Trans. Nonferrous Met. Soc. China 27(2017) 204-210
Enhancement of pentlandite surface magnetism and implications for its separation from serpentine via magnetic separation
Zhi-tao YUAN1, Ji-wei LU1, Jiong-tian LIU1,2, Li-xia LI1, Shuang-yu WANG1
1. School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China;
2. School of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, China
Received 5 November 2015; accepted 23 November 2016
Abstract:
The magnetism of pentlandite surface was enhanced through the selective precipitation of micro-fine magnetite fractions on pentlandite surfaces. This was achieved through adjustment of slurry pH and addition of surfactants. The results showed that at pH 8.8 with the addition of 100 g/t sodium hexametaphosphate, 4.5 L/t oleic acid, and 4.5 L/t kerosene, significant amount of fine magnetite particles adhered to the pentlandite surface, while trace amount of coating was found on serpentine surfaces. Thus, the magnetism of pentlandite was enhanced and pentlandite was well separated from serpentine by magnetic separation under the magnetic field intensity of 200 kA/m. Scanning electron microscopy (SEM) and zeta potential measurement were performed to characterize changes of mineral surface properties. Calculations of the extended Derjaguin-Landau-Verwey-Ocerbeek (EDLVO) theory indicated that, in the presence of surfactants the total interaction energy between magnetite and pentlandite became stronger than that between magnetite and serpentine. This enabled the selective adhesion of magnetite particles to pentlandite surfaces, thereby enhancing its magnetism.
Key words:
pentlandite; serpentine; micro-fine magnetite; magnetic separation; selective magnetic coating;
1 Introduction
Magnetic separation is based on the difference in magnetic properties of constituent mineral particles. However, in nature, most of the minerals own a very weak magnetism or no magnetism except for the iron and magnetite mineral, and consequently it is very difficult to obtain good enrichment or separation. Fortunately, there are a number of researches focused on the enhancement or artificial establishment of the magnetic susceptibility of minerals [1,2].
It is well known that the weakly magnetic properties of minerals (hematite, siderite and pyrite) can be enhanced by roasting or chemically converting them to a more magnetic phase, which consumes large amount of energy. In addition, there exists another method based on the artificial establishment, which is called magnetic coating or magnetic carrier methods without chemically altering the minerals. This is realized by incorporating a discrete magnetic phase onto the particles to be magnetized. It is easy to operate and suitable for a variety of ores or metals, with low cost. PLUMPTON [3] described and summarized diagrammatically these selective magnetic coating methods as follows: 1) selective surface decomposition of iron pentacarbonyl (Magnex process); 2) selective wetting by magnetite laden oil (Murex process); 3) selective co-flocculation with magnetite; and 4) selective surface adsorption of fine magnetite. In this process, individual magnetite fines are added in the highly dispersed slurry containing surfactants added in advance, and the magnetite is selectively attached onto the target grains’ surface based on the physicochemical properties of the target minerals and magnetite. This separation technology was initially reported by FRANGISKOS and GAMBOPOULOS [4] to separate the magnesite, serpentine, pegmatite veins, and calcite with surfactants (ARQUAD-2C of 500 g/t, diesel oil of 2.8 L/t and Flotol B of 0.4 L/t) and heavy media grade magnetite or ferrosilicon. In this case, the silica and calcite were coated whereas the magnesite was not coated and then separated using a wet belt-type separator under a magnetic field intensity of 1.0 T. Thereafter, plenty of studies have been conducted to deal with other minerals using this process [5-7].
The separation of magnesite fines from serpentine fines by magnetic carrier methods has been described in detail by ANASTASSAKIS [8]. Surfactant, 22.5 mol/L dodecylamine, was required in suspensions (1.0 g of each mineral, 100 mL of water) to allow the adsorption of fine magnetite (less than 5 μm) onto serpentine surfaces in the pH range of 6.0-11.0. At pH>9.0 only slight coating formed on magnesite surfaces [8]. The mixtures were then separated by high-intensity magnetic separation at 0.8 A and pH=8.0. The magnetic products of 92.9% serpentine and 7.1% magnesite with 99.7% recovery were obtained. Meanwhile, the selective separation of quartz coated by extremely fine magnetite particles (less than 5 μm) from magnesite was also achieved by ANASTASSAKIS [9] by adding dodecylamine (7.2×10-6 mol/L), kerosene (2.5 L/t), and pine oil (250 g/t), adjusting pH value, and optimizing the amount of magnetite. Quartz particles were strongly coated by fine magnetite in the pH range of 6.0-11.0. After being separated by the magnetic separator (1.0 A), the magnetic products of 96.0% quartz and 4.0% magnesite were obtained at pH=6.0 and the recovery of quartz was 89.5%. SINGH et al [10] recovered iron minerals from the Barsua iron ore slimes containing 56% Fe, 4.8% SiO2, and 7.2% Al2O3 by the addition of synthetic colloidal magnetite and oleate colloidal coating followed by high gradient magnetic separation technique. Meanwhile, the effects of content of colloidal magnetite, pH, and magnetic field strength were studied in detail [10]. Besides, the separation of minerals with weak magnetism, such as separating chalcocite from silica, sphalerite from gangue, coal from ash, ferrihydrite from wastewater, and metallic copper from lead using magnetic coating technique has been also investigated by several researchers [11-13].
So far, magnetic coating method, however, has not been a well-known process in the separation of sulfide ores or copper-nickel sulfide ores. As we all know, in nickel sulfide flotation, serpentine, as a common magnesium silicate, is usually reported into the flotation concentrate via absorption on the pentlandite surfaces as slime coatings, causing downstream processing problems and increasing smelting costs [14,15]. In addition, hydrophilic serpentine minerals may interfere with the flotation of pentlandite and decrease the hydrophobicity of sulfide minerals. In order to eliminate the effect of serpentine minerals, high dosage of dispersants such as sodium hexametaphosphate, sodium silicate, or carboxymethyl cellulose are usually added to depress these magnesium silicates and hence may bring about considerable economic pressures [16-18]. Therefore, the challenging issues during the processing of such a nickel sulfide are to reduce the serpentine (MgO) content and increase nickel recovery. The present work provides a new method of separating pentlandite from serpentine by magnetic coating. Surfaces of pentlandite were coated selectively by fine magnetite particles to enhance its magnetic property for magnetic separation.
2 Experimental
2.1 Materials
The experimental samples, pentlandite, serpentine and magnetite were obtained from Jinchuan Group Co., Ltd., Xiuyan Serpentine Mine and Benxi Steel Group Co., Ltd., China, respectively. Sodium hexametaphosphate (SHMP), oleic acid and kerosene were chemical reagents supplied from Tianjin Kermil Inc., China. Pentlandite and serpentine were mixed in a mass ratio of 1:2 and different amounts of magnetite were added to mixtures.
The mineral compositions of pentlandite, serpentine, and magnetite samples were identified by XRD and the XRD patterns are shown in Fig. 1. The XRD patterns of pentlandite confirmed that the sample was of high purity with minor amount of pyrite. The fraction of particle size less than 45 μm was used for the magnetic coating tests. The XRD patterns of serpentine showed that the sample contained 99% serpentine with trace amount of quartz. The natural magnetite mineral with 69.98% Fe was ground in a stirred mill for 90 min so that mineral particles were less than 5 μm. The ground sample was used as magnetic seeds.
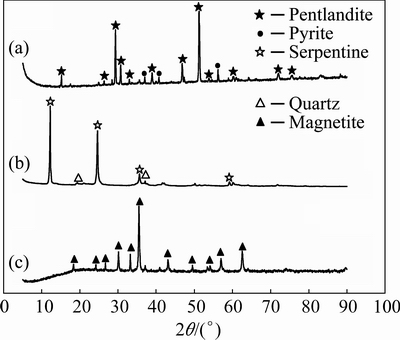
Fig. 1 XRD patterns of pentlandite (a), serpentine (b) and magnetite (c)
The particle size distributions of samples were analyzed by a Malvern laser particle size analyzer (model 2000). The results showed that the volume average diameters of pentlandite, serpentine and magnetite were 32.22, 24.46 and 2.28 μm, respectively, as shown in Table 1. The chemical analysis results of pentlandite, serpentine, and magnetite samples are given in Tables 2, 3 and 4, respectively.
Table 1 Grain size distribution of samples
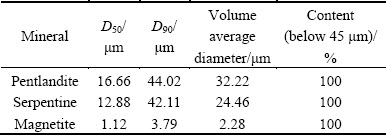
Table 2 Multi-element chemical analysis results of pentlandite sample (mass fraction, %)
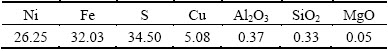
Table 3 Multi-element chemical analysis results of serpentine sample (mass fraction, %)

Table 4 Multi-element chemical analysis results of magnetite sample (mass fraction, %)

2.2 Magnetic coating tests
The magnetic coating tests of pentlandite- serpentine were carried out in a 200 mL XFGC batch flotation cell with continuous stirring to keep the particles in suspension at a fixed solid-water ratio of 15 g to 100 mL deionized water. The suspension pH was initially adjusted to the desired values by HCl or NaOH solution. Then, the prepared SHMP (100 g/t) was added and conditioned for 3 min. In order to compare the effect of magnetite content on surface coating, solutions with different contents of magnetite were prepared. Different amounts of fine magnetite fractions were added to 10 mL oleic acid (4.5 L/t) solution and the suspensions were then ultrasonicated for 4 min. After that, the mixed solutions were added to the former slurry and then agitated for 3 min. In the final step, kerosene (4.5 L/t) was added and conditioned for another 3 min at a constant stirring speed of 2400 r/min. Then, the magnetic separation was conducted by an XCSQ-50×70 wet high gradient magnetic separator (WHGMS). The magnetic concentrates and tailings were dried and weighed for the following ICP analysis.
2.3 Zeta potential measurements and SEM study
Zeta potential measurement (Nano-ZS90, Malvern, UK) was performed to investigate the changes in surface properties of samples with or without surfactants. Dilute suspensions of pentlandite, serpentine and magnetite used for zeta potential analyses were prepared at 0.5% solid, with or without surfactants in 0.001 mol/L KNO3 electrolyte, and magnetically stirred for 20 min. The suspension pH was adjusted to a desired value with HCl and KOH solutions, and allowed to reach equilibrium for 5 min before samples were directly injected into a disposable capillary cell for zeta potential measurement. The measurements were started from the pristine pH down to 2 or up to 11.
The scanning electron microscope equipped with an energy dispersive X-ray spectrometer (SEM-EDS, Hitachi, S-3400N, Japan) was used to take images of magnetic concentrates and tailings and characterize their elemental composition.
3 Results and discussion
3.1 Effect of magnetite on separation of mixed minerals after magnetic coating
Figure 2 shows the influence of magnetite’s amount on the recoveries and grades of Ni (pentlandite) and MgO (serpentine) from concentrates, under the condition of magnetic field intensity of 200 kA/m and pH 8.8. The pentlandite and serpentine were mixed at a mass ratio of 1:2.
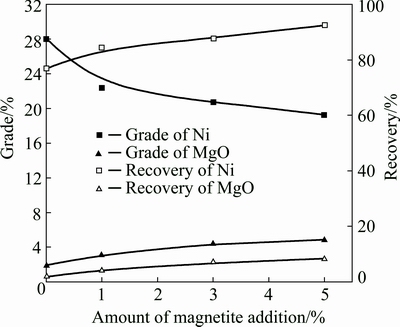
Fig. 2 Changes in grade and recovery of magnetic products with amount of magnetite addition
The Ni recovery in the absence of magnetite was low while the Ni grade was very high due to part of pentlandite with certain magnetism. Upon the addition of fine magnetite, Ni recovery markedly increased up to 92.46% with the magnetite addition of 5%, with further addition of magnetite little gain in recovery was produced and simultaneously the grade decreased. The grade and recovery of MgO marginally increased with magnetite addition (grades from 1.97% up to 4.85%; recoveries from 2.03% up to 8.32%). This is probably due to the mechanical entrainment of magnetic serpentine by minerals. The adsorption of fine serpentine fractions on the surfaces of pentlandite and magnetite may also lead to its report to concentrates. These also explained why Ni grade decreased with magnetite addition. The SEM and EDS analyses further confirmed the above inferences as shown in Fig. 3. Besides, magnetite adhering to pentlandite particles as nickel concentrate could also reduce Ni grade.
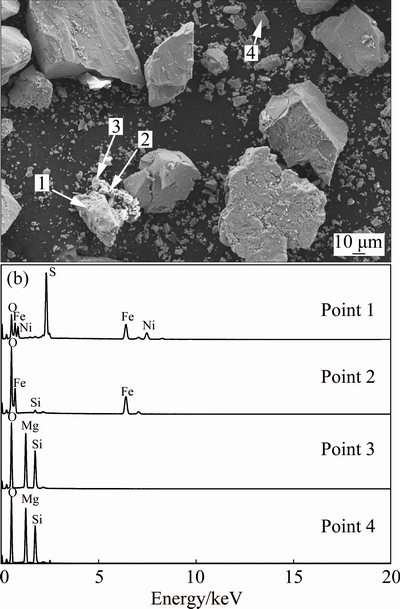
Fig. 3 SEM image of magnetic concentrates (a) and EDS patterns (b) of points 1, 2, 3 and 4 in Fig. 3(a)
3.2 Effect of surfactants on zeta potential of minerals
Figure 4 shows zeta potentials of pentlandite, serpentine and magnetite particles measured in the absence and presence of surfactants.
From Fig. 4, it can be observed that serpentine, magnetite and pentlandite were positively charged at pH below 9.6, 6.5 and 3.9, respectively. When SHMP was added, the surface charge of serpentine became more negative in the whole pH range examined. The isoelectric point for magnetite shifted to lower pH values with the addition of oleate to a less extent compared with that of serpentine. However, the surface charge of pentlandite was insensitive to the addition of SHMP and kept almost the same as that without SHMP. From the zeta potential measurements before and after the addition of surfactants, it seems that surfactants were adsorbed on the particle surfaces due to electrostatic attraction in low pH range and chemical interaction in high pH range [19,20]. Meanwhile, it can also be concluded that electrostatic repulsive force existed between each mineral (pentlandite, serpentine) and magnetite fractions at pH higher than 5.0, but the electrostatic repulsion of pentlandite-serpentine and serpentine-magnetite became even stronger, leading to the dispersion of pentlandite-serpentine and serpentine-magnetite. In spite of the electrostatic repulsion between the pentlandite and magnetite fractions, magnetite fines could still adhere to the surface of pentlandite by the van der Waals interactions and hydrophobic interactions, which has been confirmed by the following calculations of EDLVO theory.
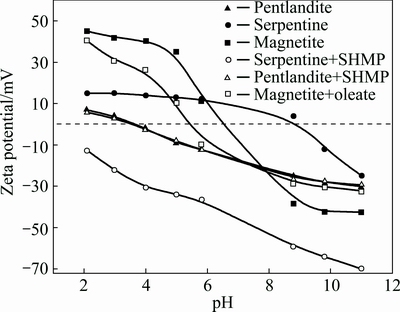
Fig. 4 Zeta potentials of pentlandite, serpentine and magnetite at different pH values in the absence or presence of surfactants with 0.001 mol/L KNO3
3.3 Morphology of pentilandite and serpentine surfaces after magnetic coating
SEM was employed to investigate the possible interaction of magnetite with pentlandite and serpentine particles, as shown in Fig. 5. Figure 5(b) shows that the morphology of the magnetic concentrates treated with magnetite fractions was completely different from that of the pentlandite. It was obviously observed that the surfaces of concentrates were coated with micro-fine magnetite particles. The EDS spectrum of point B shows the presence of Fe and O, confirming the adsorption of magnetite fines onto pentlandite which was confirmed by EDS spectrum of point A (containing elements of Fe, Ni and S).
As seen in Fig. 5(c), compared with the magnetic concentrates, the magnetic tailings were mainly serpentine particles which were confirmed by the EDS spectrum of point C. The serpentine surfaces were smooth without coating formed by the micro-fine magnetite particles. In other words, only pentlandite surfaces were selectively magnetized. Therefore, pentlandite minerals could be separated from serpentine by the magnetite coating method, which has been proved by the magnetic separation tests. However, there still existed trace quantities of magnetite fines as the single bright white particles as shown in the SEM image of tailings and the EDS patter of point D.

Fig. 5 SEM images of pentlandite (a), magnetic concentrates (b) and magnetic tailings after magnetic coating (c), and EDS patterns (d)
3.4 Changes of interaction energies with or without surfactants
Interaction (dispersion and agglomeration) between particles in suspension is mainly determined by their electrostatic interaction (UE) and van der Waals interaction (UA) according to the classical DLVO (Derjaguin-Landau-Verwey-Ocerbeek) theory. In mineral processing, surfactants are usually added to improve the mineral surface hydrophobicity or hydrophilicity. This further produces additional hydrophobic interaction (UHA) based on the EDLVO theory which usually is given as follows [21-24]:
UT=UA+UE+UHA (1)
where UT, UA, UE, and UHA are the total interaction energy, van der Waals interaction energy, electrostatic interaction energy and hydrophobic interaction energy, respectively.
The van der Waals interaction energy of two spherical particles given by GREGORY [21] is as follows:
 (2)
(2)
where A132 is the combined Hamaker constant for two different minerals (1 and 2) interacting across water (3) given by Eq. (3); H is the distance between two mineral particles; R is the radius of spherical particles; λL is the wavelength of intrinsic oscillations of atoms.
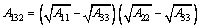 (3)
(3)
wher A11 is the Hamaker constant of mineral 1; A22 is the Hamaker constant of mineral 2; A33 is the Hamaker constant of water in the vacuum, and its value is equal to 4.0×10-20 J.
The Hamaker constants of magnetite, pentlandite and serpentine here are 23.9×10-20, 22.8×10-20, and 8.7×10-20 J, respectively. The radii of magnetite, pentlandite and serpentine particles are 1.14, 16.11 and 12.23 μm, respectively.
The electrical interaction energy of two spherical particles is determined by Eq. (4) combined with LSA model and Derjaguin model.
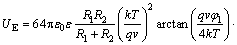
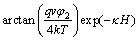 (4)
(4)
where q is the electronic charge of 1.602×10-20 C; ε0 is the permittivity of free space (8.854×10-12 F/m); ε is the relative permittivity (for water, ε=81 F/m); k is the Boltzmann constant of 1.30806×10-20 J/K; T is the temperature (298 K); κ is the reciprocal of the Debye length of 1.04×108 m-1; and φ is the zeta potential of minerals.
For two different spherical particles, with adsorbed layer thicknesses δ1 and δ2 correspondingly, the energy of hydrophobic interaction is expressed in the following equation:
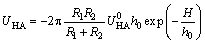 (5)
(5)
where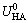 is the hydrophobic interaction constant, 7.98×10-3 J/m2; h0 is the hydrophobic force decay length (1.0×10-9 m).
is the hydrophobic interaction constant, 7.98×10-3 J/m2; h0 is the hydrophobic force decay length (1.0×10-9 m).
According to Eq. (1), the total interaction energy between magnetite and pentlandite (or serpentine) was calculated at pH 8.8 in the absence or presence of surfactants as shown in Fig. 6.
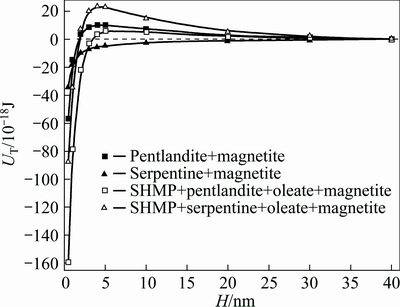
Fig. 6 Changes in total interaction energy of pentlandite-magnetite and serpentine-magnetite in the absence or presence of surfactants
From Fig. 4, the zeta potentials of pentlandite and magnetite minerals without the addition of surfactants were both negative at pH 8.8, while the serpentine surface became slightly positively charged. The affinity between magnetite and serpentine was stronger than that between magnetite and pentlandite particles that started at a separation distance of around 5 nm. In Fig. 6, at the separation distance of 4 nm, when the surfactants were added in the suspension, the interaction of magnetite-serpentine particles was reversed from attractive to repulsive. Simultaneously, magnetite-pentlandite particles began to attract each other. Below this distance, the attraction of magnetite-pentlandite particles became stronger in range and magnitude. This is a result of the serpentine surfaces being reversed to a more negative value (-64 mV) with the addition of SHMP, whereas with the addition of SHMP, the zeta potential of pentlandite mineral was almost unaffected and that of magnetite surfaces produced a slight left shift. As a result, pentlandite particles were coated by magnetite fines and surface magnetism of pentlandite was enhanced to enable its separation from serpentine by the magnetic separation method.
4 Conclusions
1) Pentlandite could be separated from serpentine at pH 8.8 and a magnetic intensity of 200 kA/m, and by adding 5% magnetite fines. A nickel concentrate of 19.26% Ni and 4.85% MgO with a satisfactory recovery of 92.46% was obtained.
2) SEM images and EDS spectra further confirm that quantities of magnetite fines are adhered to the pentilandite surfaces in the presence of surfactants.
3) The calculation of EDLVO theory showed that when the surfactant of SHMP was added in the mixed mineral suspension, the interaction between magnetite and serpentine was reversed from attractive to repulsive; whereas attractive interaction occurred between magnetite and pentlandite particles at the same separation distance of 5 nm.
References
[1] XING Wei-zhong. Theory and practice of magnetic seeds separation [M]. Beijing: Metallurgical Industry Press, 1994: 112-170. (in Chinese)
[2] WU Xi-qing, XU Peng-yun, DUAN Yun-feng, HU Cong, LI Guo-ping. Surface magnetization of siderite mineral [J]. International Journal of Mining Science and Technology, 2012, 22(6): 825-830.
[3] PLUMPTON A J. Production and processing of fine particles [M]. Pergamon, Amsterdam: Elsevier, 1988: 465-474.
[4] FRANGISKOS A, GAMBOPOULOS T. Magnetic beneficiation of magnesite ores by enhancing magnetic properties of gangue material: China, CA1013709 [P]. 1977-07-12.
[5] LUO Zhao-jun, QIAN xin, WANG Wen-qian. A new method of phosphorus separation-magnetic coating [J]. China Mining Magazine, 2000, 8(1): 56-58. (in Chinese)
[6] SINGH S, SAHOO H, RATH S S, PALEI B B, DAS B. Separation of hematite from Banded Hematite Jasper (BJH) by magnetic coating [J]. Journal of Central South University, 2015, 22(1): 437-444.
[7] UCBAS Y, BOZKURT V, BILIR K, IPEK H. Separation of chromite from serpentine in fine sizes using magnetic carrier [J]. Separation Science and Technology, 2014, 49(6): 946-956.
[8] ANASTASSAKIS G N. A study on the separation of magnesite fines by magnetic carrier methods [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 149: 585-593.
[9] ANASTASSAKIS G N. Separation of fine mineral particles by selective magnetic coating [J]. Journal of Colloid and Interface Science, 2002, 256(1): 114-120.
[10] SINGH S, SAHOO H, RATH S S, SAHU A K, DAS B. Recovery of iron minerals from Indian iron ore slimes using colloidal magnetic coating [J]. Powder Technology, 2015, 269: 38-45.
[11] KARAPINAR N. Magnetic separation of ferrihydrite from wastewater by magnetic seeding and high-gradient magnetic separation [J]. International Journal of Mineral Processing, 2003, 71(1-4): 45-54.
[12] ZU Zhan-liang, DU Yu-cheng, LUO Jia-ke, YANG Jiu-liu. Study on application of selective magnetic seeding separation process in coal gangue [J]. Multipurpose Utilization of Mineral Resources, 1998, 3: 1-4. (in Chinese)
[13] LIU Q X, FRIEDLAENDER F J. Fine particle processing by magnetic carrier methods [J]. Minerals Engineering, 1994, 7(4): 449-463.
[14] FENG Bo, LU Yi-ping, FENG Qi-ming, DING Peng, LUO Na. Mechanisms of surface charge development of serpentine mineral [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(4): 237-242.
[15] EDWARDS G R, KIPKIE W B, AGAR G E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite on pentlandite flotation [J]. International Journal of Mineral Processing, 1985, 7(1): 33-42.
[16] KIRJAVAINEN V, HEISKANEN K. Some factors that affect beneficiation of sulphide nickel–copper ores [J]. Minerals Engineering, 2007, 21(7): 629-633.
[17] WELLHAM E J, ELBER L, YAN D S. The role of carboxy methyl cellulose in the flotation of a nickel sulphide transition ore [J]. Minerals Engineering, 1992, 5(3): 381-395.
[18] LU Yi-ping, ZHANG Ming-qiang, FENG Qi-ming, LONG Tao, OU Le-ming, ZHANG Guo-fang. Effect of sodium hexametaphosphate on separation of serpentine from pyrite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1): 208-213.
[19] PRAKASH S, DAS B, MOHANTY J K, VENUGOPAL R. The recovery of fine iron minerals from quartz and corundum mixtures using selective magnetic coating [J]. International Journal of Mineral Processing, 1999, 57(1): 87-103.
[20] BREMMELL K E, FORNASIERO D, RALSTON J. Pentlandite– lizardite interactions and implications for their separation by flotation [J]. Colloids and Surfaces A, 2005, 252(2-3): 207-212.
[21] GREGORY J. Approximate expressions for retarded van der Waals interaction [J]. Journal of Colloid and Interface Science, 1981, 83(1): 138-145.
[22] JIANG Hao, XIE Zhen, LIU Guo-rong, YU Ya-wen, ZHANG Ding. Interaction forces between muscovite and silica surfaces in electrolyte solutions measured with AFM [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1783-1788.
[23] KUSUMA A M, LIU Q X, ZENG H B. Understanding interaction mechanisms between pentlandite and gangue minerals by zeta potential and surface force measurements [J]. Minerals Engineering, 2014, 69: 15-23.
[24] YIN Wang-zhong, WANG Ji-zhen. Effects of particle size and particle interactions on scheelite flotation [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3682-3687.
镍黄铁矿表面磁性增强及其与蛇纹石的磁选分离
袁致涛1,卢冀伟1,刘炯天1, 2,李丽匣1,王双玉1
1. 东北大学 资源与土木工程学院,沈阳 110819;
2. 郑州大学 化工与能源学院,郑州 450001
摘 要:通过控制矿浆pH值和添加表面活性剂,实现微细粒磁铁矿在镍黄铁矿表面选择性粘附而使其表面磁性增强。结果表明,在pH=8.8时,添加六偏磷酸钠(用量为100 g/t)、油酸(用量为4.5 L/t)和煤油(用量为4.5 L/t)后,微细磁铁矿选择性粘附在镍黄铁矿表面,使其磁性增强;而蛇纹石表面几乎不被磁铁矿粘附。然后,在磁场强度为200 kA/m条件下磁选,实现了二者的良好分离。扫描电镜结果也进一步证实了微细粒磁铁矿在镍黄铁矿表面发生了选择性粘附,而蛇纹石表面未观察到磁铁矿的存在。zeta电位测试和扩展DLVO理论计算结果表明,添加表面活性剂后,磁铁矿和镍黄铁矿颗粒间存在很强的吸引作用,从而使微细粒磁铁矿选择性粘附在镍黄铁矿表面,增强了其表面磁性。
关键词:镍黄铁矿;蛇纹石;微细磁铁矿;磁选;选择性磁罩盖
(Edited by Wei-ping CHEN)
Foundation item: Project (51574061) supported by the National Natural Science Foundation of China; Project (N150106004) supported by the Fundamental Research Funds for the Central Universities, China; Project (2014SKY-WK011) supported by the Open Fund Project of Shaanxi Key Laboratory of Comprehensive Utilization of Tailings Resources, China
Corresponding author: Ji-wei LU; Tel: +86-24-83687694; E-mail: lujiwei20041202@163.com
DOI: 10.1016/S1003-6326(17)60023-2
Abstract: The magnetism of pentlandite surface was enhanced through the selective precipitation of micro-fine magnetite fractions on pentlandite surfaces. This was achieved through adjustment of slurry pH and addition of surfactants. The results showed that at pH 8.8 with the addition of 100 g/t sodium hexametaphosphate, 4.5 L/t oleic acid, and 4.5 L/t kerosene, significant amount of fine magnetite particles adhered to the pentlandite surface, while trace amount of coating was found on serpentine surfaces. Thus, the magnetism of pentlandite was enhanced and pentlandite was well separated from serpentine by magnetic separation under the magnetic field intensity of 200 kA/m. Scanning electron microscopy (SEM) and zeta potential measurement were performed to characterize changes of mineral surface properties. Calculations of the extended Derjaguin-Landau-Verwey-Ocerbeek (EDLVO) theory indicated that, in the presence of surfactants the total interaction energy between magnetite and pentlandite became stronger than that between magnetite and serpentine. This enabled the selective adhesion of magnetite particles to pentlandite surfaces, thereby enhancing its magnetism.


