
Electrochemical codeposition of Mg-Li-Gd alloys from LiCl-KCl-MgCl2-Gd2O3 melts
WEI Shu-quan1, 2, ZHANG Mi-lin1, HAN Wei1, YAN Yong-de1, ZHANG Meng1, ZHANG Bin1, 3
1. Key Laboratory of Superlight Materials and Surface Technology of Ministry of Education,
Harbin Engineering University, Harbin 150001, China;
2. College of Chemistry and Chemical Engineering, Harbin Normal University, Harbin 150025, China;
3. Institute of Petrochemistry, Heilongjiang Academy of Sciences, Harbin 150040, China
Received 25 September 2010; accepted 21 December 2010
Abstract:
Mg-Li-Gd alloys were prepared by electrochemical codeposition from LiCl-KCl-MgCl2-Gd2O3 melts on molybdenum electrode with constant current density at 823 and 973 K. The microstructure of the Mg-Li-Gd alloys was analyzed by X-ray diffraction (XRD), optical microscopy (OM) and scanning electron microscopy (SEM). The results show that magnesium and gadolinium deposit mainly in the first 30 min, and the alloy obtained contains 96.53% Mg, 0.27% Li and 3.20% Gd (mass fraction). Then, the reduction of lithium ions occurs quickly. The composition of alloy can be adjusted by controlling electrolysis time or Gd2O3 concentration in LiCl-KCl melts. With the addition of Gd into Mg-Li alloys, the corrosion resistance of the alloys is enhanced. XRD results suggest that Mg3Gd and Mg2Gd can be formed in Mg-Li-Gd alloys. The distribution of Gd element in Mg-Li-Gd alloys indicates that Gd element mainly distributes at the grain boundaries of Mg-Li-Gd alloys.
Key words:
electrochemical codeposition; Mg-Li-Gd alloy; chloride melt; galvanostatic electrolysis; Gd2O3;
1 Introduction
Mg-Li alloys are the lightest metallic materials with high specific strength, good machining property, good magnetic screen and shock resistance ability. They are widely used in the fields of aerospace, aircraft and weapons[1-3]. Because Li is a very active element, the stability of Mg-Li alloys is relatively poor[4-6].
To improve the mechanical properties and corrosion resistance of Mg-Li based alloys, rare earth elements are added; consequently, Mg-Li-RE alloys are widely studied[7-11]. The addition of rare earth elements can effectively increase the mechanical properties and high temperature performance of Mg-Li alloys, and can improve the corrosion resistance and the performance of creep. The effect of Gd on mechanical properties of Mg alloys was investigated by directly melting high purity magnesium and Mg-Gd master alloys in an electric resistance furnace. After the addition of Gd, the grain size of Mg alloys was refined, and the strength, the ultimate tensile strength and hardness were increased [12-14].
The electrochemical codeposition has been widely used to prepare binary or ternary alloys[15-19]. Electrochemical deposition of Gd has been investigated. ZHENG et al[20] synthesized Gd-Co film using an electrodeposition method. LI et al[21] codeposited Gd-Co film in urea-acetamide-NaBr-KBr-GdCl3-CoCl2 baths by potentiostatic electrolysis at 353 K. ZHAN and WANG[22] investigated the possibility of gadolinium electrodeposition in different ionic liquids at 373 K.
However, electrochemical codeposition of Mg-Li- Gd ternary alloys has not been investigated. In this work, Mg-Li-Gd ternary alloys were prepared on a molybdenum electrode in LiCl-KCl-MgCl2-Gd2O3 melts by constant current density electrolysis. XRD, ICP-AES, OM, SEM-EDS were used to characterize the Mg-Li-Gd alloys. Corrosion resistance of the alloy was investigated in sodium chloride solution.
2 Experimental
The mixture of LiCl-KCl-MgCl2-Gd2O3 was melted in 150 cm3 alumina crucible placed in a quartz cell inside an electric tubular furnace. The temperature of melt was measured with a nickel-chromium thermocouple sheathed by an alumina tube. The LiCl and KCl were dried in electric furnace for more than 48 h at 573 K and 873 K, respectively. Gadolinium and magnesium were introduced into the bath in the form of Gd2O3 (≥99.0%) and dehydrated MgCl2 powder. All experiments were carried out under an argon atmosphere.
X-ray diffraction (Rigaku TRR Ⅲ), scanning electron microscopy (SEM JSM-6480A; JEOL Co., Ltd.), optical microscopy (DFC320, Leica Microsystems) and inductively coupled plasma atomic emission spectrometer (ICP-AES, IRIS Intrepid II XSP, Thermo Elemental) analyses were also used.
3 Results and discussion
3.1 Electrochemical codeposition of Mg-Li-Gd alloys
Electrochemical codeposition of Mg-Li-Gd alloys was carried out by galvanostatic electrolysis on a Mo electrode from LiCl-KCl molten salts containing 10% MgCl2 and 2% Gd2O3 (mass fraction, the same below if not mentioned) at 823 K. Figure 1 shows the change of the molten salt concentration and the metal content in the Mg-Li-Gd alloys. From Fig.1(a), the Mg (II) and Gd (III) concentrations in the 2%Gd2O3-10%MgCl2-LiCl-KCl molten system, determined by ICP-AES, are 5.73% and 0.19% before electrolysis, respectively. According to Ref.[23], MgCl2 can react with Gd2O3 at high temperature as follows:
MgCl2+ Gd2O3 = 2GdOCl + MgO (1)
GdOCl + MgCl2=GdCl3 + MgO (2)
The concentration of MgCl2 reduces to less than 10% and so we can detect Gd ions by ICP-AES in Gd2O3-MgCl2-LiCl-KCl melts at 823 K.
From Fig.1(a) it can be also seen that the MgCl2 and Gd2O3 concentrations in the molten salts decrease quickly in the first 30 min of electrolysis, and then more slowly. After 30 min, LiCl concentration starts to decrease. This indicates that magnesium and gadolinium are deposited in the initial 30 min, and then metal lithium is reduced.
The above results are also confirmed in Fig.1(b), which shows the metal contents of Mg-Li-Gd alloys obtained by constant current density at various electrolysis periods. The alloy contains 96.53% Mg, 0.27% Li and 3.20% Gd after electrolysis for 30 min. The Li content of the alloys increases rapidly, and 43.07% Li in alloy is deposited after electrolysis for 40 min. This suggests that the composition of alloy can be adjusted by controlling the electrolysis period.
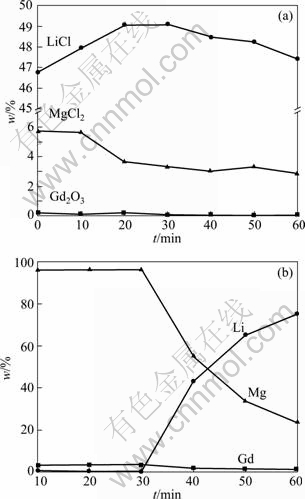
Fig.1 Change of molten salt concentration (a) and composition of alloy obtained by constant current density (b) at different periods
Galvanostatic electrolysis was also carried out in LiCl-KCl melts containing different concentrations of MgCl2 and Gd2O3 on a molybdenum electrode at 973 K (Table 1). Li content of Mg-Li-Gd alloys increases with the increase of cathode current density and electrolysis period. A higher Gd2O3 concentration in LiCl-KCl melts results in a higher Gd content in Mg-Li-Gd alloys. According to these results, the Gd content and Li content of Mg-Li-Gd alloys can be adjusted by changing the cathode current density and Gd2O3 concentration in LiCl-KCl melts.
3.2 Microstructure and composition of alloys
The microstructures of the Mg-Li-Gd alloys listed in Table 1 are shown in Fig.2. According to Mg-Li phase diagram[24], alloys A and B exhibit dual-phase microstructure (α+β phase), alloys C and D are β phases. As given in Fig.2(a), the bright and dark zones of the optical micrograph correspond to α and β phases, respectively. The dual-phase microstructure includes a β matrix and a distributed phase in lath form with a width of about 9 μm and a length of approximately 100 μm. Fig.2(c) exhibits only β matrix.
Table 1 ICP analysis of all samples obtained by galvanostatic electrolysis on Mo electrodes (S=0.322 cm2) from LiCl-KCl- MgCl2-Gd2O3 melts at 973 K
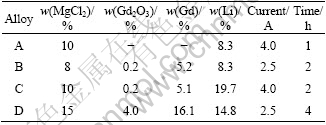
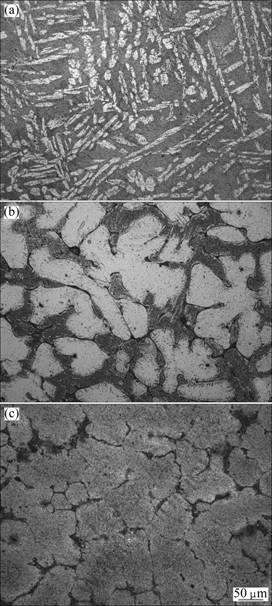
Fig.2 Optical micrographs of Mg-Li-Gd alloys alloy A (a), alloy B (b) and alloy C (c)
Figure 3 shows the XRD patterns of Mg-Li-Gd alloys obtained by galvanostatic electrolysis on a Mo electrode from LiCl-KCl-MgCl2-Gd2O3 melts at 973 K (Table 1). The XRD results are in good agreement with the one obtained in the analysis of optical micrograph. In alloys A and C, there are dual-phase structures (α + β phases). Gd has no effect on the phase structure of Mg-Li-Gd alloys because of the relatively low Gd content in Mg-Li-Gd alloys. When cathode current density is increased, phase transformation from α + β to β is observed (alloy C in Fig.3). However, in alloy D, there are Mg-Gd intermetallic compound phases besides β phase.
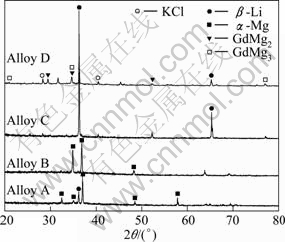
Fig.3 XRD patterns of alloys A, B, C and D
3.3 Gd element distribution and corrosion behavior of Mg-Li-Gd alloys
SEM and EDS mapping analysis of alloy B were employed to examine the distribution of Mg and Gd elements. The results show that elements Mg and Gd distribute in gray zones and bright zones in lath form, respectively (Fig.4). The gray zone in the SEM micrograph corresponds to the bright zone (α phase) in optical micrograph, and the bright lath form in the SEM micrograph corresponds to the black zone in the optical micrograph. According to the optical micrograph (Fig.5), element Gd mainly distributes at the grain boundary of Mg-Li-Gd alloys.
The corrosion behavior of alloy B with increasing content of gadolinium was evaluated in sodium chloride solution and compared with that of alloy A without containing gadolinium. Scanning electron microscopy microanalysis was used to characterize the samples. The experiments were carried out in 3.5% NaCl aqueous solution for 30 min at 298 K. Figure 6 shows that alloy A (Mg-8.3Li) has a relatively low corrosion resistance. After immersion in sodium chloride aqueous solution for 30 min at 298 K, severe corrosion is evident. In contrast, SEM characterization of alloy B (Mg-8.3Li-5.2Gd) after immersion in sodium chloride aqueous solution for 30 min at 298 K shows a less accentuated corrosion attack (Fig.6(b)).
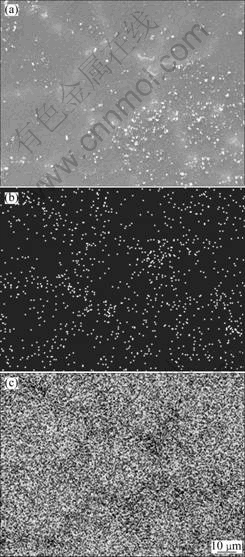
Fig.4 SEM image (a), EDS mapping analysis Gd (b) and Mg (c) of alloy B
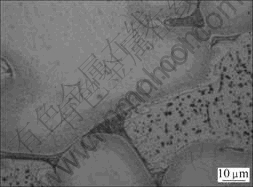
Fig.5 Microstructure of alloy B
As commonly known, corrosion at the grain boundary is most likely to occur. From above results, element Gd mainly distributes at the grain boundary of Mg-Li alloys. Mg-Gd intermetallic compounds enhance corrosion resistance of Mg-Li alloys.
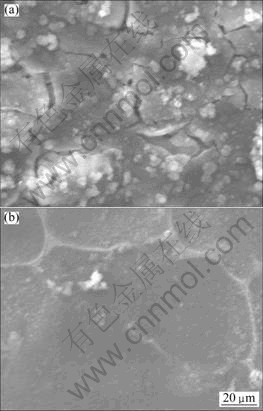
Fig.6 SEM images of alloy A (a) and B (b) after immersion for 30 min in 3.5% NaCl solution at 298 K
4 Conclusions
1) Mg-Li-Gd alloys were successfully prepared by electrochemical codeposition from LiCl-KCl-MgCl2- Gd2O3 melts on molybdenum electrode with constant current density at 823 and 973 K. The Gd content and Li content of Mg-Li-Gd alloys can be adjusted by changing the cathode current density and Gd2O3 concentration in LiCl-KCl melts.
2) The Gd element in Mg-Li-Gd alloys mainly distributes at the grain boundary of the alloy. Corrosion resistance of the alloy is enhanced with the addition of Gd in Mg-Li alloys.
References
[1] LI J F, ZHENG Z Q, LI S C, REN W D, ZHANG Z. Preparation and galvanic anodizing of a Mg-Li alloy [J]. Materials Science and Engineering A, 2006, 433: 233-240.
[2] CRAWFORD P, BARROSA R, MENDEZ J, FOYOS J, ES-SAID O S. On the transformation characteristics of LA141A (Mg-Li-Al) alloy [J]. Journal of Materials Processing Technology, 1996, 56: 108-118.
[3] WANG S J, WU G Q, LI R H, LUO G X, HUANG Z. Microstructures and mechanical properties of 5 wt.% Al2Yp/Mg-Li composite [J]. Materials Letters, 2006, 60(15): 1863-1865.
[4] DONG Han-wu, WANG Li-dong, WU Yao-ming, WANG Li-min. Effect of Y on microstructure and mechanical properties of duplex Mg-7Li alloys [J]. Journal of Alloys and Compounds, 2010, 506: 468-474.
[5] TENG Hai-tao, ZHANG Xiao-li, ZHANG Zhong-tao, LI Ting-ju, COCKCROFT S. Research on microstructures of sub-rapidly solidified AZ61 magnesium alloy [J]. Materials Characterization, 2009, 60: 482-486.
[6] LIU Bin, ZHANG Mi-lin, WU Rui-zhi. Effects of Nd on microstructure and mechanical properties of as-cast LA141 alloys [J]. Materials Science and Engineering A, 2008, 487: 347-351.
[7] YANG Xiao-wei, WANG Gui-xiang, DONG Guo-jun, GONG Fan, ZHANG Mi-lin. Rare earth conversion coating on Mg-8.5Li alloys [J]. Journal of Alloys and Compounds, 2009, 487: 64-68.
[8] WANG Tao, ZHANG Mi-lin, NIU Zhong-yi, LIU Bin. Influence of rare earth elements on microstructure and mechanical properties of Mg-Li alloys [J]. Journal of Rare Earths, 2006, 24: 797-800.
[9] HAN Wei, TIAN Yang, ZHANG Mi-lin, YE Ke, ZHAO Quan-you, WEI Shu-quan. Preparing different phases of Mg-Li-Sm alloys by molten salt electrolysis in LiCl-KCl-MgCl2-SmCl3 melts [J]. Journal of Rare Earths, 2010, 28: 227-231.
[10] WANG Tao, ZHANG Mi-lin, WU Rui-zhi. Microstructure and properties of Mg-8Li-1Al-1Ce alloy [J]. Materials Letters, 2008, 62: 1846-1848.
[11] WU Rui-zhi, QU Zhi-kun, ZHANG Mi-lin. Effects of the addition of Y in Mg-8Li-(1,3)Al alloy [J]. Materials Science and Engineering A, 2009, 516: 96-99.
[12] YANG Jie, XIAO Wen-long, WANG Li-dong, WU Yao-ming, WANG Li-min, ZHANG Hong-jie. Influences of Gd on the microstructure and strength of Mg-4.5Zn alloy [J]. Materials Characterization, 2008, 59: 1667- 1674.
[13] ZHANG Kui, LI Xing-gang, LI Yong-jun, MA Ming-long. Effect of Gd content on microstructure and mechanical properties of Mg-Y-RE-Zr alloys [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 12-16.
[14] GAO L, CHEN R S, HAN E H. Effects of rare-earth elements Gd and Y on the solid solution strengthening of Mg alloys [J]. Journal of Alloys and Compounds, 2009, 481: 379-384.
[15] IIDA T, NOHIRA T, ITO Y. Electrochemical formation of Sm-Co alloys by codeposition of Sm and Co in a molten LiCl-KCl-SmCl3-CoCl2 system [J]. Electrochimica Acta, 2003, 48: 2517-2521.
[16] HAN Wei, TIAN Yang, ZHANG Mi-lin, YAN Yong-de, JING Xiao-yan. Preparation of Mg-Li-Sm alloys by electrocodeposition in molten salt [J]. Journal of Rare Earths, 2009, 27: 1046-1050.
[17] YAN Yong-de, ZHANG Mi-lin, XUE Yun, HAN Wei, CAO Dian-xue, WEI Shu-quan. Study on the preparation of Mg-Li-Zn alloys by electrochemical codeposition from LiCl-KCl-MgCl2-ZnCl2 melts [J]. Electrochimica Acta, 2009, 54: 3387-3393.
[18] YAN Yong-de, ZHANG Mi-lin, XUE Yun, HAN Wei, CAO Dian-xue, HE Li-yi. Electrochemical study of Mg-Li-Al alloys by codeposition from LiCl-KCl-MgCl2-AlCl3 melts [J]. Journal of Applied Electrochemistry, 2009, 39: 455-461.
[19] YAN Yong-de, ZHANG Mi-lin, XUE Yun, HAN Wei, CAO Dian-xue, JING Xiao-yan, HE Li-yi, YUAN Yi. Electrochemical formation of Mg-Li-Ca alloys by codeposition of Mg, Li and Ca from LiCl-KCl- MgCl2-CaCl2 melts [J]. Physical Chemistry Chemical Physics, 2009, 11: 6148-6155.
[20] ZHENG Guang-ping, ZHAN Yang-wen, LIU Peng. Preparation and characterization of nanostructured Gd-Co films [J]. Journal of Alloys and Compounds, 2003, 358: 65-70.
[21] LI Jia-xin, LAI Heng, FAN Bi-qiang, ZHUANG Bin, GUAN Lun-hui, HUANG Zhi-gao. Electrodeposition of RE-TM (RE = La, Sm, Gd; TM= Fe, Co, Ni) films and magnetic properties in urea melt [J]. Journal of Alloys and Compounds, 2009, 477: 547-551.
[22] ZHAN Jun-jie, WANG Sen-lin. Preparation and character of Co-Gd films electroplated in urea-acetamide-NaBr melt [J]. Rare Metal Materials and Engineering, 2009, 38(10): 1698-1702. (in Chinese)
[23] DU Sen-lin, WU Mei-huang, DU Fu-ying, LIU Ying-ming. The solubility of rare earth oxides in alkali metal and alkaline metal fluorides melts [J]. Chinese Rare Earths, 1987(2): 59-62.(in Chinese)
[24] MASSALAKI T B, MURRAY J L, BENETT L H, BAKER H: Binary alloy phase diagrams [M]. America: America Society for Metals, 1990.
LiCl-KCl-MgCl2-Gd2O3熔盐共电沉积制备Mg-Li-Gd合金
魏树权1, 2, 张密林1, 韩 伟1, 颜永得1, 张 萌1, 张 斌1, 3
1. 哈尔滨工程大学 材料科学与化学工程学院,教育部超轻材料与表面技术重点实验室,哈尔滨 150001;
2. 哈尔滨师范大学 化学化工学院,哈尔滨 150025;
3. 黑龙江省科学院 石油化学研究院,哈尔滨 150040
摘 要:在823 K和973 K的条件下,采用恒电流密度共电沉积法在LiCl-KCl-MgCl2-Gd2O3熔盐体系中制备Mg-Li-Gd合金,并运用XRD、SEM、EDS和OM对所制备合金进行微观组织分析。结果表明:在开始的30 min内,主要是Mg和Gd的沉积,所得合金含96.53% Mg, 3.20% Gd和0.27% Li(质量分数),然后Li迅速沉积。可以通过控制电解时间或改变Gd2O3的浓度调节Mg-Li-Gd合金的组成。XRD分析可知,在Mg-Li-Gd合金中存在Mg3Gd相和Mg2Gd相。从Gd元素的面扫描分析可知,Gd元素主要分布在Mg-Li-Gd合金的晶界处。Gd的添加增强了合金的抗腐蚀能力。
关键词:共电沉积;Mg-Li-Gd合金;氯化物熔盐;恒电流电解;Gd2O3
(Edited by LI Xiang-qun)
Foundation item: Project (2009AA050702) supported by the National High-tech Research and Development Program of China; Project (GC06A212) supported by the Scientific Technology Project of Heilongjiang Province, China; Project (50871033) supported by the National Natural Science Foundation of China; Project (208181) supported by the Key Project of Ministry of Education, China; Project (HEUCF101002) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: ZHANG Mi-lin; Tel/Fax: +86-451-82533026; E-mail:zhangmilin2009@sina.com
DOI: 10.1016/S1003-6326(11)60788-7


