
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 2204-2211
Thermodynamic re-assessment of Fe-Ti binary system
BO Hong1, 2, WANG Jiang2, Liliana DUARTE 2, Christian LEINENBACH 2,
LIU Li-bin1, 3, LIU Hua-shan1, 3, JIN Zhan-peng1, 3
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Laboratory for Joining and Interface Technology, Swiss Federal Laboratories for
Materials Science and Technology, ?berlandstrasse 129, CH-8600, Dübendorf, Switzerland;
3. Key Laboratory of Nonferrous Metal Materials Science and Engineering, Ministry of Education,
Central South University, Changsha 410083, China
Received 30 September 2011; accepted 10 January 2012
Abstract:
The Fe-Ti binary system was re-assessed using the CALPHAD method in order to improve the capability of being extrapolated to a ternary or higher-order system. Compared with previous assessments, the main focus was put on the thermodynamic description of the two intermetallic compounds Fe2Ti and FeTi. The C14_Laves phase Fe2Ti was described by the two-sublattice model, which is widely used at present. By checking the homogeneity range on the boundary of the ternary systems involving the binary, the phase boundary of this compound was further confirmed. The FeTi phase with a BCC_B2 crystal structure was treated as the ordered phase of the BCC_A2 phase and a unified Gibbs energy function was used to describe both the ordered and disordered phases. Reproduction of the specific heat capacities of these compounds was another aspect paid particular attention to. Comprehensive comparisons of the calculated and experimental results regarding the phase diagram and thermodynamic properties show a good agreement between them and prove the validity of the present thermodynamic description.
Key words:
Fe-Ti system; order-disorder transition; thermodynamic assessment; phase diagram; CALPHAD;
1 Introduction
Fe-based alloys have been in the research focus for a long time and still remain attractive due to technical applications of all kinds of steels. It was found that the addition of up to 2% (mass fraction) Ti for the formation of carbides enhances the oxidation resistance because it acts as grain refiner [1]. Most recently, XU et al [2] carried out an investigation on the effect of Ti on the yield strength of martensitic steel fabricated by vacuum induction melting. It turned out that the addition of Ti can improve the yield strength of steel by 188 MPa, which is ascribed to precipitation hardening from TiC precipitates in the martensitic matrix. Moreover, the yield strength can be further enhanced through the process of tempering and quenching due to the formation of superfine sized grains and a large amount of nano-precipitates.
Besides the traditional applications in steels, Fe and Ti are also the important components in hydrogen storage materials. Metallic materials of either hydride forming transition metals MHn (n=1, 2, 3), metallic hydrides of intermetallic compounds ABxHn (x=0.5, 1, 2, 5) or complex hydrides forming an ionic or covalent compound can be used for hydrogen storage [3,4]. In the Fe-Ni-Ti system, the FeTi compound with partial substitution of Fe with Ni [5,6] and Ti2Ni [4] is capable of high hydrogen absorption.
Since the Fe-Ti binary is the key sub-system of the Fe-Ti-C and Fe-Ni-Ti ternary systems with applications as mentioned above, a critical evaluation and assessment of the phase diagram of the binary system is extremely important and urgent. Up to now, there is no a generally accepted version of the phase diagram. Yet, the optimized results of KUMAR et al [7] are mostly cited. Their results are in good agreement with all the available experimental data on phase diagram and thermodynamic properties except the specific heat capacities of the FeTi and Fe2Ti compounds. In addition, the extrapolation to ternary systems of interest is not reliable. Thus, in this work, a re-assessment of the Fe-Ti binary system is presented, hoping to give a reasonable and reliable thermodynamic description with a good ability of being extrapolated to any ternary or higher-order system.
2 Literature review
2.1 Information on phase diagram
The phase boundaries and invariant reactions above 1250 ℃ from 0 to 52% (mole fraction) Ti were determined by HELLAWELL and HUME-ROTHERY [8] using thermal analysis. KO and NISHIZAWA [9] reported the equilibrium composition of ferrite obtained by X-ray diffraction and electron probe micro-analysis (EPMA) and coexisting Fe2Ti compound only by EPMA. Their results regarding the solubility of Ti in BCC_Fe are consistent with those given by SPEICH [10] and ABRAHAMSON and LOPATA [11]. By means of micrographic analysis, thermal analysis and X-ray diffraction, van THYNE et al [12] determined the partial phase diagram from the composition of FeTi (50%Ti, mole fraction) to pure Ti. This is in general accordance with the BCC_Ti/BCC_Ti+HCP_Ti phase boundary given by McQUILLAN [13] and the Ti-rich liquidus and solidus by KIVILAHTI and TARASOVA [14]. The solubility of Fe in HCP_Ti has been measured by RAUB et al [15], BALESIUS and GONSER [16], MATYKA et al [17] and STUPEL et al [18]. Only the recent investigation by STUPEL et al [18] was considered because of the relatively large scatter of different sets of data.
For the investigations of the homogeneity range of the Fe2Ti compound, the results by HELLAWELL and HUME-ROTHERY [8], DEW-HUGHES [19] and QIU and JIN [20] are consistent, indicating homogeneity range centered on Fe2Ti. However, the results by MURAKAMI et al [21], KO and NISHIZAWA [9], BOOKER [22] and RAMAEKERS et al [23] are different, showing a homogeneity range leaning toward the Fe-rich side of Fe2Ti. In order to determine which set of data is more accurate, the homogeneity ranges of the Fe2Ti compound at 1173 K on the boundary of the Fe-Ti-Zr and Fe-Ti-Nb ternary systems are checked. It turns out to be 29%-34% (mole fraction) Ti [24,25]. This confirms that the results by MURAKAMI et al [21], KO and NISHIZAWA [9], BOOKER [22] and RAMAEKERS et al [23] are more reliable and are thus taken into consideration during the optimization. The width of the single phase field for Fe2Ti was reported to be 25%-37% (mole fraction) Ti at the eutectic temperature, which was recommended by MURRAY [26]. For the FeTi compound, MURAKAMI et al [21], BOOKER [22] and DEW-HUGHES [19] investigated its homogeneity range and 51.5%-52.0% (mole fraction) Ti was recommended by MURRAY [26].
The gamma loop determined by HELLAWELL and HUME-ROTHERY [8], MOLL and OGILVIE [27], WADA [28] and FISCHER et al [29] is consistent. The maximum extension of the FCC_Fe phase was reported to be 0.74% (mass fraction) Ti at 1423 K with the corresponding composition of BCC_Fe 1.24% (mass fraction) Ti [29].
2.2 Information on thermodynamic properties
The enthalpy of mixing of the liquid phase at 1873 K was measured calorimetrically by BATALIN et al [30] and WANG et al [31]. Subsequently, THIEDEMANN et al [32] measured the enthalpy of mixing of the liquid phase using levitation alloying calorimetry at 1950 K from 0 to 42% (mole fraction) Ti and at 2112 K from 0 to 31% (mole fraction) Fe. The partial enthalpies of mixing in the liquid phase were determined by ESIN et al [33] at 2000 K from 0 to 42% (mole fraction) Ti. Besides, the activities of Ti in the liquid phase at 1873 K were measured by FRUEHAN [34] and FURUKAWA and KATO [35] using the electromotive force (emf) method and the Knudsen cell-mass spectrometer combination technique, respectively.
The specific heat capacities of the intermetallic compounds FeTi and Fe2Ti were measured by WANG et al [36] from 120 to 700 K employing differential scanning calorimetry. The enthalpy of formation of FeTi was determined by KUBASCHEWSKI and DENCH [37] and GACHON et al [38] using direct reaction calorimetry. The reaction product was checked with X-ray diffraction in both of their work to ensure the stoichiometry and the structure. KUBASCHEWSKI and DENCH [37] reported that the Fe0.5Ti0.5 alloy consisted of almost entirely FeTi with some traces of Fe2Ti, while the Fe0.67Ti0.33 alloy consisted of FeTi and BCC_Fe with little amount of Fe2Ti. However, GACHON et al [38] found that Fe0.67Ti0.33 was pure while Fe0.5Ti0.5 contained traces of Fe2Ti. Moreover, DINSDALE et al [39] measured the enthalpy of formation of these two intermetallic compounds, as quoted in Ref. [40]. The results given by GACHON et al [38] and DINSDALE et al [39] are consistent with each other and thus used for the optimization.
2.3 Previous assessments
The Fe-Ti binary phase diagram was first assessed by KAUFMAN and NESOR [41] and then by many others [7,26,42-45]. Only the recent assessments will be discussed here to save space. In the work of DUMITRESCU et al [43] and JONSSON [44], the optimized phase boundary of the Fe2Ti compound is inappropriate, as discussed in section 2.1. In addition, the FeTi compound was treated as a stoichiometric phase, without considering the solubility of Fe and Ti components. No order-disorder transition between the FeTi and BCC_A2 phases was taken into account. KUMAR [7] made an improvement in his work and has thus been cited often. Yet, it was found that the experimental heat capacities of FeTi and Fe2Ti phases are not well reproduced. Most recently, KEYZER et al [45] re-optimized this binary system based on the work of KUMAR [7], with two major modifications. One is the use of a 3-sublattice model to describe the C14_Laves phase Fe2Ti. The other is changing the sign of the mixing enthalpy of the HCP phase to be the same as that of the FCC phase since both HCP and FCC phases are of closely packed structure with similar coordination.
In addition to maintaining the virtue of the optimized results of KUMAR [7], the optimization carried out in this work gives a good description of the specific heat capacities for Fe2Ti and FeTi compounds, uses 2-sublattice model for Fe2Ti phase, and makes the sign of the enthalpy of mixing of the HCP phase the same as that of the FCC phase.
3 Thermodynamic models
3.1 Solution phases
The solution phases including liquid, FCC, BCC and HCP are all described with the subsitutional solution model. The mole Gibbs energy can be expressed as a sum of the Gibbs energy of the pure elements, the Gibbs energy contributed from ideal entropy of mixing and the excess Gibbs energy, i.e.,
![]() (1)
(1)
where f denotes the solution phase; xi denotes the mole fraction of component i (i=Fe, Ti); ![]() is the molar Gibbs energy of pure component i in the structure of phase f, which is taken from the SGTE data compiled by DINSDALE [46]. The excess term is written as a Redlich-Kister polynomial, i.e.,
is the molar Gibbs energy of pure component i in the structure of phase f, which is taken from the SGTE data compiled by DINSDALE [46]. The excess term is written as a Redlich-Kister polynomial, i.e.,
![]() (2)
(2)
where
![]() (3)
(3)
A and B are the parameters to be optimized in this work.
3.2 Laves phase
Following the common rule of describing the Laves phase, the Fe2Ti compound is modeled using two sublattices with a ratio of 2:1. The Gibbs energy function per formula unit is written as:
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() (4)
(4)
where
![]() (5)
(5)
![]() (6)
(6)
![]() (7)
(7)
To fulfill the Wagner-Schottky defect model [47], the Gibbs energy of the Ti2Fe end-member is expressed as:
![]() (8)
(8)
In this work, the contribution of the end-members to the Gibbs energy of Fe2Ti is enough to fit the experimental data well including the phase boundary, the specific heat capacity and the enthalpy of formation of this compound.
3.3 Ordered phase
The BCC_B2 ordering of the FeTi phase is modeled with two sublattices (Fe,Ti)0.5(Fe,Ti)0.5, each sublattice corresponding to a site of the crystallographic structure. The molar Gibbs energy is formalized as:
![]() (9)
(9)
where
![]() (10)
(10)
![]() (11)
(11)
![]()
![]() (12)
(12)
with
![]() (13)
(13)
![]() (14)
(14)
The subscripts i, j, k and l denote the component Fe or Ti. ![]() is the site fraction of i in the first sublattice, and
is the site fraction of i in the first sublattice, and![]() is that in the second sublattice.
is that in the second sublattice.![]() and
and ![]() are expressed in the same formulas as those given in Eq. (7).
are expressed in the same formulas as those given in Eq. (7).
The two sites represented by two sublattices are crystallographically equivalent. Then the exchange of the occupation of the two sublattices should not lead to a change in Gibbs energy, and the relations ![]() and
and![]() are thus obtained.
are thus obtained.
Furthermore, this model allows the thermodynamic properties of the disordered phase to be evaluated independently. This is achieved by splitting the Gibbs energy into three terms, i.e.,
![]() (15)
(15)
where ![]() is the Gibbs energy of the disordered state, which has the same expression as the substitutional solution phase;
is the Gibbs energy of the disordered state, which has the same expression as the substitutional solution phase;![]() is the Gibbs energy described by the sublattice model, containing implicitly a contribution from the disordered state;
is the Gibbs energy described by the sublattice model, containing implicitly a contribution from the disordered state;![]() denotes the energy contribution of the disordered state to the ordered phase. The last two terms cancel each other when they are equal, thus corresponding to a disordered state.
denotes the energy contribution of the disordered state to the ordered phase. The last two terms cancel each other when they are equal, thus corresponding to a disordered state.
4 Results and discussion
The optimization of the Fe-Ti binary phase diagram was performed using the Parrot module in the Thermo-Calc? software developed by SUNDMAN et al [48]. All the parameters optimized in this work are listed in Table 1. The calculated phase diagram of the Fe-Ti binary system along with the experimental data is shown in Fig. 1. It can be seen that the experimental data are reproduced very well. The calculated invariant reactions and compositions of the equilibrium phases are summarized in Table 2. Figure 2 shows the calculated gamma loop, which agrees well with all the experimental data as well as the assessed work of KUMAR et al [7]. For the Ti-rich phase diagram, the calculated result in this work fits better with the most recent experimental data given by STUPEL et al [18] than the work of KUMAR et al [7], as indicated by Fig. 3.
A comparison was made between the calculated enthalpy of mixing of the liquid phase at 1873 K and the measured values by BATALIN et al [30], WANG et al [31] and THIEDEMANN et al [32]. They are consistent with each other as seen from Fig. 4. The calculated partial enthalpy of mixing of the liquid phase at 2000 K and the activities of Fe and Ti in the liquid phase at 1873 K are shown in Fig. 5 and Fig. 6, respectively. The little discrepancy between the calculated and experimental results is within the experimental error. Seen from Figs. 4-6, the thermodynamic properties for liquid phase calculated in this work and by KUMAR et al [7] are almost the same.
Table 1 Thermodynamic parameters optimized in this work
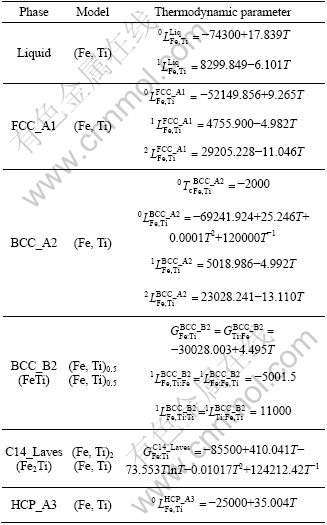
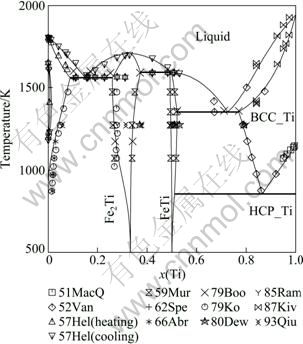
Fig. 1 Calculated phase diagram of Fe-Ti binary system
Table 2 Invariant reactions and critical points in Fe-Ti binary system
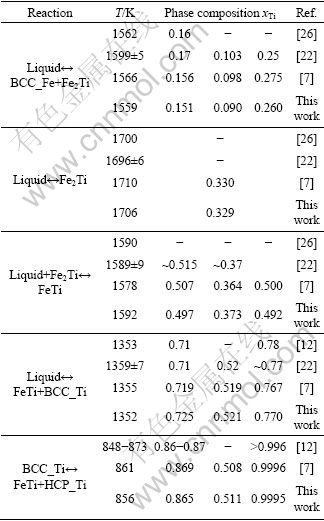

Fig. 2 Calculated gamma loop in Fe-rich side
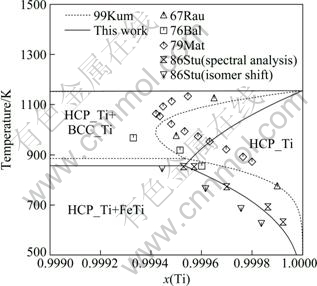
Fig. 3 Calculated phase diagram of Fe-Ti binary system in Ti-rich side
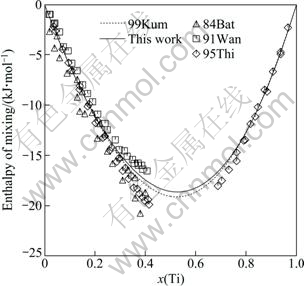
Fig. 4 Calculated enthalpy of mixing of liquid Fe-Ti alloys at 1873 K
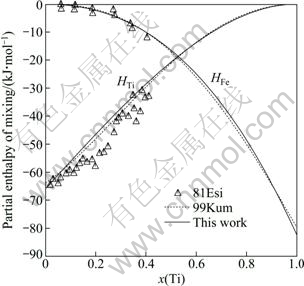
Fig. 5 Calculated partial enthalpy of mixing of liquid Fe-Ti alloys at 2000 K
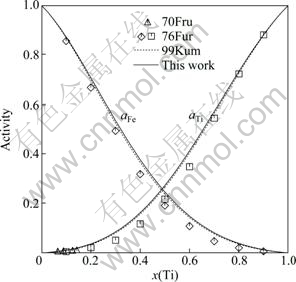
Fig. 6 Calculated activities of Fe and Ti in liquid phase at 1873 K
For the thermodynamic properties of the Fe2Ti and FeTi compounds, many efforts have been made to reproduce the measured values while maintaining a good fit for the phase boundaries. The calculated specific heat capacities of Fe2Ti and FeTi compounds are shown in Figs. 7 and 8, respectively, superimposed with the experimental data from WANG et al [36] and the calculated results of KUMAR et al [7]. It is found that the calculated specific heat capacities for these two compounds agree better with the experimental data than those given by KUMAR et al [7]. For the FeTi compound, the third and subsequent terms in the Gibbs energy expression as given in Eq. (7) almost have no effects on the calculated heat capacity, while the interaction parameter of Fe and Ti components in the disordered phase does. Therefore, the thermodynamic parameters of both the ordered and disordered phases are optimized in this work. It should be noted that the Curie temperature for the disordered BCC_A2 phase is assessed by adjusting the interaction parameter between Fe and Ti ![]() since it also has an influence on the calculated heat capacity of the FeTi phase. The calculated enthalpy of formation of the Fe2Ti phase at 1413 K is in good agreement with the experimental data given by DINSDALE et al [39], as shown in Fig. 9. The formation enthalpy measured by GACHON et al [38] at 1514 K is also given in Fig. 9 for comparison, although the temperatures for these two sets of experimental data are different. Figure 10 shows the calculated enthalpy of formation at 1513 K for both BCC_A2 and BCC_B2 (FeTi) phases, along with the experimental data from GACHON et al [38] and DINSDALE et al [39]. A good agreement has been achieved.
since it also has an influence on the calculated heat capacity of the FeTi phase. The calculated enthalpy of formation of the Fe2Ti phase at 1413 K is in good agreement with the experimental data given by DINSDALE et al [39], as shown in Fig. 9. The formation enthalpy measured by GACHON et al [38] at 1514 K is also given in Fig. 9 for comparison, although the temperatures for these two sets of experimental data are different. Figure 10 shows the calculated enthalpy of formation at 1513 K for both BCC_A2 and BCC_B2 (FeTi) phases, along with the experimental data from GACHON et al [38] and DINSDALE et al [39]. A good agreement has been achieved.
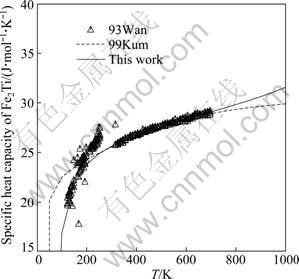
Fig. 7 Calculated specific heat capacity of Fe2Ti compound, compared with experimental data from WANG et al [36] and optimized results of KUMAR et al [7]
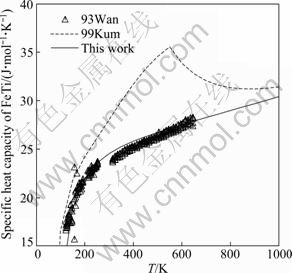
Fig. 8 Calculated specific heat capacity of FeTi compound, compared with experimental data from WANG et al [36] and optimized results of KUMAR et al [7]
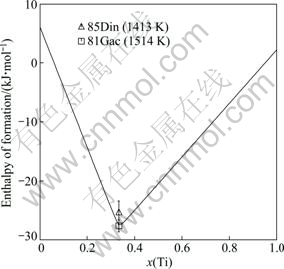
Fig. 9 Calculated enthalpy of formation of Fe2Ti compound at 1413 K

Fig. 10 Calculated enthalpy of formation of BCC_A2 and BCC_B2 (FeTi) phases at 1513 K
5 Conclusions
1) A new assessment of the Fe-Ti binary phase diagram was performed in this work and a set of reasonable thermodynamic parameters were obtained.
2) The experimental data on phase diagram and thermodynamic properties especially those for Fe2Ti and FeTi intermetallic compounds were well reproduced.
3) The good agreement between the calculated and experimental results guarantees the extrapolation capability of the Fe-Ti binary system to interesting ternary and higher-order systems.
References
[1] CACCIAMANi G, KEYZER J D, FERRO R, KLOTZ U E, LACAZE J, WOLLANTS P. Critical evaluation of the Fe-Ni, Fe-Ti and Fe-Ni-Ti alloy systems [J]. Intermetallics, 2006, 14(10-11): 1312-1325.
[2] XU L, SHI J, CAO W Q, WANG M Q, HUI W J, DONG H. Yield strength enhancement of martensitic steel through titanium addition [J]. J Mater Sci, 2011, 46(10): 3653-3658.
[3] SCHLAPBACH L. Hydrogen in intermetallic compounds I+II [M]. Heidelberg: Springer, 1988.
[4] Z?TTEL A. Materials for hydrogen storage [J]. Mater Today, 2003, 6(9): 24-33.
[5] OGURO K, OSUMI Y, SUZUKI H, KATO A, IMAMURA Y, TANAKA H. Hydrogen storage properties of TiFe1-xNiyMz alloys [J]. J. Less Common Met, 1983, 89(1): 275-279.
[6] LEE S M, PERNG T P. Correlation of substitutional solid solution with hydrogenation properties of TiFe1-xMx (M=Ni, Co, Al) alloys [J]. J Alloys Compd, 1999, 291(1-2): 254-261.
[7] HARI KUMAR K C, DUMITRESCU L, SUNDMAN B, WOLLANTS P. Thermodynamic assessment of the Fe-Ti system with special emphasis on the modeling of the FeTi(B2) phase [C]//ANSARA I, BERNARD C. Proceedings of the Calphad XXVIII Conference. Grenoble: INPG, 1999: 95.
[8] HELLAWELL A, HUME-ROTHERY W. The constitution of alloys of iron and manganese with transition elements of the first long period [J]. Phil Trans R Soc Lond A, 1957, 249(968): 417-459.
[9] KO M, NISHIZAWA T. Effect of magnetic transition on the solubility of alloying elements in alpha iron [J]. J Jpn Inst Met, 1979, 43(2): 118-126.
[10] SPEICH G R. Precipitation of Laves phases from iron-niobium (columbium) and iron-titanium solid solutions [J]. Trans AIME, 1962, 224: 850-858.
[11] ABRAHAMSON E P, LOPATA S L. The lattice parameters and solubility limits of alpha iron as affected by some binary transition-element additions [J]. Trans AIME, 1966, 236: 76-87.
[12] van THYNE R J, KESSLER H D, HANSEN M. The systems titanium-chromium and titanium-iron [J]. Trans ASM, 1952, 44: 974-989.
[13] McQUILLAN A D. The application of hydrogen equilibrium- pressure measurements to the investigation of titanium alloy systems [J]. J Inst Metals, 1951, 79: 73-88.
[14] KIVILAHTI J K, TARASOVA O B. The determination of the Ti-rich liquidus and solidus of the Ti-Fe system [J]. Metall Trans A, 1987, 18(9): 1679-1680.
[15] RAUB E, RAUB C J, ROESCHEL E. The α-Ti-Fe solid solution and its superconducting properties [J]. J Less Common Met, 1967, 12(1): 36-40.
[16] BALESIUS A, GONSER U. Precision phase analysis [J]. J Phys Colloq, 1976, 37: C6-397-C6-399.
[17] MATYKA J, FAUDOT F, BIGOT J. Study of iron solubility in α titanium [J]. Scr Metall, 1979, 13(7): 645-648.
[18] STUPEL M M, BAMBERGER M, RON M. The solubility of iron in α-titanium in the temperature range 360-580 ℃ [J]. J Less Common Met, 1986, 123(1-2): 1-7.
[19] DEW-HUGHES D. The addition of Mn and Al to the hydriding compound FeTi: Range of homogeneity and lattice parameters [J]. Metall Trans A, 1980, 11(7): 1219-1225.
[20] QIU C A, JIN Z P. An experimental study and thermodynamic evaluation of the Fe-Ti-W system at 1000 ℃ [J]. Scr Metall, 1993, 28(1): 85-90.
[21] MURAKAMI Y, KIMURA H, NISHIMURA Y. An investigation on the titanium-iron-carbon system [J]. Trans Nat Res Inst Met, 1959, 1(1): 7-21.
[22] BOOKER P H. Ternary phase equilibria in the systems Ti-Fe-C, Ti-Co-C and Ti-Ni-C [D]. Beaverton, OR: Oregon Graduate Center, 1979.
[23] RAMAEKERS P P J, van LOO F J J, BASTIN G F. Phase relations, diffusion paths and kinetics in the system Fe-Ti-C at 1273 K [J]. Z Metallkd, 1985, 76(4): 245-248.
[24] ZHOU G J, JIN S, LIU L B, LIU H S, JIN Z P. Determination of isothermal section of Fe-Ti-Zr ternary system at 1173 K [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(5): 963-966.
[25] XU H H, DU Y, YUAN Z H, LI S T, SCHUSTER J C, HE Y H. Phase equilibria of the Fe-Nb-Ti system at 900 ℃ [J]. J Alloys Compd, 2005, 396(1-2): 151-155.
[26] MURRAY J L. The Fe-Ti (Iron-Titanium) system [J]. Bull Alloy Phase Diagrams, 1981, 2(3): 320-334.
[27] MOLL S H, OGILVIE R E. Solubility and diffusion of titanium in iron [J]. Trans AIME, 1959, 215: 613-618.
[28] WADA T. Austenite loop in Iron-Titanium system [J]. Trans Nat Res Inst Met, 1964, 6(2): 43-46.
[29] FISCHER W A, LORENTZ K, FABRITIUS H, HOFFMANN A, KALWA G. Investigation of phase transformations in iron alloys using a magnetic balance [J]. Arch Eisenhüttenwes, 1966, 37: 79-87.
[30] BATALIN G I, KURACH V P, SUDAVTSOVA V S. Enthalpies of mixing of Fe-Cr and of Fe-Ti melts [J]. Russ J Phys Chem, 1984, 58(2): 289-291.
[31] WANG H, L?CK R, PREDEL B. Calorimetric determination of the enthalpy of mixing of liquid iron-titanium alloys [J]. Z Metallkd, 1991, 82(8): 659-665.
[32] THIEDEMANN U, QIN J P, SCHAEFERS K, R?SNER-KUHN M, FROHBERG M G. Mixing enthalpy measurements of liquid Fe-Ti alloys by levitation alloying calorimetry and calculation of the thermodynamic properties of mixing [J]. ISIJ Int, 1995, 35(12): 1518-1522.
[33] ESIN Y O, VALISHEV M G, ERMAKOV A F, GELD P V, PETRUSHEVSKII M S. Partial and integral enthalpy of mixing of liquid Fe-Ti alloys [J]. Izv Akad Nauk SSSR Met, 1981, 3: 30-32.
[34] FRUEHAN R J. Activities in liquid Fe-Al-O and Fe-Ti-O alloys [J]. Metall Trans, 1970, 1: 3403-3410.
[35] FURUKAWA T, KATO E. Thermodynamics of binary liquid iron-titanium alloys by mass spectrometry [J]. Trans ISIJ, 1976, 16: 382-387.
[36] WANG H, L?CK R, PREDEL B. Heat capacities of intermetallic compounds in the iron-titanium system [J]. Z Metallkd, 1993, 84(4): 230-236.
[37] KUBASCHEWSKI O, DENCH W A. The heats of formation in the systems titanium-aluminium and titanium-iron [J]. Acta Metall, 1955, 3(4): 339-346.
[38] GACHON J C, NOTIN M, HERTZ J. The enthalpy of mixing of the intermediate phases in the systems FeTi, CoTi and NiTi by direct reaction calorimetry [J]. Thermochim Acta, 1981, 48(1-2): 155-164.
[39] DINSDALE A T, CHART T G, PUTLAND F H. Enthalpies of formation of binary phases in the Fe-Ni system [R]. Middlesex: National Physical Laboratory, 1985.
[40] GACHON J C, HERTZ J. Enthalpies of formation of binary phases in the systems FeTi, FeZr, CoTi, CoZr, NiTi and NiZr by direct reaction calorimetry [J]. CALPHAD, 1983, 7(1): 1-12.
[41] KAUFMAN L, NESOR H. Coupled phase diagrams and thermochemical data for transition metal binary systems—I [J]. CALPHAD, 1978, 2(1): 55-80.
[42] HARI KUMAR K C, WOLLANTS P, DELAEY L. Thermodynamic reassessment and calculation of Fe-Ti phase diagram [J]. CALPHAD, 1994, 18(2): 223-234.
[43] DUMITRESCU L F S, HILLERT M, SAUNDERS N. Comparison of Fe-Ti assessments [J]. J Phase Equilib, 1998, 19(5): 441-448.
[44] JONSSON S. Assessment of the Fe-Ti system [J]. Metall Mater Trans B, 1998, 29(2): 361-370.
[45] KEYZER J D, CACCIAMANI G, DUPIN N, WOLLANTS P. Thermodynamic modeling and optimization of the Fe-Ni-Ti system [J]. CALPHAD, 2009, 33(1): 109-123.
[46] DINSDALE A T. SGTE data for pure elements [J]. CALPHAD, 1991, 15(4): 317-425.
[47] WAGNER C, SCHOTTKY W. Theory of ordered mixed phases [J]. Z Phys Chem, 1930, B11: 163-210.
[48] SUNDMAN B, JANSSON B, ANDERSSON J O. The Thermo-Calc databank system [J]. CALPHAD, 1985, 9(2): 153-190.
Fe-Ti二元体系的热力学重新优化
薄 宏1, 2,王 江2,Liliana DUARTE 2,Christian LEINENBACH 2,
刘立斌1, 3,刘华山1, 3,金展鹏1, 3
1. 中南大学 材料科学与工程学院,长沙 410083;
2. Laboratory for Joining and Interface Technology, Swiss Federal Laboratories for Materials
Science and Technology, ?berlandstrasse 129, CH-8600, Dübendorf, Switzerland;
3. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083
摘 要:为提高Fe-Ti二元系外推到三元或多元体系的能力,应用CALPHAD方法重新优化了该二元系。与前人的优化工作相比,重点放在对两个二元金属间化合物Fe2Ti和FeTi的热力学描述上。因目前普遍采用双亚点阵模型来描述C14_Laves相,所以采用双亚点阵模型来描述Fe2Ti相。通过检验包含Fe-Ti二元系的三元体系Fe-Ti边界上Fe2Ti相的均匀化范围进一步证实了Fe2Ti相的相边界。FeTi相具有BCC_B2晶体结构,因而将其处理成为BCC_A2相的有序相,并且用统一的Gibbs能函数来描述有序和无序相。另外一个特别关注的方面就是重现这两个化合物的实测热容。计算结果与有关相图和热力学性质实验结果的广泛对比显示两者符合得很好,从而证明了所得热力学描述的有效性。
关键词:Fe-Ti体系;有序无序转变;热力学优化;相图;相图计算
(Edited by YUAN Sai-qian)
Foundation item: Project (IP08-092009) supported by Sino Swiss Science and Technology Cooperation (SSSTC); Project (50971136) supported by the National Natural Science Foundation of China; Project (1343-71134001013) supported by the Scholarship Award for Excellent Doctoral Student granted by Ministry of Education of China
Corresponding author: LIU Li-bin; Tel: +86-731-88877732; Fax: +86-731-88876692; E-mail: PDC@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)61450-7
Abstract: The Fe-Ti binary system was re-assessed using the CALPHAD method in order to improve the capability of being extrapolated to a ternary or higher-order system. Compared with previous assessments, the main focus was put on the thermodynamic description of the two intermetallic compounds Fe2Ti and FeTi. The C14_Laves phase Fe2Ti was described by the two-sublattice model, which is widely used at present. By checking the homogeneity range on the boundary of the ternary systems involving the binary, the phase boundary of this compound was further confirmed. The FeTi phase with a BCC_B2 crystal structure was treated as the ordered phase of the BCC_A2 phase and a unified Gibbs energy function was used to describe both the ordered and disordered phases. Reproduction of the specific heat capacities of these compounds was another aspect paid particular attention to. Comprehensive comparisons of the calculated and experimental results regarding the phase diagram and thermodynamic properties show a good agreement between them and prove the validity of the present thermodynamic description.
[3] SCHLAPBACH L. Hydrogen in intermetallic compounds I+II [M]. Heidelberg: Springer, 1988.
[4] Z?TTEL A. Materials for hydrogen storage [J]. Mater Today, 2003, 6(9): 24-33.
[16] BALESIUS A, GONSER U. Precision phase analysis [J]. J Phys Colloq, 1976, 37: C6-397-C6-399.
[28] WADA T. Austenite loop in Iron-Titanium system [J]. Trans Nat Res Inst Met, 1964, 6(2): 43-46.
[44] JONSSON S. Assessment of the Fe-Ti system [J]. Metall Mater Trans B, 1998, 29(2): 361-370.
[46] DINSDALE A T. SGTE data for pure elements [J]. CALPHAD, 1991, 15(4): 317-425.
[47] WAGNER C, SCHOTTKY W. Theory of ordered mixed phases [J]. Z Phys Chem, 1930, B11: 163-210.


