文章编号:1004-0609(2011)05-1159-06
含碳铬铁矿球团的固态还原特性
李建臣1, 2, 白国华1, 李光辉1
(1. 中南大学 资源加工与生物工程学院,长沙 410083;2. 中冶华天工程技术有限公司 烧结部,南京 210019)
摘 要:通过含碳铬铁矿粉矿成球及还原实验,研究还原温度、内配碳量、还原时间和添加剂对铬铁矿球团预还原结果的影响。结果表明:还原温度和内配碳量对预还原球团的金属化指标影响非常明显,温度高于1 200 ℃时,铬铁矿预还原球团的金属化指标开始明显增加,内配碳比IC/O=1.2为铬铁矿预还原球团的较佳配比;高温 (≥1 300 ℃)时,可实现含碳铬铁矿球团的快速还原(t ≤1 h);低温时,铁优先于铬还原,总还原反应完成时间需3~4 h。添加剂强化含碳铬铁矿球团预还原的实验结果表明,不同添加剂对还原反应的影响具有较大差异;不同温度下,相同添加剂的催化能力也有一定的差异;实验所涉及的添加剂中以NaCl、Na2B4O7·10H2O和Na2CO3的催化效果较好。
关键词:
中图分类号:TF046;TF641 文献标志码:A
Solid-state reduction properties of carbon-bearing chromite pellets
LI Jian-chen1, 2, BAI Guo-hua1, LI Guang-hui1
(1. School of Resources Processing and Bio-engineering, Central South University, Changsha 410083, China;
2. Sintering Department, Huatian Engineering and Technology Corporation, MCC, Nanjing 210019, China)
Abstract: The balling and reducing experiments of carbon-bearing chromite fines were carried out in laboratory. The effects of reduction temperature, amount of carbon, reduction time and types of additives on the metallization of carbon-bearing chromite pellets were investigated. The results show that the reductivity of carbon-bearing chromite pellet is greatly influenced by the reduction temperature and C/O mole ratio. At the temperature above 1 200 ℃, the metallization of prereduction pellet begins to increase obviously, and IC/O=1.2 is better for the prereduction of chromite pellet. The fast reduction of carbon-bearing chromite pellet can be achieved at high temperature (≥1 300 ℃); but it needs 3-4 h to finish the reduction reaction at low temperature, and the reduction reaction of iron is easier than that of chromium. The intensifying reduction of carbon-bearing chromite pellet with the help of additives was studied. The results indicate that different additives have great difference in the influence of reduction reaction, and the catalytic property of the same additive also has some difference at different reduction temperatures. For all involved additives in the experiment, NaCl, Na2B4O7·10H2O and Na2CO3 have excellent catalytic performance.
Key words: chromite; pellet; solid-state reduction; metallization
铬铁合金是生产不锈钢和高铁素体合金的最重要合金材料的一种,它可作为钢的添加料生产多种高强度、抗腐蚀、耐磨、耐高温、耐氧化的特种钢[1]。目前,工业生产中为降低铬铁生产设备的造价,各厂都趋向使用大型还原封闭电炉,这些电炉必须使用硬块铬铁矿[2-3]。目前,世界铬铁矿年开采量中块矿约占20%,粉矿(<8 mm)约占80%[4]。由于硬块铬铁矿供应困难,这就迫使各厂使用廉价的铬铁矿粉矿,但这类矿必须经过预处理才能入炉。
铬铁矿粉矿预处理技术包括烧结、造球等工艺。由于铬尖晶石熔点很高,且难以形成低熔点的液相,烧结法处理铬铁矿存在产量低、燃耗高及烧结矿强度差等缺点。铬铁矿粉矿球团工艺最具代表性的为固态还原法(SRC),即预还原法。实践表明,预还原球团冶炼铬铁,可提高电炉生产能力,降低电耗;其缺点是还原操作温度较高(>1 300 ℃)、还原时间较长(> 4 h)[5-6]、炉衬损耗较大且球团金属化率不超过70%。科研人员对铬铁矿球团还原工艺进行了许多研 究[7-17],但还没有开发出成熟的、与工业生产相匹配的适宜流程,因此有待进一步探索。本文作者通过研究铬铁矿球团预还原的特性,探讨了还原温度、还原时间、内配碳及添加剂对铬铁矿球团还原的影响规律,以期实现节能降耗、增产增效,并为在铬铁合金生产中利用铬铁矿粉矿制备优质炉料的研究提供参考。
1 实验
实验所用铬铁粉矿来自辽阳进口矿;其外观颜色为黑色,具有半金属光泽。铬铁矿样品的化学成分见表1,其粒度组成见表2,其XRD谱见图1。
铬铁矿样品的XRD分析结果表明,原矿中主物相为(Mg,Fe)O·(Cr,Al)2O3和(Mg,Fe)Cr2O4;次物相为Fe3O4和FeO·(Cr,Al)2O3;光学显微结构鉴定结果表明,原矿中还含镁橄榄石、铁镁橄榄石等极微量物相。
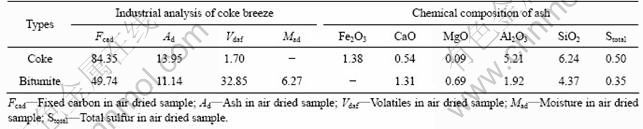
实验所用还原剂为烟煤和焦粉。焦粉来自于上海宝钢烧结厂;烟煤来自于新疆哈密。内配焦粉的工业分析结果及其灰分的化学组成如表3所列。使用时,内配焦粉磨细至90%的颗粒粒径小于0.074 mm;外用还原剂烟煤经破碎、筛分,分离出粒径为0~3 mm的烟煤供还原使用。实验所用添加剂主要由钠盐组成,均为分析纯(AR)级别。使用时,将添加剂磨细至粒度不大于0.074 mm,按一定质量比与铬铁矿粉、焦粉混匀造球。
表1 铬铁矿样品的化学成分
Table 1 Chemical composition of chromite sample (mass fraction, %)
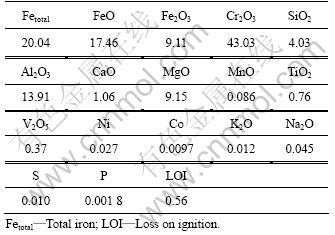
表2 铬铁矿样品的粒度组成
Table 2 Size distribution of chromite sample

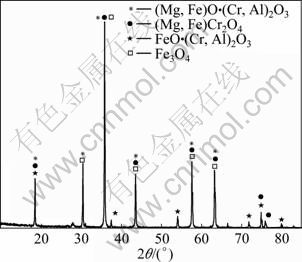
图1 铬铁矿样品的XRD谱
Fig.1 XRD pattern of chromite fines sample
表3 焦粉工业分析结果及其灰分的化学组成
Table 3 Industrial analysis results of coke breeze and chemical compositions of ash (mass fraction, %)
根据实验要求,按一定质量比在铬铁矿粉中配入焦粉和添加剂,经混匀后,加水润湿,再人工制成直径为10~12 mm的球团;造好的球团放入温度为 (105±5) ℃的FN101-1型鼓风烘箱中至少干燥2 h,以确保球团烘干;每次取5个烘干的球团配加一定量的焦炭或还原煤置于d 50 mm×100 mm石墨坩埚(坩埚内球团的上下部均加入一定量的还原煤),再一并放入设定好温度的竖式高温气氛管式炉(发热元件为Si-Mo棒,炉管型号为d 100 mm×1 000 mm),以2 L/min氮气为保护气体,调整还原所需的时间进行还原反应;待达到试验所设定的时间,取出料罐,并在2 L/min氮气流里冷却至室温,得到铬铁矿预还原球团。
还原产品经破碎、制样后,采用化学物相分析并结合XRD分析测定预还原球团中金属化铬(MCr)、金属化铁(MFe)的含量,采用氧化还原容量法测定还原样中TCr、TFe含量。实验以ηCr、ηFe、ηtotal来评价还原效果。计算方法如下:
ηCr = MCr/TCr (1)
ηFe = MFe/TFe (2)
ηtotal = (MCr+ MFe)/(TCr+TFe) (3)
式中:ηCr、ηFe和ηtotal分别表示还原样的铬、铁和总金属化率,%;MCr为还原样中金属化铬含量,%;MFe为还原样中金属化铁含量,%;TFe为还原样中全铁的含量,%;TCr为还原样中全铬的含量,%。
2 结果与分析
徐荣军等[17]以CO作还原气体,研究了内配碳铬矿球团的还原特性。本实验拟采用储量丰富、价格低廉的煤作还原剂,进行煤基还原含碳铬铁矿球团的研究,考虑到还原时间较长可能造成还原剂的不足,还原过程中每隔30 min,向料罐内添加30 g烟煤,以确保炉内还原气氛,并保护还原产品。还原温度对含碳铬铁矿球团预还原的影响如图2所示。实验条件如下:球团内配焦比IC/O=1.2(IC/O为球团内固定碳物质中的碳与被还原的氧化物中的氧的摩尔比,下同),还原时间为4 h,以烟煤为外部还原剂。
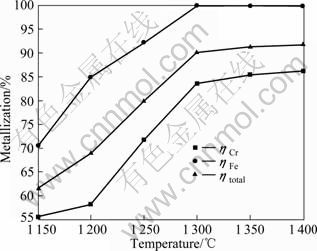
图2 还原温度对含碳铬铁矿球团预还原的影响
Fig.2 Effect of reduction temperature on prereduction of carbon-bearing chromite pellet
结果显示,随还原温度的升高,各金属化指标均呈增加趋势;超过1 300 ℃,增加趋势变缓,这是由于产生了密实的金属壳层,覆盖在未还原的矿物周围,阻碍了还原气体的扩散反应;此时铬金属化率83.56%,铁已还原完毕。在1 150和1 200 ℃时,铬金属化率分别为55.59%和58.18%,铁的金属化率分别为70.58%和84.88%,铁优先于铬还原;铬从1 200 ℃以后才开始快速还原,还原温度对铬金属化率的影响远比对铁的大,这是因为Cr2O3的还原是强吸热反应,因此,温度越高,越有利于Cr2O3的还原。
图3所示为不同温度下铬铁矿预还原球团的显微矿相结构。由图3可以看出,还原温度对铬铁矿球团预还原的影响非常显著, 1 250~1 300 ℃为铬铁矿预还原的温度过渡区间,达到此区间,铬铁矿球团才开始快速还原。随还原温度的升高,铬铁矿中铬矿物和铁矿物得到了大量还原,生成的金属化铬铁(白色)由星点颗粒或小片状逐渐增多、长大,最后聚结为大片状,并由铬铁矿边缘向中心扩散,覆盖在未还原的铬铁矿表面形成金属结圈,阻碍了扩散反应的通道,对后期反应不利,使还原反应几乎停滞。这进一步证明了上述实验现象的正确性。
当球团内配碳比IC/O为0.8、1.0和1.2,还原时间为4 h时, 不同温度下内配碳量对预还原结果的影响见图4。由图4可见,内配碳量对铬铁矿球团还原的影响十分明显,且温度为1 200~1 300 ℃时更显著;温度为1 150 ℃时,内配碳素不能充分消耗,致使还原球内部仍残留大量游离碳,碳热自还原反应的程度较弱,导致球团金属化率较低;温度高于1 300 ℃时,还原温度的影响成为了绝对主导,配碳量的影响开始变弱。当IC/O=1.2时,铬铁矿预还原球团的各金属化指标表现出很大增幅。铬的碳热还原主要为借助碳气化反应的间接还原,紧贴着氧化物的精细碳粒降低了还原进程中气相的氧位,为碳热自还原反应创造了条件,使CO分压增大,促进了扩散交换,因此,强化了还原反应效果。
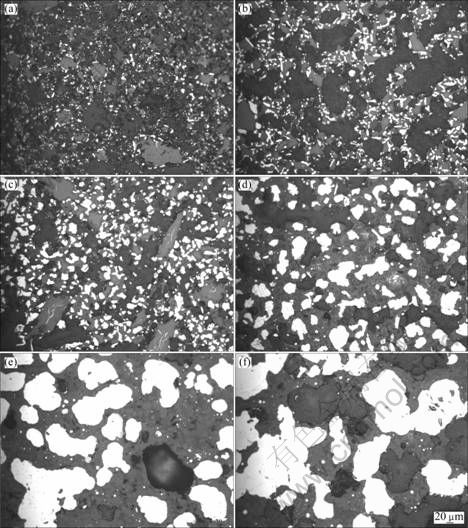
图3 不同温度下铬铁矿预还原球团的显微结构
Fig.3 Microstructures of preproduction balls of chromite ores at different temperatures: (a) 1 150 ℃, IC/O=1.2, 4 h; (b) 1 200 ℃, IC/O=1.2, 4 h; (c) 1 250 ℃, IC/O=1.2, 4 h; (d) 1 300 ℃, IC/O=1.2, 4 h; (e) 1 350 ℃, IC/O=1.2, 4 h; (f) 1 400 ℃, IC/O=1.2, 4 h
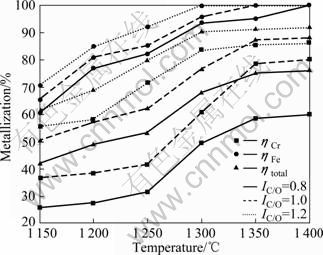
图4 内配碳比对铬铁矿球团预还原的影响
Fig.4 Effects of C/O mole ratio on prereduction of chromite pellet
球团内配碳比IC/O=1.2,不同还原温度下,还原时间对预还原结果的影响如图5所示。结果表明,高温(≥1 300 ℃)时,碳的气化反应速率以及还原过程扩散速率均较大,可实现快速还原,还原1 h已基本完成;温度越高,金属化指标的增幅受还原时间的制约越小。低温时,铬铁矿表面某些质点上的铁矿物优先得到还原,并逐渐向中心推进,2 h后其金属化率趋于稳定;受界面反应和形核势垒的限制,铬矿物还原及其产物形核速率较慢,故铬的大幅还原则在1~2 h后,整个还原需要3~4 h才能完成。
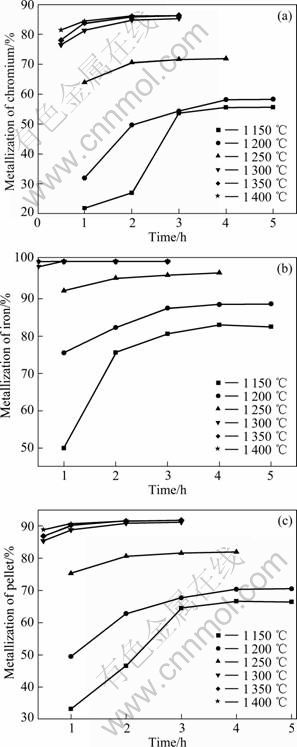
图5 还原时间对金属化率的影响
Fig.5 Effects of reduction time on metallization of chromium (a), iron (b) and pellet (c)
上述实验表明,铬铁矿球团还原要达到较高的金属化率需要较高的温度(≥1 300 ℃),这不利于节能降耗,为了强化铬铁矿球团的预还原效果,国内外科研人员进行了许多相关的研究,其中配加添加剂(催化剂)被认为是一种较有效的方法[16-17]。前期研究已表明,铬铁矿球团还原主要受铬还原的制约,为了考察实验条件下,不同添加剂对铬还原的催化规律,本实验以2%Na2B4O7·10H2O为基准,其他添加剂换算成与其含有相同阳离子(Na+)质量分数。计算结果如下:0.74% Na2SO4,0.42% NaOH,0.55% Na2CO3,0.88% NaHCO3,0.6% NaCl,1.33% Na3PO4·12H2O,0.89% NaNO3。不同添加剂对铬金属化率的影响见图6。实验条件为:球团内配碳比IC/O=1.2,还原时间为4 h,以烟煤为外部还原剂。
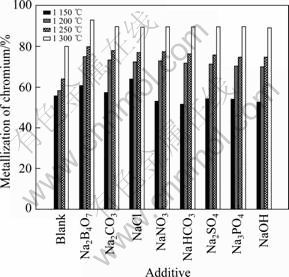
图6 添加剂对铬金属化率的影响
Fig.6 Effects of additives on metallization of chromium in prereduction of pellet
由图6可见,不同温度下,不同添加剂的催化能力具有一定的差异:1 150 ℃时,NaCl>Na2B4O7·10H2O>Na2CO3>未加添加剂>其它添加剂;1 200~1 250 ℃时,Na2B4O7·10H2O>Na2CO3>NaNO3>NaCl>NaHCO3>Na2SO4>Na3PO4·12H2O>NaOH>未加添加剂;1 300 ℃时,Na2B4O7·10H2O>Na2CO3>NaHCO3>NaCl >NaNO3>Na2SO4 >Na3PO4·12H2O>NaOH>未加添加剂。对于添加剂强化铬铁矿还原的机理,研究者提出了不同的理论[18],概括为添加剂强化了碳的气化反应;促进了固相扩散反应;导致了矿物晶格的畸变,降低了其表观反应活化能等理论。因此,添加剂强化含碳铬铁矿球团还原的作用机理较复杂,受还原温度、还原时间和添加剂类别等因素的影响,是多种因素耦合作用的结果,具体机制有待进一步探索和研究。综合而言,本实验所涉及的添加剂中以NaCl、Na2B4O7·10H2O和Na2CO3的催化效果较好,可为降低铬铁矿预还原温度以及缩短还原时间提供帮助。
3 结论
1) 还原温度和内配碳量对预还原球团的金属化指标影响非常明显,温度高于1 200 ℃时,铬铁矿预还原球团的金属化指标开始明显增加,内配碳比IC/O=1.2为铬铁矿预还原球团的较佳配比;高温 (≥1 300 ℃)时,可实现铬铁矿球团的快速还原(t≤ 1 h);低温时,铁优先于铬还原,总反应完成时间需3~4 h。
2) 添加剂强化含碳铬铁矿球团还原的作用受还原温度、添加剂类别等因素的影响,是多种因素耦合作用的结果;实验所涉及的添加剂中以NaCl、Na2B4O7·10H2O和Na2CO3的催化效果较好。
REFERENCES
[1] WEBER P, ERIC R H. The reduction of chromite in the presence of silica flux[J]. Minerals Engineering, 2006, 19(3): 318-324.
[2] TAKANO C, ZAMBRANO A P, NOGUEIR A E A, MOURAO M B, IGUCHI Y. Chromites reduction reaction mechanisms in carbon-chromites composite agglomerates at 1 773 K[J]. ISIJ International, 2007, 47(11): 1585-1589.
[3] YANG Y D, SOMMERVILLE I D, JOHNSTON R F, MCLEAN A. Use of solid state carbothermic reduction in production of transition metals and their carbides[J]. J Iron & Steel Res, 2000, 7(2): 15-22.
[4] ZHU D Q, LI J, PAN J, HE A P. Sintering behaviours of chromite fines and the consolidation mechanism[J]. Int J Miner Process, 2008, 86 (1/4): 58-67.
[5] 阎江峰, 陈加希, 胡 亮. 铬冶金[M]. 北京: 冶金工业出版社, 2007: 119-120.
YAN Jiang-feng, CHEN Jia-xi, HU Liang. Chromium metallurgy[M]. Beijing: Metallurgical Industry Press, 2007: 119-120.
[6] SINGH V, MOHAN RAO S. Study the effect of chromite ore properties on pelletisation process[J]. Int J Miner Process, 2008, 88(1/2): 13-17.
[7] CHEN J, ZHAO J, ZHANG M, YAN H, ZHOU J X. Carburization of ferrochromium metals in chromium ore fines containing coal during voluminal reduction by microwave heating[J]. Journal of Central South University of Technology, 2009, 16(1): 43-48.
[8] SOYKAN O, ERIC R H, KING R P. Kinetics of the reduction of Bushveld complex chromite ore at 1 416 ℃[J]. Metallurgical Transactions B, 1991, 22(6): 801-810.
[9] SOYKAN O, ERIC R H, KING R P. Reduction mechanism of a natural chromite at 1 416 ℃[J]. Metallurgical Transactions B, 1991, 22(1): 53-63.
[10] VAZARLIS H G, LEKATOU A. Pelletising-sintering, prereduction, and smelting of greek chromite ores and concentrates[J]. Ironmaking and Steelmaking, 1993, 20(1): 42-53.
[11] LEKATOU A, WALKER R D. Solid state reduction of chromite concentrate: Melting of prereduced chromite[J]. Ironmaking and Steelmaking, 1995, 22(5): 378-392.
[12] LEKATOU A, WALKER R D. Mechanism of solid state reduction of chromite concentrate[J]. Ironmaking and Steelmaking, 1995, 22(5): 393-404.
[13] HAZAR-YORUC A B. Reduction mechanism of chromite spinel with carbon[J]. Minerals and Metallurgical Processing,2007, 24(2): 115-120.
[14] ATASOY A, SALE F R. An investigation on the solid state reduction of chromite concentrates[C]//Mechatronic Systems and Materials Ⅲ. Diffusion and Defect Data Part. B: Solid State Phenomena. Switzerland: Trans Tech Publications Ltd, 2009: 752-757.
[15] ZAMBRANO A P, TAKANO C, MOURAO M B. Enhanced reduction of self-reducing pellet of chromites with Fe-Si addition in the reductant[C]//TMS 2008 EPD Congress. Warrendale, PA: Minerals, Metals & Materials Society, 2008: 449-454.
[16] ZAMBRANO A P, TAKANO C, NOGUEIRA A E A, RIBEIRO T R, MOURAO M B. Reduction behavior of chromite carbon composite pellets, at temperature of 1 873 K[C]//ANNALS-Int Meet Ironmak Int Symp Iron Ore 3rd International Meeting on Ironmaking and 2nd International Symposium on Iron Ore. Maranhao, Brazil: Int. Meet. Ironmak. Int. Symp. Iron Ore, 2008: 667-677.
[17] 徐荣军, 倪瑞明, 张圣弼, 马中庭. 含碳铬矿的造球及还原催化研究[J]. 烧结球团, 1995, 20(2): 10-14.
XU Rong-jun, NI Rui-ming, ZHANG Sheng-bi, MA Zhong-ting. Balling and reduction experiments of carbon-bearing chromite powder[J]. Sintering and Pelletizing, 1995, 20(2): 10-14.
[18] DING Y L, WARNER N A. Catalytic reduction of carbon- chromite composite pellets by lime[J]. Thermochimica Acta, 1997, 292(1/2): 85-94.
(编辑 何学锋)
基金项目:国家杰出青年基金资助项目(50725416)
收稿日期:2010-03-08;修订日期:2010-07-05
通信作者:李建臣,硕士研究生, 助理工程师;电话:025-86991297;E-mail: lijianchencsu@sina.com


