
J. Cent. South Univ. (2020) 27: 3320-3333
DOI: https://doi.org/10.1007/s11771-020-4549-x

Field trial on use of soybean crude extract for carbonate precipitation and wind erosion control of sandy soil
GAO Yu-feng(高玉峰), MENG Hao(孟浩), HE Jia(何稼), QI Yong-shuai(亓永帅), HANG Lei(杭磊)
Key Laboratory of Ministry of Education for Geomechanics and Embankment Engineering,Hohai University, Nanjing 210098, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Wind erosion is a major cause of land desertification and sandstorm formation in arid and semi-arid areas. The objective of this study was to evaluate the potential of soybeans crude extract induced calcium carbonate precipitation (SICP) on reducing wind erosion risk of sandy soil. Field tests were carried out in Ulan Buh Desert, Ningxia Hui Autonomous Region, China. Results showed that the SICP method could significantly enhance the surface strength and wind erosion resistance of the topsoil. The optimal cementation solution (urea-CaCl2) concentration and spraying volume, according to experiments conducted on sandy land, were 0.2 mol/L and 4 L/m2, respectively. Under this condition, the CaCO3 content was approximately 0.45%,the surface strength of sandy soil could reach 306.2 kPa, and the depth of wind erosion was approximately zero, after 30 d completion of SICP treatment. Soil surface strength declined with the increase of time, and long-term sand fixation effects of SICP treatment varied depending on topography. Whereas wind erosion in the top area of the windward slope was remarkable, sandy soils on the bottom area of the windward slope still maintained a relatively high level of surface strength and a low degree of wind erosion 12 month after SICP treatment. Scanning electron microscopy (SEM) tests with energy dispersive X-ray (EDX) confirmed the precipitation of CaCO3 and its bridge effect. These findings suggested that the SICP method is a promising candidate to protect sandy soil from wind erosion in desert areas.
Key words:
Cite this article as:
GAO Yu-feng, MENG Hao, HE Jia, QI Yong-shuai, HANG Lei. Field trial on use of soybean crude extract for carbonate precipitation and wind erosion control of sandy soil [J]. Journal of Central South University, 2020, 27(11): 3320-3333.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4549-x1 Introduction
Land desertification is one of the most serious environmental problems all over the world [1]. Wind erosion is a major cause of desertification and sandstorm in arid and semi-arid areas. Sandy soils in these regions have low cohesion and loose structure. During the dry season, high-speed wind can easily erode exposed surfaces containing organic matter that is necessary for vegetation, resulting in deterioration of farmland [2]. Meanwhile, the dust suspended in the atmosphere obscures visibility, pollutes the air, and endangers human health [3]. Therefore, due to the consideration of agriculture, environment, and human health, it is imperative to control wind erosion of sandy soil in arid and semi-arid areas.
The existing methods to control wind erosion can be classified into three categories, known as vegetation methods, mechanical methods, and chemical methods [4]. The most efficient method for wind erosion control is to cover the land surface with vegetation, and the greater the vegetation cover is, the more effective it would be [5]. However, vegetation methods may be difficult in some areas where soils are agriculturally unsuitable, and the strong winds tend to uproot seeding or bury them with drifting sand [6]. In the case of potential failure to establish the vegetation, mechanical and chemical methods could be used [7, 8]. Fences and windbreaks employed by mechanical methods to create barriers to wind flow can effectively reduce the erosivity of wind, whereas they often have limited protection height and can easily be buried by drifting sand [6]. Over the past half-century, various materials, such as petroleum products, cements, and synthetic polymers, have been evaluated to find suitable reagents for reducing the erodibility of the soil [4, 9]. However, some of the chemical soil stabilizers may not be cost-effective and eco-compatible.
The impressionability of topsoil to the wind is a key factor in wind erosion, and soil crusts can significantly reduce the risk of wind erosion [10, 11]. Generally, the sheath-forming and exopolysaccharide-excreting microbes such as cyanobacteria, algae, fungi, and bacteria in soil can form a highly specialized microbial community structure named biological soil crusts (BSCs) [12]. The BSCs, which are ubiquitous on the sand surface of the desert, play a significant role in soil stabilization and water conservation [12, 13]. Numerous studies have documented erosion- resistant properties of biocrusts [12-14]. Meanwhile, land restoration using induced biological soil crusts (IBSCs) in the desert land at the southern fringe of the Hobq Desert in China has reported over the past few years [15, 16]. Though certain achievements were reaped, some remaining problems need to be solved. In the initial phase of artificial BSCs, the BSCs are fragile and weakly attached to the soil surface and are vulnerable to wind, rain, hoofed animals, etc [13].
Biocementation may be an alternate approach for wind erosion control of sandy soils. Biocementation is based on biologically mediated mineralization to form crystals between soil particles; the precipitations can modify soil properties, such as strength and stiffness [17-23]. In most of the application of biocementation to date, CaCO3 crystals were formed on the base of urease- catalyzed urea hydrolysis, which can be described as follows:
 (1)
(1)
 (2)
(2)
Carbonate is firstly produced from the decomposition of urea induced by urease. Then precipitation of calcium carbonate crystals occurs in situ when soluble calcium sources are provided, which can fill pores and bind loose particles [18, 19].
Urease plays a crucial role in bio-cementation and is found naturally in micro-organisms, such as bacteria and fungus [24, 25]. The most commonly used urease producing micro-organism is Sporosarcina pasteurii (precious known as Bacillus pasteurii) [26-31]. The microbially induced calcium carbonate precipitation (MICP) method can potentially be used in a wide range of applications, such as ground improvement [32, 33], rehabilitation of contaminated soil [34, 35], healing cracks in concrete [36], and reducing wind- and water- induced erosion [37-41]. However, the high cost of bacterial cultivation is an obstacle for the successful commercialization of biocementation [42]. Furthermore, legislation in some countries makes it challenge to introduce foreign bacteria into the soil as their influence on native micro-organisms is still unknown.
Besides micro-organisms, urease is also widely distributed in the plant, such as soybeans, Canavalia ensiformis, and mulberry leaves [42]. Application of purified plant urease to induce the generation of CaCO3 crystals has been proved effective in sand fixing [43]. However, for one thing, it is still too expensive to use purified urease for large-scale applications. For another, a wide variation in soil conditions is frequent in the windblown regions and, the complex and changeable weather conditions in-situ are quite different from those in laboratories. Therefore, it is imperative to find a low-cost urease source and to carry out the field-scale sand-fixing test. Soybeans crude extract, containing an appreciable amount of urease, may be an appropriate alternative of in situ formations of CaCO3 to stabilize sandy soils that are vulnerable to wind erosion [44].
The objective of this research was to exploit the feasibility of using soybeans crude extract induced calcium carbonate precipitation (SICP) to reduce wind erosion risk of sandy soils in desert areas. For this purpose, field tests were carried out on the edge of Ulan Buh Desert, Ningxia Hui Autonomous Region, China. The optimum dosage of biological curing agents was obtained from the short-term (30 d) experiments conducted on sandy land. Specifically, long-term (1 year) performance of SICP treatment on the sandy land and windward slope of the dune was also investigated.
2 Materials and methods
2.1 Soybeans crude extract and cementation solutions
Soybeans were purchased from a nearby market. A shredding machine was employed, and soybeans powder passing through a 60-mesh sieve was soaked in tap water, according to solid (soybeans powder)-liquid (tap water) ratio of 1: 25. Then, the homogenate was stirred until the soybeans powder was evenly dispersed in the tap water. The supernatants (soybeans crude extract) were collected 1 h later and used without further purification. It should be noted that soybeans crude extract was prone to deterioration, owing to the high temperature in situ, and therefore, they should not be stored for long periods. Urease activity, reflecting the rate of the bio-catalyzed urea hydrolysis, was measured based on the change of electric conductivity under the condition of 1 mol/L urea at 20 °C [28]. The rate of conductivity increase mS/(min·cm-1) was concentrated into a urea hydrolysis rate (mmol/L urea hydrolysis/min) with a ratio of 1: 11.1 [28]. Urease activity of the soybeans crude extract was 4.44 mmol/L urea hydrolysis/min in this study.
Agricultural grade urea (total nitrogen ≥46.4%) and industrial-grade calcium chloride (CaCl2 content ≥94%) used in this experiment were purchased from a farmer market nearby. Four kinds of cementation solutions including 0.1 mol/L urea-0.1 mol/L CaCl2, 0.2 mol/L urea-0.2 mol/L CaCl2, 0.4 mol/L urea-0.4 mol/L CaCl2, and 1 mol/L urea- 1 mol/L CaCl2 were used in field tests.
2.2 Test sites and schemes
In order to evaluate the effects of the SICP method on the stabilization treatment of sandy soils under field conditions, field tests were carried out. The test sites (106°20′E, 38°46′N) were on the southwestern fringe of Ulan Buh Desert, Ningxia Hui Autonomous Region, China. The test area was mainly composed of sandy land and dunes. The dominant components of the sandy soil were quartz grains with a size between 0.075 and 0.4 mm (Figure 1). This region was located in the continental arid and semi-arid climate zones with annual precipitation of 172.5 mm and an annual evapotranspiration rate of 1756.8 mm. Low rainfall and strong evaporation deteriorated the ecological environment in general. The average annual temperature was 9.0 °C, with the maximum temperature of 38.5 °C and the minimum temperature of -27.7 °C. The average wind speed was in the range of 2.0-3.0 m/s, with the maximum wind speed exceeding 28.5 m/s. The test sites were adjacent to highways, farmlands, and villages. Sandy soils in this region were generally unprotected, and they experienced severe erosion, especially during the winter and spring. Prevailing winds transported the uncovered surface soils and deposited them elsewhere, which was a tremendous threat to local traffic and cultivation.
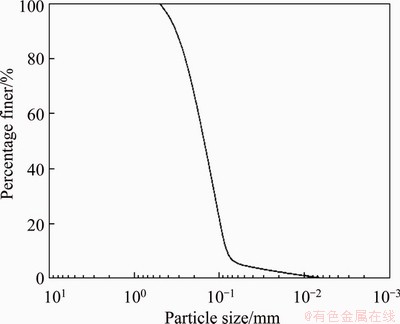
Figure 1 Particle size distribution of topsoil
Field tests were carried out on two types of sand structure (Figure 2): The first one is bare sandy land (Figure 2(a)). Seventeen test plots (TPs 1-17) at intervals of 30 cm and each measuring 2 m×2 m were established in the sandy land. Sand berms, approximately 10.2 cm in height, were constructed around the perimeter of each test plot to prevent surrounding erodible sands from being blown onto test plots during the study. Sixteen test plots (TPs 2-17) received SICP treatment, while a control test plot (TP 1) was absent of any treatment. The second type is windward slope of the dune (Figure 2(b)). Two neighboring windward slopes with similar shapes (height of 7.2 m and slope angle of 19°-26°) were selected as the test plots (TPs 18 and 19). TP 19 was treated with SICP, using the optimum dosage of biological curing agent obtained from TPs 1-17, while TP 18 was absent of any treatment. The windward slopes were divided into three regions, namely, bottom area (TP 18B and TP 19B, altitude: 0-1 m), middle area (TP 18M and TP 19M, altitude: 3.1- 4.1 m), and top area (TP 18T, and TP 19T, altitude: 6.2-7.2 m), according to the altitude. The trials lasted for one year. The performance of SICP in natural circumstances was evaluated by wind erosion resistance and penetration resistance assessment. Table 1 presents the treatment schemes for all test plots.

Figure 2 (a) TPs 1-17 in sandy land; (b) TPs 18 and 19
2.3 Application methods
Field tests were carried out on April 30th, 2018. Soybeans crude extract and cementation solution were prepared separately in two containers, and SICP treatment was accomplished using an agricultural sprayer. In this study, soybeans crude extract and cementation solutions were mixed adequately in the agricultural sprayer, according to a proportion of 1:1 in volume. After that, it was sprayed immediately onto the surface of the test plots. The short retention time (less than 5 min) of the mixture was conducted to minimize the biochemical reaction process in the sprayer [6]. Furthermore, to reduce evaporation caused by the high sand surface temperature and excessive solar exposure and to increase the efficiency of the biochemical reaction, the spray treatment was completed near sunset. After being treated, all test plots were left to the effects of local weather conditions 3 d, and then, the other experiments were carried out. During the 3 d, the field temperature was in the range of 5-27 °C, and the total precipitation was zero.
Table 1 Treatment schemes for sandy land (TPs 1-17) and dune (TPs 18 and 19)

2.4 Surface penetration and erosion pins tests
Wind erosion depends on how easily the particles can be detached from the surface and is thus closely related to the surface strength of sandy soil. In this study, a handhold micro-penetrometer (HP-50, Aidebao, China) with a diameter of 6 mm was adopted to assess soil surface strength of the test plots, as recommended by ROLSTON et al [45]. Note that three points were selected randomly for each test plot and the average surface strength at the three points was calculated, and presented herein.
Erosion pins were employed to examine wind erosion resistance of sandy soils. The use of erosion pins is convenient to measure sand erosion as well as deposition for their negligible obstruction to sand movement [46]. For TPs 1-17, three erosion pins, 30 cm long and 1 m apart, were inserted halfway into the soil at random positions before SICP treatment, i.e., 15 cm above the ground in each test plot. For TPs 18 and 19, three erosion pins with the same altitude, i.e., an altitude of 0.5 m for TP 18B and TP 19B, altitude of 3.6 m for TP 18M and TP 19M, and altitude of 6.7 m for TP 18T and TP 19T, were inserted into each region as mentioned above. The change in erosion pin heights was measured, and the average value obtained from the three pins was calculated and presented.
2.5 Measurement of calcium carbonate content
Soil samples (around 5 grams each) taken from the topsoil of TPs 1-17 were rinsed in deionized water and dried. Then, the soils were placed in 50 mL of 1 mol/L HCl to dissolve CaCO3. The concentration of the aqueous calcium in the hydrochloric acid was measured using the EDTA titrimetric method [32]. The effective amount of CaCO3 (produced from the SICP) of the cemented sandy soils is the difference between the value evaluated experimentally (TPs 2-17) and the initial value of CaCO3 content exhibited by that of TP 1.
2.6 SEM and EDX analysis
Scanning electron microscopy (SEM) (JSM-7600F, Jeol, Japan) coupled with energy dispersive X-ray (EDX) was used in this study to investigate the development of microstructure within the treated soil samples and to analyze the composition of the bio-mediate product. Before conducting the microscopy investigation, two samples, prepared from the surface layer of TP 1 and TP 15 (30 d after the SICP treatment), were oven-dried, and then were crushed into small pieces. The samples were sputter-coated with gold and then examined by SEM at voltages ranging from 0.5 to 30 kV.
3 Results and discussion
3.1 Effect of SICP treatment on surface strength of sandy land and CaCO3 precipitation
Figure 3(a) shows the surface strength of sandy land (TPs 1-17) 30 d after SICP treatment. To note that, the sandy land received about 63.8 mm (63.8 L/m2) of rainfall, approximately 16.0-63.8 times the spraying volumes used in this study (1-4 L/m2), during this period and the residue of the unreacted CaCl2 and urea as well as the biochemical reaction by-product NH4Cl in soil was considered “negligible”. In general, the SICP treatment significantly improved surface strength of the sandy land, and the improvement was intensively affected by both the cementation solution concentration and spraying volume (Figure 3(a)). It is shown that an increase in the total application rate from 1 to 4 L/m2 at the concentration of CaCl2-urea 0.1-1 mol/L enhanced the penetration resistance of the reinforced layers from 25.2 to 306.2 kPa, 30 d after SICP treatment. Overall, the application of 4 L of medium solution per square meter resulted in greater soil surface strength. The general trend of the surface strength-spraying volume behavior obtained after biocementation was similar to that obtained after chemical stabilization conducted by TELYSHEVA et al [47]. The mechanical test proceeded by TELYSHEVA et al [47] demonstrated that the penetration resistance of sandy soil increased with the spraying volume (2, 3 and 4 L/m2).
For the same spraying volume, soil surface strength improved when cementation solution concentration increased from 0.1 to 0.2 mol/L. Nevertheless, no further significant improvement of soil surface strength could be seen as the cementation solution concentration increased from 0.2 to 1 mol/L. The effective CaCO3 content (produced from the SICP) in the topsoil 30 d after the SICP treatment is illustrated in Figure 3(b). The amount of CaCO3 precipitation tended to grow with the increase of cementation solution concentration from 0.1 to 0.4 mol/L, and thereafter hardly any obvious improvement could be observed. AL-THAWADI [48] confirmed that the increase of calcium concentration results in inhibition of urease activity. OKWADHA et al [49] reported that a high concentration of urea and CaCl2 (>0.5 mol/L) decreases the efficiency of CaCO3 precipitation and de MUYNCK et al [50] demonstrated that the best CaCl2 concentration for CaCO3 precipitation is 0.25 mol/L. The results in this study were consistent with them and showed that it is impractical to increase the calcium carbonate content only by increasing the concentration of urea-CaCl2 under the field conditions.

Figure 3 (a) Surface strength and (b) CaCO3 content of sandy land 30 d after SICP treatment as functions of cementation solution concentration and spraying volume; (c) Surface strength versus CaCO3 content (Each bar represents the mean±standard deviation of triplicate tests)
The results also imply that when the spray volume remains constant, the surface strength increased with the augment of the CaCO3 content though some dispersion existed, i.e., a higher amount of CaCO3 precipitation potentiated the establishment of bonds between the soil particles, inducing a greater level of improvement in terms of soil surface strength (Figure 3(c)). The increase of surface strength was more significant for CaCO3 content until 0.45%, and after that, no significant change was observed. A consistent result was obtained by VENDA et al [51]. They claimed that the strength of MICP-treated coarse-grained soils dramatically increased with the amount of CaCO3 at low content (<0.5%). The negligible improvement in soil surface strength at higher CaCO3 content (>0.45%) may be due to limited bond-making areas between sand grains [51, 52]. The bonding-making area is defined as the possible binding points between soil particles that play an essential role in soil aggregation and consolidation [6]. As a result, increasing the amount of CaCO3 crystals may not be conducive to improve wind erosion resistance of sandy soil when the bonding-making area is filled. To note that, in addition to the CaCO3 content, the amount of spraying was another important factor intimately impacting the surface strength in this study. For instance, although the calcium carbonate content was approximately 0.4%, the surface strength of sand was 35.6, 59.3, 166.5 and 306.2 kPa when the spraying volume was 1, 2, 3 and 4 L/m2, respectively. The thickness of soil crusts increased with the increase of spraying volume [47, 53]. Penetration resistance (surface strength) reflects the crust hardness and bonding strength between particles, and higher bonding strength and crust thickness can definitely contribute to better surface strength [47, 53].
In the short term (30 d in this study), 4 L/m2 at a cementation solution concentration of 0.2 mol/L was an optimum dosage for sandy land from the aspect of soil stabilization. In this condition, the soil surface strength could reach 306.2 kPa, 17.4 times higher than that of the untreated site, 30 d after SICP treatment. The soil surface strength (25.2-306.2 kPa) obtained from the application of SICP was comparable to that obtained from chemical stabilization using lignin-based polymer (0.16-0.98 MPa) while significantly higher than that obtained from man-made algal crusts at the initial stage (<60 kPa) [47, 54]. In this sense, the SICP method may also be used to provide early strength for the formation of artificial biological soil crusts. The rapid CaCO3 precipitation induced by the SICP process, in contrast to slower microbial methods (to form man-made BSCs), makes it well-suited for surface treatments that have a relatively short time range within which they need to become effective.
3.2 Effect of SICP on erosion resistance of sandy land
Figure 4(a) illustrates the erosion depth of the sandy land (TPs 1-17) 30 d after SICP treatment.
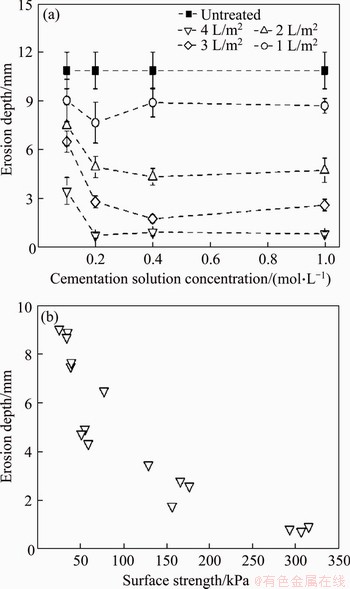
Figure 4 (a) Erosion depth of sandy land 30 d after SICP treatment as functions of cementation solution concentration and spraying volume; (b) Erosion depth of SICP treated sites versus soil surface strength (Each bar represents the mean±standard deviation of triplicate measurements)
Though surface curing of sandy soils using the SICP method could generally decrease soil loss; the reduction was severely affected by the dosage of the biological agents. When the cementation solution concentration was constant, erosion depth of the topsoil decreased dramatically with the increase of spraying volume, an opposite tendency to the surface strength mentioned above. This result was consistent with Ref. [37], in which MEYER et al evaluated the dust suppressive potential of MICP and declared that increasing the application volume while maintaining the same bacteria cell and cementation solution concentration reduced soil mass losses significantly. Also, according to FENG et al [55], if stabilizer concentration is kept as constant, crust thickness is proportional to the application rate, and the penetration resistance increases linearly with crust thickness.
Under the same spraying volume, erosion depth of the test plots (TPs 2-17) decreased with the increase of cementation solution concentration (≤0.2 mol/L) and thereafter remained roughly constant though higher cementation solution concentrations (>0.2 mol/L) were employed. At a low concentration of cementation solution, increasing the concentration contributed to the formation of a larger amount of calcium carbonate and thus resulted in higher erosion resistance (Figure 3(b)). The subtle discrimination of erosion depth at high concentrations of cementation solution (≥0.2 mol/L) may also be due to the inhibition of urease activity by CaCl2 solutions and the limited bond-making area [48-52].
Figure 4(b) illustrates the erosion depth of the SICP treated sites versus the soil surface strength. A strong relationship existed between the erosion depth and the soil surface strength, although the data were for the test sites treated with different dosages of the biological agents. Penetration resistance (surface strength) reflects the crust hardness and bonding strength between particles; higher bonding strength, crust thickness, and viscosity can definitely contribute to better surface strength, and such improvements are representations of higher wind erosion resistance [53]. The relationship between the erosion depth and the soil surface strength indicated that the surface strength was a good indicator of the wind erosion resistance.
Considering the enhancement in wind erosion resistance and the economic cost, the optimal cementation solution concentration and spraying volume were 0.2 mol/L and 4 L/m2, respectively. In this case, the final concentration of CaCl2·2H2O was 3%, approximately 12.5 times less than the concentration (38%) allowed to use commercially [6]. In addition, the concentrations of NH4Cl (2.2%) and urea (12%) were much lower than the urea concentration (43%) allowed as a frost protectant pesticide without any evidence of adverse chronic effects [37].
3.3 Long-term sustainability of SICP treatment
The surface strength and wind erosion depth of TP 1 (untreated site) and TP 15 (SICP-treated site) in 12 mon are shown in Figures 5(b) and (c). The surface strength of TP 15 was 314.6 kPa, about 19.1 times higher than that of TP 1 (16.7 kPa), immediately after SICP treatment, and then decreased gradually with the increase of time. The drop in surface strength of the SICP-treated sandy land was nearly 57% and 77%, 6 and 12 month later, respectively. However, it should be noted that the surface strength of the SICP-treated sandy land was still much higher (5.6 and 3.1 times, respectively) than that of the untreated one. Also, a distinct difference in soil erosion existed between TP 1 and TP 15. Whereas the former had changes in pin height of 10.9, 20.5, 35.7 and 40.5 mm; the latter had changes of 0.7, 2, 4.7 and 5.6 mm (6.4%, 9.8%, 13.2% and 15.7% of the former) after 1, 2, 6 and 12 month of exposure to the local wind erosion conditions (Figure 5(a)), respectively. Soil surface strength and wind erosion resistance of the sandy land were significantly improved by the SICP treatment over one year in this study.
Figure 6 illustrates surface strength and erosion depth obtained from the bottom (TP 18B and TP 19B), middle (TP 18M and TP 19M), and top (TP 18T and TP 19T) area of the windward slopes. Similar to that of the sandy land, for TP 19 (SICP-treated windward slope), soil surface strength decreased while erosion depth increased as time went on. However, the surface strength and erosion depth showed a clear transition from the bottom to the top area. Whereas the bottom area had a reduction in surface strength of 54.3% and a change in pin height of 3.2 mm, six month after SICP treatment, the top area, had a reduction in surface strength of 91.2% and a change in pin height of 19.5 mm. The middle area had an intermediate value with a reduction in surface strength of 75.6% and a change in pin height of 8.9 mm, six month after SICP treatment. Sandy soils in the bottom area of the SICP-treated windward slope still maintained a relatively high level of surface strength (84.2 kPa) and a low degree of wind erosion (4.8 mm) 12 mon after SICP treatment, compared with the untreated windward slope. However, sandy soils in the middle and top area, 12 month after the SICP treatment, were fiercely eroded (29.2 and 61.6 mm, respectively).
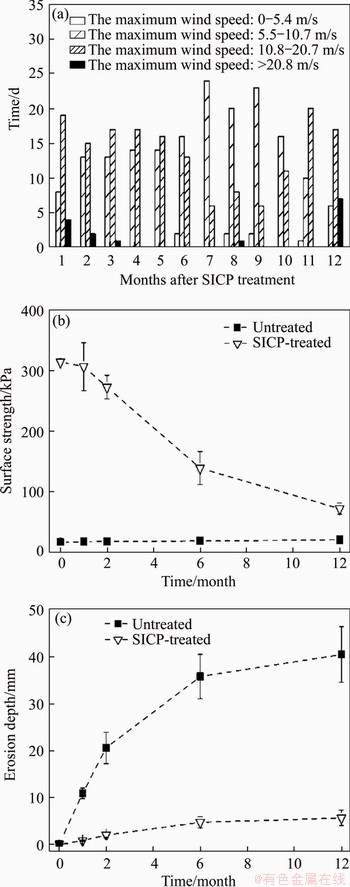
Figure 5 (a) Time with the different maximum wind speed months after SICP treatment; (b) Surface strength of sandy land (TP1 and TP 15) in 1 year; (c) Erosion depth of sandy land (TP 1 and TP 15) in 1 year (Each bar represents mean±standard deviation of triplicate measurements)

Figure 6 (a) Surface strength of the windward slopes (TP 18 and TP 19) in 1 year; (b) Erosion depth of windward slopes (TP 18 and TP 19) in 1 year (Each bar represents the mean±standard deviation of triplicate measurements)
Environmental deterioration such as winds, rains, ultraviolet rays, wet-dry cycles, and freeze-thaw cycles may cause disturbance to soil crusts, leading to the decrease of surface strength and wind erosion resistance in the long term [12, 16]. According to the field measurements taken by ARENS et al [56], wind speed at foredunes increased by 10% from the beach to an altitude of 6 m and thereafter increased by 50% at an altitude of 10 m. The substantial decrease of soil surface strength and erosion resistance at the top area (altitude: 6.2-7.2 m) of the SICP treated windward slope may reflect the substantial increase in the erosion power of the wind [57]. As a result, in the application of SICP technology to sand-dune stabilization, a more reasonable treatment option should be determined according to the erosive power of wind. For instance, larger spraying volumes or multiple treatments are suggested in the areas suffering from severe wind erosion to enhance the ductility of SICP treatment.
The situation of TP 15 6 month after SICP treatment is shown in Figure 7. It is clear from Figure 7 that plants in the SICP treatment sandy cyanobacteria, algae, lichens, bryophytes, bacteria, land grew comparatively well, and soil crusts were distributed in succession on the soil surface. Soil crusts play significant roles in soil stabilization and desertification control [58]. Generally, and fungi in soil can form the so-called biological soil crusts through the cementation effect of the extracellular polymeric substance (EPS) [12-16]. However, on the one hand, it takes a long time for the formation of BCSs in the natural environment; on the other hand, high wind power may impede BSCs establishment [12, 13]. In this study, the formation of soil crusts was due to the CaCO3 precipitation induced by soybeans crude extract, which is environmentally-friendly and controllable.

Figure 7 Situation of TP 15, 6 month after SICP treatment
3.4 SEM imaging and EDX analysis
The morphologic properties of the precipitated mineral crystals and the microstructure within the surface layer of the SICP treated sandy soil were evaluated by SEM (Figures 8(a)-(c)). For the SEM and EDX analysis, the calcium carbonate content of the soil sample is 0.45%. Precipitated CaCO3 was present both at particle contacts and on particle surfaces of the SICP treated sandy soil (Figures 8(b) and (c)), but not in the absence of treatment (Figure 8(a)). Large pore spaces still existed after SICP treatment, indicating that the porosity of the sandy soil is largely intact (Figures 8(b) and (c)). Ideal sand stabilizers should offer good adhesion and rapid infiltration of sand when they are sprayed onto the surface, and they should not reduce water infiltration significantly [59]. Energy dispersive X-ray (EDX) analysis (Figure 8(d)) shows that the elemental composition of the substrate (spectrum 1 in Figure 8(b)) was primarily of Si and O, while the elemental composition of the precipitate (spectrum 2 in Figure 8(b)) was in consistence with CaCO3. Because the mineral type cannot be differentiated through EDX, crystal morphology was used as a tool to differentiate between types of minerals [60]. Spherical crystals with the irregular and disordered structure were widely observed in the SICP treated sandy soil (Figure 8(c)), which indicates that the dominant CaCO3 phase was vaterite [61]. Similar CaCO3 deposition patterns have been observed in sand specimens treated by enzyme-induced calcium carbonate precipitation [43, 60].

Figure 8 SEM analysis results of (a) untreated sandy soils from TP 1 and (b-c) SICP-treated sandy soils from TP 15;(d) EDX results of sampling point
In urease-based reactions, NH4+ and CO32- released by the enzymatic hydrolysis of urea. The generated NH4+ increases pH, and precipitation of calcium carbonate crystal occurs in situ when soluble calcium sources are provided. In this study, the biochemical reaction was induced by soybeans crude extract. Binding by precipitated CaCO3 of sand particles (Figures 8(b) and (c)) explains the improvement of wind erosion resistance and surface strength of the sandy soil samples after SICP treatment.
4 Conclusions
The potential of soybeans crude extract induced calcium carbonate precipitation on reducing wind erosion risk of sandy soils in field conditions was investigated. Surface strength and erosion resistance of SICP-treated sandy soil were significantly influenced by the combination of cementation solution concentration and spraying volume. The optimal cementation solution concentration and spraying volume, considering the stabilization effect of sandy land and economic cost, were 0.2 mol/L and 4 L/m2, respectively. In this dosage, the CaCO3 content was approximately 0.45%, the surface strength of sandy soil could reach 306.2 kPa, and the depth of wind erosion was nearly zero 30 d after SICP treatment. Soil surface strength declined, and erosion depth rose with time. Long-term sand fixation effects of SICP treatment varied depending on topography in this study. Soil surface strength and erosion depth of the sandy land, one year after SICP treatment based on the optimum dosage of biological curing agent, were 72.3 kPa and 5.6 mm, respectively. An obvious and durable improvement in wind erosion resistance of sandy soils, compared with the untreated site, was observed in the SICP-treated sandy land. For the SICP-treated windward slope, whereas wind erosion in the top area was remarkable, sandy soils in the bottom area still maintained a relatively high level of surface strength and a low degree of wind erosion even 12 month later. It is clearly indicated that the SICP, a new biological treatment technology, is useful for reducing wind erosion of sandy soils. The technique is worth popularizing for topsoil protection in desert areas.
Contributors
The overarching research goals were developed by GAO Yu-feng. GAO Yu-feng oversight the research activity planning and execution. MENG Hao, HE Jia, QI Yong-shuai, and HANG Lei conducted the field tests and analyzed the measured data. The initial draft of the manuscript was written by GAO Yu-feng, MENG Hao, and HE Jia. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
GAO Yu-feng, MENG Hao, HE Jia, QI Yong- shuai, and HANG Lei declare that they have no conflict of interest.
References
[1] JIANG Liang-liang, JIAPAER G, BAO An-ming, KURBAN A, GUO Hao, ZHENG Guo-xiong, DE MAEYER P. Monitoring the long-term desertification process and assessing the relative roles of its drivers in Central Asia [J]. Ecological Indicators, 2019, 104: 195-208. DOI: 10.1016/ j.ecolind.2019.04.067.
[2] KHEIRABADI H, MAHMOODABADI M, JALALI V, NAGHAVI H. Sediment flux, wind erosion and net erosion influenced by soil bed length, wind velocity and aggregate size distribution [J]. Geoderma, 2018, 323: 22-30. DOI: 10.1016/j.geoderma.2018.02.042.
[3] REYNOLDS J F, SMITH D M S, LAMBIN E F, TURNER II B L, MORTIMORE M, BATTERBURY S P J, DOWNIN T E, DOWLATABADI H, FERNANDEZ, R J, HERRICK J E, HUBER-SANNWALD E, JIANG Hong, LEEMANS R, LYNAM T, MAESTRE F T, AYARZA M, WALKER B. Global desertification: Building a science for dryland development [J]. Science, 2007, 316(5826): 847-851. DOI: 10.1126/science.1131634.
[4] FEIZI Z, AYOUBI S, MOSADDEGHI M R, BESALATPOUR A A, ZERAATPISHEH M, RODRIGO- COMINO J. A wind tunnel experiment to investigate the effect of polyvinyl acetate, biochar, and bentonite on wind erosion control [J]. Archives of Agronomy and Soil Science, 2019, 65(8): 1049-1062. DOI: 10.1080/03650340.2018.154 8765.
[5] LAN Shu-bin, ZHANG Qing-yi, WU Li, LIU Yong-ding, ZHANG De-lu, HU Chun-xiang. Artificially accelerating the reversal of desertification: cyanobacterial inoculation facilitates the succession of vegetation communities [J]. Environmental Science & Technology, 2013, 48(1): 307-315. DOI: 10.1021/es403785j.
[6] MALEKI M, EBRAHIMI S, ASADZADEH F, TABRIZI M E. Performance of microbial-induced carbonate precipitation on wind erosion control of sandy soil [J]. International Journal of Environmental Science and Technology, 2016, 13(3): 937-944. DOI: 10.1007/s13762-015-0921-z.
[7] MA Rui, LI Jun-ran, MA Yan-jun, SHAN Li-shan, LI Xue-lin, WEI Lin-yuan. A wind tunnel study of the airflow field and shelter efficiency of mixed windbreaks [J]. Aeolian Research, 2019, 41: 100544. DOI: 10.1016/j.aeolia.2019. 100544.
[8] MA Guo-fu, RAN Fei-tian, FENG En-ke, DONG Zhi-bao, LEI Zi-qiang. Effectiveness of an eco-friendly polymer composite sand-fixing agent on sand fixation [J]. Water, Air, & Soil Pollution, 2015, 226(7): 221. DOI: 10.1007/ s11270-015-2490-7.
[9] SAIEDI N, BESALATPOUR A A, SHIRANI H, ABBASZADEH DEHAJI P, ESFANDIARPOUR I, FARAMARZI M. Aggregation and fractal dimension of aggregates formed in sand dunes stabilized by Pistachio PAM and PistachioPVAc mulches [J]. European Journal of Soil Science, 2017, 68(5): 783-791. DOI: 10.1111/ejss. 12458.
[10] BELNAP J, WEBER B, BUDEL B. Biological soil crusts as an organizing principle in drylands [M]// Biological Soil Crusts: An Organizing Principle in Drylands. Cham: Springer, 2016. DOI: 10.1007/978-3-319-30214-0_1.
[11] CHANG I, PRASIDHI A K, IM J, SHIN H D, CHO G C. Soil treatment using microbial biopolymers for anti- desertification purposes [J]. Geoderma, 2015, 253: 39-47. DOI: 10.1016/j.geoderma.2015.04.006.
[12] CHOCK T, ANTONINKA A J, FAIST A M, BOWKER M A, BELNAP J, BARGER N N. Responses of biological soil crusts to rehabilitation strategies [J]. Journal of Arid Environments, 2019, 163: 77-85. DOI: 10.1016/j.jaridenv. 2018.10.007.
[13] PENG Cheng-rong, ZHENG Jiao-li, HUANG Shun, LI Shuang-shuang, LI Dun-hai, CHENG Ming-yu, LIU Yong-ding. Application of sodium alginate in induced biological soil crusts: enhancing the sand stabilization in the early stage [J]. Journal of Applied Phycology, 2017, 29(3): 1421-1428. DOI: 10.1007/s10811-017-1061-2.
[14] ROSSI F, MUGNAI G, DE PHILIPPIS R. Complex role of the polymeric matrix in biological soil crusts [J]. Plant and Soil, 2018, 429(1, 2): 19-34. DOI: 10.1007/s11104-017- 3441-4.
[15] ZHOU Xiang-jun, KE Tan, LI Shuang-xi, DENG Song-qiang, AN Xiao-liang, MA Xiao, PHILIPPIS R D, CHEN Lan-zhou. Induced biological soil crusts and soil properties varied between slope aspect, slope gradient and plant canopy in the Hobq desert of China [J]. CATENA, 2020, 190: 104559. DOI: 10.1016/j.catena.2020.104559.
[16] DENG Song-qiang, ZHANG Da-yi, WANG Gao-hong, ZHOU Xiang-jun, YE Chao-ran, FU Tao-ran, KE Tan, ZHANG Yu-rui, LIU Yong-ding, CHEN Lan-zhou. Biological soil crust succession in deserts through a 59-year-long case study in China: How induced biological soil crust strategy accelerates desertification reversal from decades to years [J]. Soil Biology and Biochemistry, 2020, 141: 107665. DOI: 10.1016/j.soilbio.2019.107665.
[17] DEJONG J T, FRITZGES M B, NUSSLEIN K. Microbially induced cementation to control sand response to undrained shear [J]. Journal of Geotechnical and Geoenvironmental Engineering, 2006, 132(11): 1381-1392. DOI: 10.1061/ (ASCE)1090-0241(2006)132:11(1381).
[18] IVANOV V, CHU Jian. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ [J]. Reviews in Environmental Science and Bio/Technology, 2008, 7(2): 139-153. DOI: 10.1007/s11157- 007-9126-3.
[19] van PAASSEN L A, GHOSE R, van DER LINDEN T J, van DER STAR W R, van LOOSDRECHT M C. Quantifying biomediated ground improvement by ureolysis: Large-scale biogrout experiment [J]. Journal of Geotechnical and Geoenvironmental Engineering, 2010, 136(12): 1721-1728. DOI: 10.1061/(ASCE)GT.1943-5606.0000382.
[20] IVANOV V, STABNIKOV V. Soil surface biotreatment [M]. Singapore: Springer, 2017. DOI: 10.1007/978-981-10- 1445-1.
[21] MUJAH D, SHAHIN M A, CHENG Liang. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization [J]. Geomicrobiology Journal, 2017, 34(6): 524-537. DOI: 10.1080/01490451.2016.1225866.
[22] HE Jia, GAO Yu-feng, GU Zhang-xiang, CHU Jian, WANG Li-ya. Characterization of crude bacterial Urease for CaCO3 precipitation and cementation of Silty sand [J]. Journal of Materials in Civil Engineering, 2020, 32(5): 04020071. DOI: 10.1061/(ASCE)MT.1943-5533.0003100.
[23] Al QABANY A, SOGA K, SANTAMARINA C. Factors affecting efficiency of microbially induced calcite precipitation [J]. Journal of Geotechnical and Geoenvironmental Engineering, 2011, 138(8): 992-1001. DOI: 10.1061/(ASCE)GT.1943-5606.0000666.
[24] de MUYNCK W, de BELIE N, VERSTRAETE W. Microbial carbonate precipitation in construction materials: A review [J]. Ecological Engineering, 2010, 36(2): 118-136. DOI: 10.1016/j.ecoleng.2009.02.006.
[25] DHAMI N K, QUIRIN M E C, MUKHERJEE A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves [J]. Ecological Engineering, 2017, 103: 106-117. DOI: 10.1016/j.ecoleng.2017.03.007.
[26] GAO Yu-feng, HANG Lei, HE Jia, CHU Jian. Mechanical behaviour of biocemented sands at various treatment levels and relative densities [J]. Acta Geotechnica, 2019, 14(3): 697-707. DOI: 10.1007/s11440-018-0729-3.
[27] JAIN S, ARNEPALLI D N. Biochemically induced carbonate precipitation in aerobic and anaerobic environments by Sporosarcina pasteurii [J]. Geomicrobiology Journal, 2019, 36(5): 443-451. DOI: 10.1080/01490451.2019.1569180.
[28] WHIFFIN V S, VAN PAASSEN L A, HARKES M P. Microbial carbonate precipitation as a soil improvement technique [J]. Geomicrobiology Journal, 2007, 24(5): 417-423. DOI: 10.1080/01490418701436185.
[29] MONTOYA B M, DEJONG J T, BOULANGER R W. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation [J]. Géotechnique, 2013, 63(4): 302-312. DOI: 10.1680/geot.SIP13.P.019.
[30] OMOREGIE A I, KHOSHDELNEZAMIHA G, SENIAN N, ONG D E L, NISSOM P M. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials [J]. Ecological Engineering, 2017, 109: 65-75. DOI: 10.1016/j.ecoleng.2017.09.012.
[31] LIU Lu, LIU Han-long, XIAO Yang, CHU Jian, XIAO Peng, WANG Yang. Biocementation of calcareous sand using soluble calcium derived from calcareous sand [J]. Bulletin of Engineering Geology and the Environment, 2018, 77(4): 1781-91. DOI: 10.1007/s10064-017-1106-4.
[32] CHU Jian, STABNIKOV V, IVANOV V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil [J]. Geomicrobiology Journal, 2012, 29(6): 544-549. DOI: 10.1080/01490419.2011.592929.
[33] YANG Shang-chuan, LESHCHINSKY B, CUI Kai, ZHANG Fei, GAO Yu-feng. Influence of failure mechanism on seismic bearing capacity factors for shallow foundations near slopes [J]. Geotechnique, 2020: 1-46. DOI: 10.1680/jgeot. 19.P.329.
[34] KHADIM H J, AMMAR S H, EBRAHIM S E. Biomineralization based remediation of cadmium and nickel contaminated wastewater by ureolytic bacteria isolated from barn horses soil [J]. Environmental Technology & Innovation, 2019, 14: 100315. DOI: 10.1016/j.eti.2019.100315.
[35] JIANG Ning-Jun, LIU Rui, DU Yan-Jun, BI Yu-zhang. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency [J]. Science of the Total Environment, 2019, 672: 722-731. DOI: 10.1016/j.scitotenv. 2019.03.294.
[36] CHEN Huai-cheng, QIAN Chun-xiang, HUANG Hao-liang. Self-healing cementitious materials based on bacteria and nutrients immobilized respectively [J]. Construction and Building Materials, 2016, 126: 297-303. DOI: 10.1016/ j.conbuildmat.2016.09.023.
[37] MEYER F D, BANG S, MIN S, STETLER L D, BANG S S. Microbiologically-induced soil stabilization: application of Sporosarcina pasteurii for fugitive dust control [C]// Geo-frontiers 2011: Advances in Geotechnical Engineering 2011. Dallas: ASCE, 2011: 4002-4011. DOI: 10.1061/41165 (397)409.
[38] STABNIKOV V, CHU Jian, MYO A N, IVANOV V. Immobilization of sand dust and associated pollutants using bioaggregation [J]. Water, Air, & Soil Pollution, 2013, 224(9): 1631. DOI: 10.1007/s11270-013-1631-0.
[39] ZOMORODIAN S M A, GHAFFARI H, O'KELLY B C. Stabilisation of crustal sand layer using biocementation technique for wind erosion control [J]. Aeolian Research, 2019, 40: 34-41. DOI: 10.1016/j.aeolia.2019.06.001.
[40] JIANG Ning-jun, TANG Chao-sheng, YIN Li-yang, XIE Yue-han, SHI Bin. Applicability of microbial calcification method for sandy-slope surface erosion control [J]. Journal of Materials in Civil Engineering, 2019, 31(11): 04019250. DOI: 10.1061/(ASCE)MT.1943-5533.0002897.
[41] MOHEBBI M M, HABIBAGAHI G, NIAZI A, GHAHRAMANI A. A laboratory investigation of suppression of dust from wind erosion using biocementation with Bacillus amyloliquefaciens [J]. Scientia Iranica, 2019, 26(5): 2665-2677. DOI: 10.24200/sci.2018.20220.
[42] NAM I H, ROH S B, PARK M J, CHON C M, KIM J G, JEONG S W, SONG H, YOON M H. Immobilization of heavy metal contaminated mine wastes using Canavalia ensiformis extract [J]. Catena, 2016, 136: 53-58. DOI: 10.1016/j.catena.2015.07.019.
[43] HAMDAN N, KAVAZANJIAN E Jr. Enzyme-induced carbonate mineral precipitation for fugitive dust control [J]. Géotechnique, 2016, 66(7): 546-555. DOI: 10.1680/jgeot. 15.P.168.
[44] GAO Yu-feng, TANG Xin-yi, CHU Jian, HE Jia. Microbially induced calcite precipitation for seepage control in sandy soil [J]. Geomicrobiology Journal, 2019, 36(4): 366-375. DOI: 10.1080/01490451.2018.1556750.
[45] ROLSTON D E, LOUIE D T, BEDAIWY M N A. Micropenetrometer for in situ measurement of soil surface strength [J]. Soil Science Society of America Journal, 1991, 55(2): 481-485. DOI: 10.2136/sssaj1991.036159918019800 20031x.
[46] JUNGERIUS P D, VAN DER MEULEN F. The development of dune blowouts, as measured with erosion pins and sequential air photos [J]. Catena, 1989, 16(4, 5): 369-376. DOI: 10.1016/0341-8162(89)90021-0.
[47] TELYSHEVA G, SHULGA G. Silicon-containing polycomplexes for protection against wind erosion of sandy soil [J]. Journal of Agricultural Engineering Research, 1995, 62(4): 221-227. DOI: 10.1006/jaer.1995.1080.
[48] Al-THAWADI S. High strength in-situ biocementation of soil by calcite precipitating locally isolated ureolytic bacteria [D]. Murdoch: Murdoch University, 2008. https:// researchrepository.murdoch.edu.au/id/eprint/721/.
[49] OKWADHA G D, LI Jin. Optimum conditions for microbial carbonate precipitation [J]. Chemosphere, 2010, 81(9): 1143-1148. DOI: 10.1016/j.chemosphere.2010.09.066.
[50] de MUYNCK W, de BELIE N, VERSTRAETE W. Microbial carbonate precipitation in construction materials: A review [J]. Ecological Engineering, 2010, 36(2): 118-136. DOI: 10.1016/j.ecoleng.2009.02.006.
[51] VENDA OLIVEIRA P J, NEVES J P. Effect of organic matter content on enzymatic biocementation process applied to coarse-grained soils [J]. Journal of Materials in Civil Engineering, 2019, 31(7): 04019121. DOI: 10.1061/(ASCE) MT.1943-5533.0002774.
[52] DEJONG J T, MORTENSEN B M, MARTINEZ B C, NELSON D C. Bio-mediated soil improvement [J]. Ecological Engineering, 2010, 36(2): 197-210. DOI: 10.1016/j.ecoleng.2008.12.029.
[53] XU Guang, DING Xu-han, KURUPPU M, ZHOU Wei, BISWAS W. Research and application of non-traditional chemical stabilizers on bauxite residue (red sand) dust control, a review [J]. Science of the Total Environment, 2018, 616: 1552-1565. DOI: 10.1016/j.scitotenv.2017.10.158.
[54] XIE Zuo-ming, LIU Yong-ding, HU Chun-hai, CHEN Lan-zhou, LI Dun-hai. Relationships between the biomass of algal crusts in fields and their compressive strength [J]. Soil Biology and Biochemistry, 2007, 39(2): 567-572. DOI: 10.1016/j.soilbio.2006.09.004.
[55] FENG Guang-long, SHARRATT B, VADDELLA V. Windblown soil crust formation under light rainfall in a semiarid region [J]. Soil and Tillage Research, 2013, 128: 91-96. DOI: 10.1016/j.still.2012.11.004.
[56] ARENS S M, van KAAM-PETERS H M E, van BOXEL J H. Air flow over foredunes and implications for sand transport [J]. Earth Surface Processes and Landforms, 1995, 20(4): 315-332. DOI: 10.1002/esp.3290200403.
[57] KIDRON G J, ZOHAR M. Wind speed determines the transition from biocrust-stabilized to active dunes [J]. Aeolian Research, 2014, 15: 261-267. DOI: 10.1016/j.aeolia. 2014.04.006.
[58] ADESSI A, de CARVALHO R C, de PHILIPPIS R, BRANQUINHO C, da SILVA, J M. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts [J]. Soil Biology and Biochemistry, 2018, 116: 67-69. DOI: 10.1016/j.soilbio.2017.10.002.
[59] AMIRASLANI F, DRAGOVICH D. Combating desertification in Iran over the last 18 years: An overview of changing approaches [J]. Journal of Environmental Management, 2011, 92(1): 1-13. DOI: 10.1016/j.jenvman. 2010.08.012.
[60] NAFISI A, SAFAVIZADEH S, MONTOYA B M. Influence of microbe and enzyme-induced treatments on cemented sand shear response [J]. Journal of Geotechnical and Geoenvironmental Engineering, 2019, 145(9): 06019008. DOI: 10.1061/(ASCE)GT.1943-5606.0002111.
[61] BEATO C, FERNANDEZ M S, FERMANI S, REGGI M, NEIRA-CARRILLO A, RAO A, FALINIB G, ARIAS J L. Calcium carbonate crystallization in tailored constrained environments [J]. Cryst Eng Comm, 2015, 17(31): 5953-5961. DOI: 10.1039/C5CE00783F.
(Edited by ZHENG Yu-tong)
中文导读
大豆粗提脲酶诱导碳酸钙沉积抑制风沙土风蚀的现场试验
摘要:风蚀作用是干旱、半干旱地区土地荒漠化和沙尘暴形成的主要原因。本文通过现场试验研究用大豆粗提脲酶诱导碳酸钙沉积法抑制风沙土风蚀的可行性。试验场地位于中国宁夏回族自治区乌兰布和沙漠地区。结果表明,该方法能够显著提高风沙土的表面强度和抗风蚀能力。适合当地的最优胶结液(尿素-氯化钙溶液)浓度为0.2 mol/L,最优喷洒量为4 L/m2。在上述用量下,大豆粗提脲酶诱导碳酸钙沉积法处理30 d后风沙土表层碳酸钙含量为 0.45%,表面强度达306.2 kPa,风蚀深度几乎为零。风沙土表面强度随时间的延长而下降,且其长期固沙效果与地形有关。大豆粗提脲酶诱导碳酸钙沉积法处理12个月后,沙丘迎风面底部和沙地风蚀程度显著降低且仍能保持较高的表面强度,而沙丘迎风面顶部风蚀较为明显。扫描电子显微镜和X射线能谱仪测试结果证实了碳酸钙晶体的形成及其桥接效应。结果表明,大豆粗提脲酶诱导碳酸钙沉积法能够有效降低风沙土的可蚀性且具有良好的耐久性,是沙漠地区抑制风沙土风蚀的候选方案。
关键词:大豆粗提脲酶诱导碳酸钙沉积;生物矿化;风沙土;风蚀;现场试验
Foundation item: Projects(51978244, 51979088, 51608169) supported by the National Natural Science Foundation of China
Received date: 2020-03-16; Accepted date: 2020-07-07
Corresponding author: GAO Yu-feng, PhD, Professor; Tel: +86-25-83787287; E-mail: yfgao66@163.com; ORCID: https://orcid.org/ 0000-0002-5837-3382
Abstract: Wind erosion is a major cause of land desertification and sandstorm formation in arid and semi-arid areas. The objective of this study was to evaluate the potential of soybeans crude extract induced calcium carbonate precipitation (SICP) on reducing wind erosion risk of sandy soil. Field tests were carried out in Ulan Buh Desert, Ningxia Hui Autonomous Region, China. Results showed that the SICP method could significantly enhance the surface strength and wind erosion resistance of the topsoil. The optimal cementation solution (urea-CaCl2) concentration and spraying volume, according to experiments conducted on sandy land, were 0.2 mol/L and 4 L/m2, respectively. Under this condition, the CaCO3 content was approximately 0.45%,the surface strength of sandy soil could reach 306.2 kPa, and the depth of wind erosion was approximately zero, after 30 d completion of SICP treatment. Soil surface strength declined with the increase of time, and long-term sand fixation effects of SICP treatment varied depending on topography. Whereas wind erosion in the top area of the windward slope was remarkable, sandy soils on the bottom area of the windward slope still maintained a relatively high level of surface strength and a low degree of wind erosion 12 month after SICP treatment. Scanning electron microscopy (SEM) tests with energy dispersive X-ray (EDX) confirmed the precipitation of CaCO3 and its bridge effect. These findings suggested that the SICP method is a promising candidate to protect sandy soil from wind erosion in desert areas.


