Figure 5 shows the diagram of △GΘ-T for ZrO2-SiO2-Al2O3-C-N2 system which were obtained from Eqs. (10)-(15). It can be seen that Fig. 5 Diagram of △GΘ-T for ZrO2-SiO2-Al2O3-C-N2 system During the CTRN process, a small amount of ZrON can be formed from ZrO2, ZrSiO4 and ZrN (Eqs. (16)-(18)), which can be confirmed by the XRD pattern as shown in Figs. 1 and 2. 2ZrO2(s)+2C(s)+N2(g)=2ZrON(s)+2CO(g) (16) ZrSiO4(s)+C(s)+N2(g)=ZrON(s)+SiO(g)+9CO(g) (17) 2ZrO2(s)+2ZrN(s)+N2(g)=4ZrON(s) (18) As shown in Figs. 1 and 2, Fe3Si and FeSi can be detected in the products synthesized at 1550 °C. The reason is that there exists Fe2O3 in fly ash and zircon. During the CTRN process, Fe2O3 can react with SiO2 and C to form Fe3Si and FeSi (Eqs. (19) and (20)). An important fact is that FeSi forms in a sample with higher carbon content (mz:mf:mc=49:100:80, 49:100:100), and Fe3Si can be detected in a sample with lower carbon content (mz:mf:mc=49:100:40, 49:100:60). There are many efforts needed to make to investigate the effect of processing parameters on the formation mechanisms of FeSi and Fe3Si. 3Fe2O3(s)+2SiO2(s)+13C(s)=2Fe3Si+13CO(g) (19) Fe2O3(s)+2SiO2(s)+7C(s)=2FeSi+7CO(g) (20) In this work, the formation process of β-Sialon/ ZrN/ZrON composites can be summarized as follows: 1) For the samples with lower carbon content (mz:mf:mc=49:100:40, 49:100:60), the CTRN process includes the formation of ZrO2 due to the decomposition of zircon, the other decomposition product (SiO2) reacts with Fe2O3 and C to synthesize Fe3Si and FeSi. 2) For the samples with higher carbon content (mz:mf:mc=49:100:80, 49:100:100), the CTRN process includes the formation of β-Sialon and the conversion of ZrO2 to ZrN and ZrON. 4 Conclusions 1) Increasing carbon content in a sample and holding time can promote the formation of β-Sialon, ZrN and ZrON. The β-Sialon/ZrN/ZrON composites can be successfully fabricated at 1550 °C for 15 h while heating the samples with mz:mf:mc value of 49:100:100. 2) The average grain size of β-Sialon and ZrN(ZrON) synthesized at 1550 °C for 15 h are about 2 and 1 μm, respectively. 3) The fabrication process of β-Sialon/ZrN/ZrON composites includes the formation of β-Sialon and ZrO2 as well as the conversion of ZrO2 to ZrN and ZrON. References [1] HOU X M, CHOU K C, LIF S. Some new perspectives on oxidation kinetics of SiAlON materials [J]. J Eur Ceram Soc, 2010, 28(6): 1243-1249. [2] MA B Y, YU J K. Influence of processing parameters on the phase composition of ZrN-Si3N4 synthesized from zircon [J]. Rare Metals, 2009, 28(4): 367-371. [3] RAWAL S K, CHAWLA A K, CHAWLA V, JAYAGANTHAN R, CHANDRA R. Structural, optical and hydrophobic properties of sputter deposited zirconium oxynitride films [J]. Mater Sci Eng B, 2010, 172(3): 259-266. [4] HYUGA H, KONDO N, KITA H. Fabrication of dense β-SiAlON ceramics with ZrO2 additions via a rapid reaction-bonding and postsintering route [J]. J Am Ceram Soc, 2011, 94(4): 1014-1018. [5] MA Bui-jue, LI Ying, YAN Chen, DING Yu-shi. Effects of synthesis temperature and raw materials composition on preparation of β-Sialon based composites from fly ash [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 12-133. [6] YI X M, AKIYAMA T. Mechanical-activated, combustion synthesis of β-SiAlON [J]. J Alloys Compd, 2010, 495(1): 144-148. [7] PANNEERSELVAM M, RAO K J. A microwave method for the preparation and sintering of β′-SiAlON [J]. Mater Res Bull, 2003, 38(4): 663-674. [8] PRADEILLES N, RECORDM C, GRANIER D, MARIN-AYRAL R M. Synthesis of β-SiAlON: A combined method using sol-gel and SHS processes [J]. Ceram Int, 2008, 34(5): 1189-1194. [9] VLASOVA M, VINOKUROV V B, GRIGOR’EV O N, PANASYUK A D, BEGA N D, KAKAZEY M, GONZALEZ- RODRIGUEZ J G, [10] [11] ZHANG H J, HAN B, LIU Z J. Preparation and oxidation of bauxite-based β-Sialon bonded SiC composite [J]. Mater Res Bull, 2006, 41(9): 1681-1689. [12] LUO X Y, SUN J L, DENG C J, HONG Y R. Synthesis of β-Sialon from coal gangue [J]. J Mater Sci Technol, 2003, 19(1): 93-96. [13] GILBERT J E, MOSSET A. Preparation of β-Sialon from fly ashes [J]. Mater Res Bull, 1998, 33(1): 117-123. [14] WANG H J, WANG Y L, JIN Z H. SiC powder prepared from fly ash [J]. J Mater Process Tech, 2001, 117(1-2): 52-55. [15] ZHANG L, YANG J J, WANG X P. Synthesis and characterization of SiC-mullite spheres by microwave heating [J]. Rare Metal Materials and Engineering, 2011, 40(3): 526-529. (in Chinese) [16] LI J H, MA H W, HUANG W H. Effect of V2O5 on the properties of mullite ceramics synthesized from high-aluminum fly ash and bauxite [J]. J Hazard Mater, 2009, 166(2-3): 1535-1539. [17] MA B Y, LI Y, CUI S G, ZHAI Y C. Preparation and sintering properties of zirconia-mullite-corundum composites using fly ash and zircon [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(12): 2331-2335. [18] MA B Y, YU J K. Phase composition of SiC-ZrO2 composite materials synthesized from zircon doped with La2O3 [J]. J Rare Earths, 2009, 27(5): 806-810. [19] CHEN Zhao-you. Chemical thermodynamics of refractories [M]. Beijing: Metallurgical Industry Press, 2005: 526-527. (in Chinese) [20] LIANG Ying-jiao, CHE Yin-chang. Handbook of thermodynamic data in inorganic [M]. Shenyang: Northeastern University Press, 1993: 449-479. (in Chinese). 马北越,孙明刚,丁玉石,闫 晨,厉 英 东北大学 材料与冶金学院,沈阳 110819 摘 要:以粉煤灰、锆英石和活性炭为原料,采用原位碳热还原氮化法成功制备β-Sialon/ZrN/ZrON复合材料。研究配料组成和保温时间对合成过程的影响,并讨论材料的生成过程。通过XRD和SEM表征材料的相组成和显微组织。结果表明:增加试样中的碳含量以及延长保温时间均能促进β-Sialon、ZrN 和ZrON 的生成。合成β-Sialon/ ZrN/ZrON复合材料的适宜工艺参数为锆英石、粉煤灰和活性炭的质量比49:100:100、合成温度 1550 °C、保温时间15 h。在1550 °C保温15 h合成的β-Sialon 和ZrN(ZrON)的平均粒径分别约为2和1 μm。β-Sialon/ZrN/ZrON复合材料的制备过程包括β-Sialon和ZrO2的生成过程以及 ZrO2 向ZrN和ZrON的转化过程。 关键词:Sialon;ZrN;ZrON;原位合成;碳热还原氮化法;粉煤灰;锆英石 (Edited by Chao WANG) Foundation item: Project (2013AA030902) supported by the National High-tech Research and Development Program of China; Projects (51074038, 51274057) supported by the National Natural Science Foundation of China; Projects (N120402006, N100302002) supported by the Fundamental Research Funds for the Central Universities, China; Project (L2012079) supported by the Educational Commission of Liaoning Province of China; Project (110215) supported by the Training Program on National College Students Innovation Experiment Corresponding author: Ying LI; Tel: +86-24-83688995; E-mail: liying@mail.neu.edu.cn DOI: 10.1016/S1003-6326(13)62779-X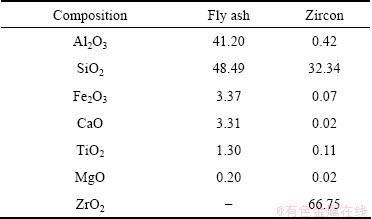
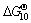 /(J·mol-1)
/(J·mol-1) Al6Si2O13(s)=3Al2O3(s)+2SiO2(s) (11)
Al6Si2O13(s)=3Al2O3(s)+2SiO2(s) (11) /(J·mol-1)
/(J·mol-1)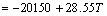
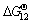 /(J·mol-1)
/(J·mol-1)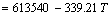
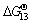 /(J·mol-1)
/(J·mol-1) (1/3)ZrSiO4(s)+(1/6)Al6Si2O13(s)+(2/3)SiO2(s)+
(1/3)ZrSiO4(s)+(1/6)Al6Si2O13(s)+(2/3)SiO2(s)+ /(J·mol-1)
/(J·mol-1)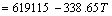
 /(J·mol-1)
/(J·mol-1)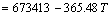
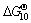 and
and  for Eqs. (10) and (11), respectively, keep positive when the temperature is less than 1850 °C, which reveals that Eqs. (10) and (11) can not easily generate at the experimental temperature of 1550 °C.
for Eqs. (10) and (11), respectively, keep positive when the temperature is less than 1850 °C, which reveals that Eqs. (10) and (11) can not easily generate at the experimental temperature of 1550 °C. 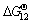 ,
, 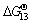 ,
,  and
and  for Eqs. (12)-(15), respectively, become negative at about 1630 °C, and they decrease sharply as increasing the temperature. At 1550 °C, the △GΘ values for Eqs. (10)-(15) are as follows:
for Eqs. (12)-(15), respectively, become negative at about 1630 °C, and they decrease sharply as increasing the temperature. At 1550 °C, the △GΘ values for Eqs. (10)-(15) are as follows: 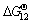 <
<  <
< 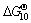 <
< <
< <
<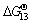 . It indicates that the β-Sialon can be easily formed from Al2O3 and SiO2 by the CTRN reaction. However, the CTRN reaction of ZrO2 to form ZrN is very difficult. Increasing holding time to 12-15 h benefits the formation of ZrN (Fig. 2).
. It indicates that the β-Sialon can be easily formed from Al2O3 and SiO2 by the CTRN reaction. However, the CTRN reaction of ZrO2 to form ZrN is very difficult. Increasing holding time to 12-15 h benefits the formation of ZrN (Fig. 2).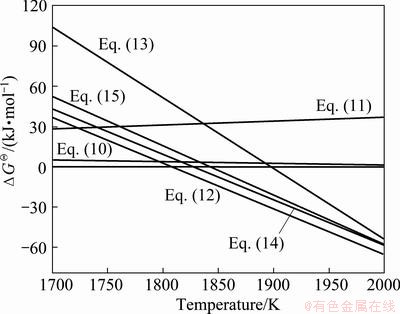
 G,
G,  M. Features of SiAlON synthesis from kaolin [J]. Mate Sci Eng A, 2004, 366(2): 325-331.
M. Features of SiAlON synthesis from kaolin [J]. Mate Sci Eng A, 2004, 366(2): 325-331. Z. Carbothermal reduction and nitridation of powder pyrophyllite [J]. J Eur Ceram Soc, 2004, 24(5): 791-796.
Z. Carbothermal reduction and nitridation of powder pyrophyllite [J]. J Eur Ceram Soc, 2004, 24(5): 791-796.用粉煤灰和锆英石制备β-Sialon/ZrN/ZrON 复合材料
Abstract: β-Sialon/ZrN/ZrON composites were successfully fabricated by an in-situ carbothermal reduction-nitridation process with fly ash, zircon and active carbon as raw materials. The effects of raw materials composition and holding time on synthesis process were investigated, and the formation process of the composites was also discussed. The phase composition and microstructure of the composites were characterized by means of XRD and SEM. It was found that increasing carbon content in a sample and holding time could promote the formation of β-Sialon, ZrN and ZrON. The proper processing parameters to synthesize β-Sialon/ZrN/ZrON composites were mass ratio of zircon to fly ash to active carbon of 49:100:100, synthesis temperature of 1550 °C and holding time of 15 h. The average grain size of β-Sialon and ZrN(ZrON) synthesized at 1550 °C for 15 h reached about 2 and 1 μm, respectively. The fabrication process of β-Sialon/ZrN/ZrON composites included the formation of β-Sialon and ZrO2 as well as the conversion of ZrO2 to ZrN and ZrON.


