DOI: 10.11817/j.ysxb.1004.0609.2021-36589
电解铜箔表面处理技术及添加剂研究进展
师慧娟1,陆冰沪2,樊小伟1,李大双2,郑小伟2,刘 耀1,谭育慧1,唐云志1
(1. 江西理工大学 材料冶金化学学部,赣州 341000;
2. 安徽铜冠铜箔有限公司,池州 247100)
摘 要:
随着5G通讯、新能源汽车等高端产业的发展,对电解铜箔产品性能提出更高的要求。表面处理技术是铜箔生产中极为重要的一项工艺技术,是解决铜箔绿色环保生产和获得高性能电解铜箔的主要途径。本文从国内外铜箔研究现状出发,归纳了包含粗化、固化、合金化、钝化、硅烷化等工艺流程的表面处理技术,并对每道工序中电解液的成分以及电沉积的影响因素进行分类总结,综述了粗化工序中添加剂的分类及研究现状与技术进展,重点阐述了包括晶粒细化剂、整平剂、光亮剂、表面活性剂与无机盐等各添加剂的作用机理及其对铜箔组织形貌和性能变化的影响规律,展望了我国铜箔的发展方向,为我国自主开发高性能铜箔生产技术提供参考。
关键词:
文章编号:1004-0609(2021)-05-1270-15 中图分类号:TG178 文献标志码:A
引文格式:师慧娟, 陆冰沪, 樊小伟, 等. 电解铜箔表面处理技术及添加剂研究进展[J]. 中国有色金属学报, 2021, 31(5): 1270-1284. DOI: 10.11817/j.ysxb.1004.0609.2021-36589
SHI Hui-juan, LU Bing-hu, FAN Xiao-wei, et al. Research progress of electrolytic copper foil surface treatment technology and additives[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(5): 1270-1284. DOI: 10.11817/j.ysxb.1004.0609.2021-36589
电解铜箔作为生产印制线路板和锂离子电池的基本导电原材料,是各种电子元器件相互组装的载体[1-3]。近年来,随着国内外电子技术和移动通讯的更新换代,电子线路板正朝着种类齐全化、功能多样化、传输快速化方向发展,相应电解铜箔的生产和应用也逐步向精细化、超薄化和高频化发展。由于电沉积制备的生箔为表面裸露的铜结晶晶粒,在高温条件下与树脂胶板压合成覆铜板的抗剥离强度低,易松脱报废;同时抗高温氧化能力差,容易出现铜扩散造成后期印制线路板短路风险;直接以生箔蚀刻线路也极易发生侧蚀造成断路风险。因此电解铜箔在印制线路板的实际生产应用中,需要经过一系列的表面处理,通常情况下,包含预处理、粗化、固化、合金化、钝化和硅烷化等工艺过程,以满足各种新兴元器件的应用要求。对于整个铜箔处理工艺,通常需要在镀液中加入少量添加剂,以改善处理层的结构形貌和组织性能,添加剂对铜箔表面粗糙度、剥离强度、抗拉强度、伸长率、抗氧化等性能起着关键作用。目前,应用于铜箔的添加剂种类繁多,功能各异,尤其是一些发挥络合作用的添加剂。我国对铜箔表面处理及添加剂研究取得了较快的发展,但还存在一些问题:1) 一些添加剂存在不同工艺条件下的离子选择性作用,即使结构相似的物质也会带来反差效果,并且相关添加剂作用机理至今还不明确,无法从理论上指导生产实践;2) 由于各企业市场竞争关系,所研究的添加剂大多是技术保密产品,通常以编号形式代替,同时,以往我国大部分铜箔企业的表面处理技术常使用含硫氰、砷、铬等有毒有害添加剂,不利于环保,这对我国电解铜箔的制备和表面处理技术提升提出了更高的要求;3) 随着5G用电解铜箔、锂电池铜箔等新产品的开发,我国对这些新型高档电解铜箔的研究还没有跟上国际步伐,落后于日本、美国、卢森堡、韩国等发达国家,或者整套技术从国外引进。针对上述问题,有必要根据现有的理论研究情况,综合当前国内外电解铜箔生产的表面处理技术进行归纳与总结,加深对生产高性能铜箔能力的理解,掌握相关处理技术及其添加剂的作用效果,为我国铜箔企业创新发展提供一些参考。
1 电解铜箔表面处理技术
电解铜箔是由硫酸及硫酸铜主液经电还沉积获得致密铜结晶组织而来。依据法拉第第一定律可对照通电量与时间计算出理论沉积量与实际沉积量的电沉积效率,通常沉积效率在98%以上。工业生产是以恒压输电形式达到稳定的恒流沉积模式,通过调整阴极辊转速可制备厚度在6~105 μm的多样性电解铜箔。由锟筒复制出的面称为光面,沉积形成的面为毛面,但沉积完成的是表面性能较弱的生箔,后续应用还需经过一系列表面处理,以改善铜箔组织性能,满足各领域的应用要求。Matsuda等[4]和UNO等[5]详细介绍了电解铜箔表面处理的工艺流程,常见的表面处理技术有粗化→固化→合金化→钝化→硅烷化等工序,具体如图1所示。
1.1 粗化及固化技术
粗化可以增加铜箔表面的活性位点,通常是在高酸低铜电镀液中以极限电流密度进行电沉积,获得均匀覆盖的细小沉积铜瘤点,取代光滑的外轮廓峰型面,提升与树脂板的黏合能力[6-9]。固化过程与粗化稍有不同,主要是为了进一步包裹和加固所得到的枝晶状的粗化瘤点,避免瘤点脱落,即在粗化松散的铜颗粒上紧固一层铜,增加与树脂胶板的抗剥离强度,处理效果见图2。此外,粗化需要各添加剂配合作用,固化仅为酸铜溶液,并配合低于极限电流密度沉积。
电解铜箔粗化及固化的机理如图3所示,反应机理如下:1) 在电场的作用下,铜离子向阴极的双电层移动;2) 在阴极表面铜离子的水合程度逐渐降低;3) 铜离子被还原为铜单质,并吸附在铜基底上;4) 吸附态的铜单质扩散到铜箔基底的活性位点,形成新的晶核并进行晶核的生长。
传统粗化工艺采用电解液中添加含砷化合物,目的是以砷的变价还原电位接近Cu2+的还原电位,从而形成竞争机制,抑制沉积铜在极限电流密度下的过快结晶形成树枝状铜晶粒竖直生长,促使电结晶组织平铺于表面峰型,得到均匀铺展的层面状铜瘤。粗化生成的瘤点在减少枝晶的同时维持较低的粗糙度值,获得大的比表面积,最终提高剥离强度。但砷化物含剧毒,不利于当前表面处理环保要求。张东等[10]采取直流电三段沉积工艺对铜箔表面进行低粗化处理,经该工艺处理的铜箔表面粗糙度Rz≤3 μm,厚度18 μm的铜箔抗剥离强度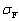 ≥12.74 N/cm;厚度12 μm的铜箔抗剥离强度
≥12.74 N/cm;厚度12 μm的铜箔抗剥离强度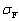 ≥11.76 N/cm,由于该工艺不采用添加剂,在整个低粗化处理过程中,不会出现砷、硒等有毒物质,实现了无毒环保的工艺环境,不仅降低了生产成本,还有利于实现可持续发展。胡旭日等[11]采用多步粗化工艺,在无添加剂体系中以30 g/L Cu2+、110~150 g/L H2SO4为最佳工艺条件,厚度35 μm的铜箔经该工艺粗化后粗糙度Rz由未粗化前的7.0 μm提高到8.4 μm以上,抗剥离强度
≥11.76 N/cm,由于该工艺不采用添加剂,在整个低粗化处理过程中,不会出现砷、硒等有毒物质,实现了无毒环保的工艺环境,不仅降低了生产成本,还有利于实现可持续发展。胡旭日等[11]采用多步粗化工艺,在无添加剂体系中以30 g/L Cu2+、110~150 g/L H2SO4为最佳工艺条件,厚度35 μm的铜箔经该工艺粗化后粗糙度Rz由未粗化前的7.0 μm提高到8.4 μm以上,抗剥离强度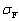 >20.97 N/cm。付强等[12]针对现有铜箔毛面粗糙度高和抗高温氧化性差,设计了一种电解铜箔表面微细粗化处理工艺,该工艺在强粗化与固化后再进行一次弱粗化,其中强弱粗化都是以Mo12Na3O40P为新型环保添加剂,所得铜箔毛面粗糙度低,铜箔抗常温、高温氧化性好。何成群等[13]在不含任何添加剂的条件下将铜箔表面处理工艺由粗化→粗化→固化→固化调整为粗化→固化→粗化→固化的多级循环处理,经该工艺处理后18 μm的铜箔表面粗糙度Rz<6.5 μm,抗剥离强度
>20.97 N/cm。付强等[12]针对现有铜箔毛面粗糙度高和抗高温氧化性差,设计了一种电解铜箔表面微细粗化处理工艺,该工艺在强粗化与固化后再进行一次弱粗化,其中强弱粗化都是以Mo12Na3O40P为新型环保添加剂,所得铜箔毛面粗糙度低,铜箔抗常温、高温氧化性好。何成群等[13]在不含任何添加剂的条件下将铜箔表面处理工艺由粗化→粗化→固化→固化调整为粗化→固化→粗化→固化的多级循环处理,经该工艺处理后18 μm的铜箔表面粗糙度Rz<6.5 μm,抗剥离强度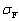 >14.01 N/cm,35 μm的铜箔表面粗糙度Rz<8.5 μm,抗剥离强度
>14.01 N/cm,35 μm的铜箔表面粗糙度Rz<8.5 μm,抗剥离强度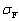 >19.01 N/cm,所得镀层形貌可以达到和含砷化合物表面处理工艺等同的效果,解决了含砷添加剂污染环境的问题,符合 IPC—4562标准。
>19.01 N/cm,所得镀层形貌可以达到和含砷化合物表面处理工艺等同的效果,解决了含砷添加剂污染环境的问题,符合 IPC—4562标准。
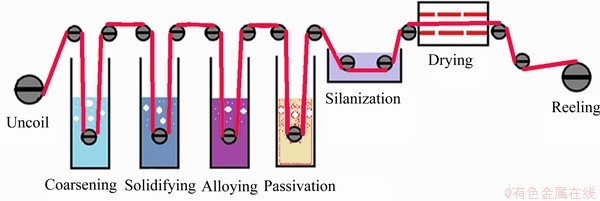
图1 电解铜箔表面处理技术流程图
Fig. 1 Flow chart of electrolytic copper foil surface treatment technology
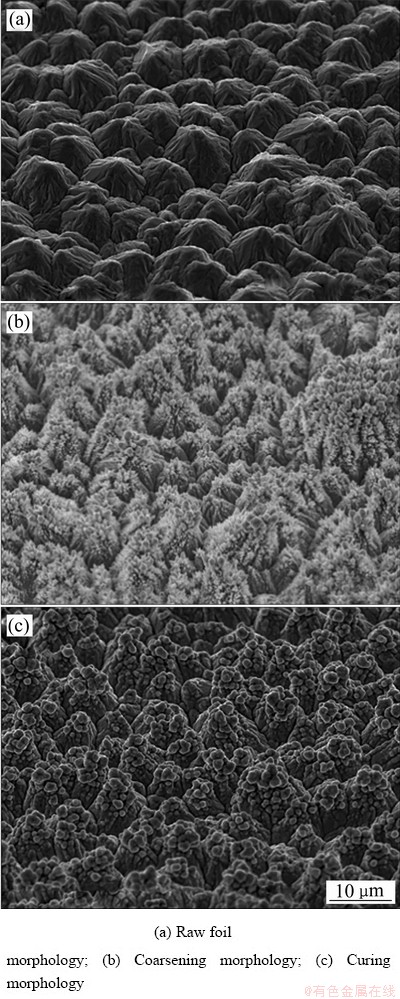
图2 电解铜箔的SME像
Fig. 2 SEM image of electrolytic copper foil
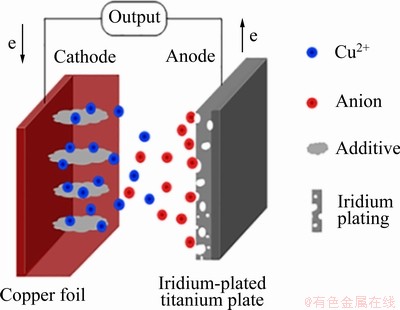
图3 电解铜箔粗化及固化机理示意图
Fig. 3 Schematic diagram of coarsening and curing mechanism of electrolytic copper foil
1.2 合金化技术
合金化技术通常是在粗化、固化工序的基础上再镀一层或多层异种金属。由合金镀构成的镀层一方面提高了覆铜箔板的耐热性及抗剥离强度,防止铜箔与树脂基板层压时铜向树脂基板扩散和在刻蚀工序中发生侧漏[14-15]][;另一方面,耐热层与铜箔基底构成无数个微型原电池,一旦发生腐蚀,耐热层会先作为阳极被腐蚀,而阴极铜箔基底受到保护(牺牲阳极的阴极保护法),进而起到防腐蚀性能。
目前,国内印制线路板已实现35 μm线宽的生产工艺,要求表面处理技术在压制成覆铜板后,也要保证蚀刻性能,若镀层的耐腐蚀性过强,可能出现蚀刻不彻底出现短路的现象,若镀层的耐腐蚀性过弱,蚀刻电子线路的宽度在0.1 mm以内时会出现侧蚀,可能出现断路现象,因此要求表面处理层具有合适的耐腐蚀性。为了提高电解铜箔的耐热性和抗剥离强度,李应恩等[16]以焦磷酸钾、硫酸镍、硫酸锌和添加剂A(A中阳离子为K+、Zn2+、Co2+中的至少一种)为镀液,开发出一种具有较高剥离强度的耐热层工艺,该工艺使12 μm铜箔的抗高温剥离强度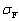 ≥11.96 N/cm。杨培霞等[17]对铜箔表面电镀三元合金Zn-Ni-Sn以取代传统有毒六价铬钝化工艺,分析认为镀层中的Sn可提高铜箔的耐蚀性,Ni可有效防止铜箔氧化变色的问题,劣化率仅为1.38%,抗剥离强度高达21.46 N/cm。谭育慧等[18]提出了一种Zn-Ni-P-La合金工艺,该工艺通过在镀层中引入稀土镧,利用镧的特异性吸附使镀层的结晶更加均匀细致,可有效防止因存放时间过长而发生锌扩散的问题。Albalat等[19]发现,在Zn-Ni镀液中加入酚类衍生物等添加剂,即使在低Ni含量时,也可以获得晶粒大小均一和针孔数量减少的镀层形貌,并且抗腐蚀性能随镀层中镍含量的增加而增加。Abedini等[20]在碱性溶液中加入无机锰盐,所获得的Zn-Ni-Mn耐热层极化电阻比Zn-Ni层增加7倍,且腐蚀电流密度显著降低,表现出优异的耐酸腐蚀性能,在蚀刻电子线路中能够蚀刻干净且无侧蚀,降低了耐酸劣化率,稳步提高线路精细化设计。
≥11.96 N/cm。杨培霞等[17]对铜箔表面电镀三元合金Zn-Ni-Sn以取代传统有毒六价铬钝化工艺,分析认为镀层中的Sn可提高铜箔的耐蚀性,Ni可有效防止铜箔氧化变色的问题,劣化率仅为1.38%,抗剥离强度高达21.46 N/cm。谭育慧等[18]提出了一种Zn-Ni-P-La合金工艺,该工艺通过在镀层中引入稀土镧,利用镧的特异性吸附使镀层的结晶更加均匀细致,可有效防止因存放时间过长而发生锌扩散的问题。Albalat等[19]发现,在Zn-Ni镀液中加入酚类衍生物等添加剂,即使在低Ni含量时,也可以获得晶粒大小均一和针孔数量减少的镀层形貌,并且抗腐蚀性能随镀层中镍含量的增加而增加。Abedini等[20]在碱性溶液中加入无机锰盐,所获得的Zn-Ni-Mn耐热层极化电阻比Zn-Ni层增加7倍,且腐蚀电流密度显著降低,表现出优异的耐酸腐蚀性能,在蚀刻电子线路中能够蚀刻干净且无侧蚀,降低了耐酸劣化率,稳步提高线路精细化设计。
1.3 钝化技术
由于铬是硬度最大的单质金属,因此传统钝化技术常采用铬酸盐钝化,在钝化过程中金属铬的表面易生成致密的碱式铬酸盐氧化膜,可以有效提高铜箔在运输过程中的耐磨性和抗氧化性,延长铜箔的储存时间。但是自2006年7月1日起,中国出口的电子电气产品开始按欧盟立法制定的《关于限制在电子电气设备中使用某些有害成分的指令》(简称ROHS指令)进行检测,指令明确规定了铅、镉、汞、六价铬等有害物质的最大添加量,这对我国电子技术提出了前所未有的挑战。随着全球环保意识的不断提高,研发新型绿色无污染的钝化技术以取代铬酸盐电镀是各铜箔企业及科研人员研究的热点,目前铬酸盐、钼酸盐、稀土盐、钛盐、硅酸盐和有机盐等钝化技术正处于试验阶段,有待在工业上推广应用。
李应恩等[21]采用植酸盐钝化技术制备的6~12 μm电解铜箔在恒温烘箱中可实现140 ℃、15 min无氧化变色现象,恒温恒湿试验结果表明,在温度80 ℃和湿度90%的条件下,可实现72 h后无氧化变色的现象。林家宝[22]开发了一种环保型电解铜箔表面无铬钝化处理液,该处理液包括50%植酸、40%硅酸钠和40%双氧水(体积分数)等6~8种配位剂或络合剂,18 μm和35 μm铜箔使用该处理液后,在湿度≥85%和温度≥60℃条件下保存72 h可实现无氧化变色的现象,同时3.3 mm线宽的18 μm铜箔在18%HCl中具有92%的抗腐蚀性,3.3 mm线宽的35 μm铜箔在18%HCl具有85%的抗腐蚀性。该工艺避免了传统工艺采用六价铬和三价铬对环境和人体的危害,可满足ROSH指令要求,并且以二段电流沉积取代传统镀铬阶段实现了无毒环保生产,降低了生产成本,为下游客户节约材料成本。杨少坤[23]在Zn-Ni耐热合金层的基础上采用钼酸盐钝化工艺,其工艺配方为钼酸钠(8 g/L),氧化锌(3 g/L),磷酸钠(4 g/L),植酸(2 mL/L),所得钝化膜表面平整,颜色均匀,可实现280 ℃、2 h无氧化变色现象,延长了铜箔的保存期限。
1.4 硅烷化技术
铜箔与树脂板压合的覆铜板,其首要指标是提高抗剥离强度。由于导体具有“集肤效应”,传输频率越高信号越趋于铜箔表层,例如1 GHz时趋肤深度为2 μm,10 GHz时趋肤深度只有0.66 μm,因此,起伏的表面轮廓易造成电子信号的失真和延迟,为了降低传输损失,需要铜箔具有低粗糙度,减少信号损失,而低粗糙度又将降低与树脂胶板的黏着力,二者的反向要求增加了粗化处理的难度。因此,迫切需要通过增强化学键力来提高抗剥离强度维持低粗糙值,对此寻找合适的硅烷偶联剂[24-25]涂覆层成为这一研究领域的共同目标。硅烷偶联剂的水解产物硅醇羟基与基底铜箔表面氧化物的羟基形成Si—O—Me特殊的化学键[26-28],大大增加树脂胶板与铜箔基底的结合力,硅烷偶联剂分子与铜箔表面氢氧根键合的简化机理如图4所示。
胡旭日等[29]在5~35 ℃条件下,以去离子水、γ- (2, 3-环氧丙氧)丙基三甲氧基硅烷(KH-560)、γ-氨丙基三乙氧基硅烷(KH-550)体积比为1:1:1混合液为复合硅烷偶联剂溶液,经测试涂覆该复合硅烷偶联剂的铜箔抗剥离强度为21.76 N /cm,煮2 h后剥离强度为21.56 N/cm,损失率为0.92%;浸288 ℃焊锡20 s后,抗剥离强度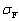 为21.76 N/cm,损失率为0,与单独使用KH-550或HK-560相比可大幅提升铜箔与树脂胶板的抗剥离强度。Li等[30]以三甲氧基丙基硅烷(TMPS)和双(三甲氧基硅烷)乙烷(BTMSE)为环保黏合剂,采用溶胶凝胶法和浸渍法对Al、Cu、和Sn的表面进行改性,证实了TMPS膜与铜表面存在Me—O共价键。但传统硅烷偶联剂与铜表面形成的Me—O键较弱,为了提高金属基底与硅烷偶联剂的黏着力和抗腐蚀性,众多研究者尝试以Me—S键取代Me—O键。Tani等[31]首先通过三聚硫氰酸将平均粗糙度仅为0.5 μm的铜箔表面功能化,然后再用含巯基的硅烷偶联剂涂覆铜箔表面,经170 ℃压板1 h后测得铜箔与树脂胶板的抗剥离强度为
为21.76 N/cm,损失率为0,与单独使用KH-550或HK-560相比可大幅提升铜箔与树脂胶板的抗剥离强度。Li等[30]以三甲氧基丙基硅烷(TMPS)和双(三甲氧基硅烷)乙烷(BTMSE)为环保黏合剂,采用溶胶凝胶法和浸渍法对Al、Cu、和Sn的表面进行改性,证实了TMPS膜与铜表面存在Me—O共价键。但传统硅烷偶联剂与铜表面形成的Me—O键较弱,为了提高金属基底与硅烷偶联剂的黏着力和抗腐蚀性,众多研究者尝试以Me—S键取代Me—O键。Tani等[31]首先通过三聚硫氰酸将平均粗糙度仅为0.5 μm的铜箔表面功能化,然后再用含巯基的硅烷偶联剂涂覆铜箔表面,经170 ℃压板1 h后测得铜箔与树脂胶板的抗剥离强度为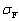 =10 N/cm,相比只用三聚硫氰酸处理铜箔表面时的抗剥离强度可提高25.9%。Balaji等[32]通过水解和缩合反应制备了不同浓度对氨基硫酚掺杂的3-环氧丙氧基丙基三甲氧基硅烷基溶胶-凝胶基质(PATP-GPTMS),傅立叶变换红外光谱和X射线光电子能谱分析表明,在0.1 mol/L PATP-GPTMS/Cu涂层中,铜表面上的PATP和硫醇单层相连接,增强了铜与树脂板的化学键力,Tafel极化曲线表明,PAPT-GPTMS/Cu涂层具有95.52%的耐腐蚀性,如图5所示。
=10 N/cm,相比只用三聚硫氰酸处理铜箔表面时的抗剥离强度可提高25.9%。Balaji等[32]通过水解和缩合反应制备了不同浓度对氨基硫酚掺杂的3-环氧丙氧基丙基三甲氧基硅烷基溶胶-凝胶基质(PATP-GPTMS),傅立叶变换红外光谱和X射线光电子能谱分析表明,在0.1 mol/L PATP-GPTMS/Cu涂层中,铜表面上的PATP和硫醇单层相连接,增强了铜与树脂板的化学键力,Tafel极化曲线表明,PAPT-GPTMS/Cu涂层具有95.52%的耐腐蚀性,如图5所示。
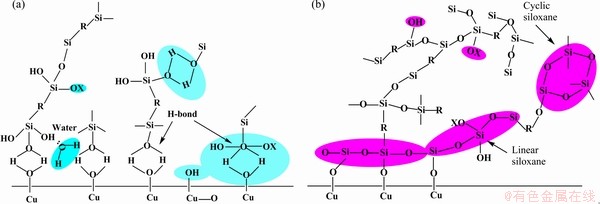
图4 脱水缩合前后氢键键面示意图
Fig. 4 Schematic diagram of hydrogen bonding surface before(a) and after(b) dehydration and condensation
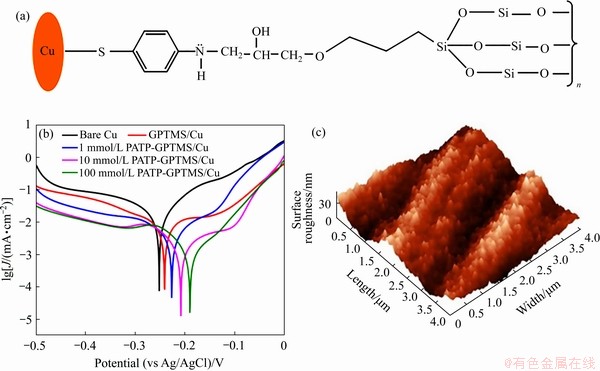
图5 PATP-GPTMS/Cu涂层的结构示意图、Tafel极化曲线以及AFM像[32]
Fig. 5 Schematic diagram of structure(a), Tafel polarization curves(b) and AFM image(c) of PATP-GPTMS/Cu layer[32]
2 粗化技术中添加剂的分类
粗化在电解铜箔表面处理中最为关键,促使铜箔表面沉积一层枝状的铜或氧化亚铜晶粒,增大铜箔的比表面积,提高剥离强度,确保覆铜板压合的完整制备。为改善沉积铜颗粒的深镀与均镀效果及防脱粉现象,需要添加剂作用沉积过程,以提升铜箔表面组织性能。
2.1 晶粒细化剂
电解铜箔粗化结晶包括形核和生长两个过程,形核速度和生长速度对镀层的结构及形貌起着非常重要的作用[33]。粗大晶粒会加剧镀层的漫反射,使镀层表面模糊无金属光泽,影响美观;最主要的是粗大晶粒容易形成铜粉,降低与覆铜箔层板间的结合力,所以需要细化晶粒改善镀层组织。常用的晶粒细化剂为一些络合物、螯合物或具有还原性的物质,通过物理吸附或化学吸附[34]作用使过电位较小的金属离子转化为过电位较大的络合离子,增大阴极极化,从而达到细化晶粒降低粗糙度值又增大比表面积的作用。
值得注意的是,若镀液中不含任何添加剂时,铜离子以水合铜离子的形式存在,当加入含配体的添加剂时,铜离子会与配体结合形成过电位增大的络合物。郑雅杰等[35]在二次镀铜体系中探究了络合剂对电沉积速度的影响,研究表明,当三乙醇胺作为主络合剂时,沉积速率随EDTA·2Na盐和a,a′-联吡啶的浓度增大而减小,分析认为络合剂可增大沉积过电位,降低沉积速率,使镀层细致均一化。Lin等[36]在化学镀铜溶液中采用甲醛作为还原剂,研究了乙二胺四乙酸二钠盐(EDTA)、三乙醇胺(TEA)、乙二胺(En)作为络合剂或还原剂对铜镀层形貌、粗糙度、择优取向等的影响。结果表明,EDTA是一种很好的络合剂,可以使沉积晶粒大小趋于均匀化,同时,TEA促使晶粒取向从(111)晶面转向(220)晶面,En作为晶粒细化剂吸附在铜箔表面可降低镀层的粗糙度。
2.2 整平剂及光亮剂
沉积层的结构及表面形貌由基底的电流分布决定[37]。在电沉积过程中,宏观平整的基底在微观下呈现凹凸不平的待镀基底表面有一层扩散层,表面凹陷处扩散层的厚度大于凸出处高度,整平剂通过扩散层到达凹陷处的时间大于凸出处,因此凸出处的整平剂含量多,增大了电极反应的极化,抑制晶粒沉积,从而增加表面的平整性。电镀工艺中常见的整平剂有2-巯基苯并咪唑、2-四氢噻唑硫酮、亚乙基硫脲、乙撑硫脲和健那绿等染料。
肖宁等[38]通过加入2 mg/L四氢噻唑硫铜(H1)整平剂,制备了显微硬度为187 HV、阴极极化值为97.4 mV的镀铜层,并且该硬度可维持14 d以上,满足电子雕刻的要求。有机染料是镀铜工艺中较早采用的整平剂和第二级添加剂,常用的有机染料品种较多,主要有吩嗪染料、嚼嗪染料、三苯甲烷染料、二苯甲烷染料、噻嗪染料、酞菁染料和酚红染料等。LIN等[39]研究发现,在60 LPM传质速率下,镀液中不含任何添加剂制备的铜箔表面含有大量褶皱,当单独使用健那绿作为带正电荷的唑类化合物时,铜晶粒表面褶皱明显减少并且表面Rz值由2.256 μm降低至1.456 μm,当健那绿、3-巯基-1-丙烷磺酸钠和聚乙二醇(20000)组合使用时,制备的铜箔表面非常光滑均匀,且表面Rz值仅为0.132 μm。
胺类及其衍生物因结构中含有不饱和键及氮原子可吸附在电极表面增大阴极极化,因此既是整平剂又是低电流密度区的光亮剂[40-41]][。Fabian等[42]用聚丙烯酰胺取代瓜尔胶可获得更高的成核和晶粒三维生长速率,使沉积表面更均匀和粗糙度更低。根据SCHARIFKER和HILLS提出的三维生长模型,有关瞬时成核和连续成核机理被广泛应用于金属电沉积研究中。丁辛城等[43]研究了整平剂TS-L (含氮杂环化合物的季胺盐)对铜沉积电位、成核方式及力学性能的影响,结果表明,随着整平剂TS-L浓度的增加,铜沉积电位逐渐负移,分析认为TS-L结构式中带正电的基团与Cu2+竞争阴极表面的活性位点阻碍铜的沉积,利于镀层晶粒的细化;进一步将计时电流法所测数据转换为无因次曲线,结果表明,在不含TS-L的镀液中铜的成核模型为瞬时成核,当镀液中含有20 mg/L TS-L时,铜电沉积的初始阶段为瞬时成核,但随着TS-L浓度的增加及测量时间的延长又转为连续成核方式;铜箔力学性能测试表明,其横向面的抗拉强度大于290 MPa,伸长率高达20.99%,满足IPC-TM-650《印制电路协会试验方法指南》中提出的要求。Varvara等[44-46]研究发现,丁炔二醇乙氧基化合物、三乙基苄基氯化铵可以阻碍铜离子从溶液主体向双电层的传质,有促进铜成核抑制晶体生长过程的作用,并可以使铜镀层的织构由未加添加剂时的(111)晶面转变为晶粒较细的(110)晶面;当丁炔二醇乙氧基化合物和三乙基苄基氯化铵以15:1混合(IT-85)时,细化晶粒的作用将进一步增强,并且其诱导期与缓慢成核有关。
对于含硫添加剂一般可加速铜沉积,表现出相应的光亮或整平作用,其解释有:一是这些添加剂对抑制层的置换促进了铜离子接近电极表面,提高了沉积速率;二是通过Cu+硫醇盐直接催化Cu2+还原为Cu+,由于在铜沉积的过程中Cu2+还原为Cu+是整个过程中的速控步,可加速铜的沉积。Chen等[47]研究了不同光亮剂及整平剂的去极化效果。通过对3种添加剂(3-巯基-1-丙烷磺酸盐(MPS)、3-S-噻吩鎓丙烷磺酸盐(UPS)和3-(苯并噻 唑-2-巯基)-1-丙烷磺酸钠(ZPS)[48])的恒电流测试对比(见图6),结果表明,当没有加入3种整平剂及光亮剂时,电压稳定在-0.7 V左右,当分别加入25 mg/L MPS、100 mg/L UPS和20 mg/L ZPS时电位显著正移,表现出明显的去极化能力,但UPS和ZPS的去极化能力比MPS弱;同时三者均可降低镀层粗糙度。
由此可见,整平剂的作用是改善二次电流分布,通过扩散吸附在电极上并在电极上消耗。整平剂和光亮剂没有明显的区分界限,但它们有一个共同点,即在分子结构中含有碳碳双键(—C=C—)、碳碳三键(—C≡C—)、氮氮双键(—N=N—)、大π键等不饱和键。
2.3 表面活性剂
表面活性剂在电镀工艺中起着至关重要的作用。主要作用有三点[49-50]:1) 降低镀层的表面张力,使阴极的析氢反应更容易发生;2) 使基底表面的油污分散,达到去除油污的目的;3) 表面活性剂属于双亲分子,容易在基底表面形成双电层,增加过电位,可细化镀层晶粒。常用的表面活性剂有聚乙二醇(PEG)、十二烷基硫酸钠、聚二硫二丙烷磺酸钠、烷基聚醚磺酸盐等。
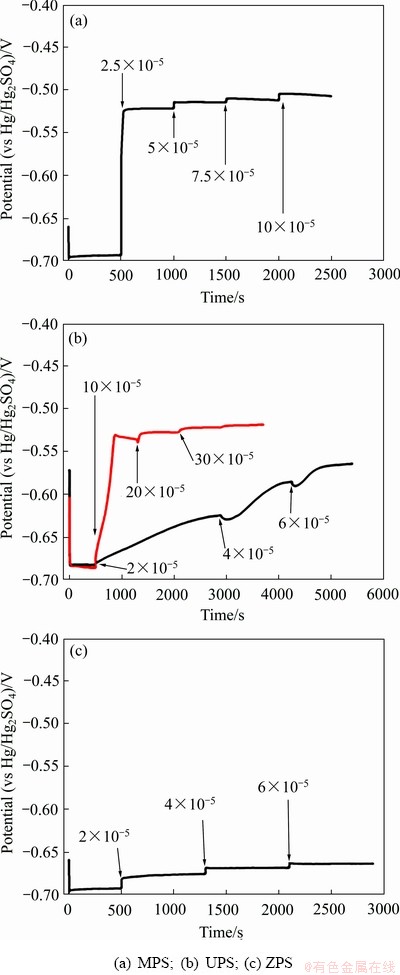
图6 三种光亮剂的恒电流测试图[47]
Fig. 6 Schematic diagram of constant current of three brighteners analyzed at 1000 r/min[47]
Xue等[51]以铜为阳极,铅为阴极,研究了表面活性剂对铜粉组成、粒度分布以及电流效率的影响。结果表明,当聚乙烯吡咯烷酮(PVP)浓度为5 g/L时,电流效率最高,粒径分布范围较窄;但是当聚乙烯吡咯烷酮(PVP)和聚氧乙烯烷基酚磺酸钠(APES)混合时,扩大了粒径分布使所得镀层铜粉粒径减小,深镀能力提高,可以达到实际生产需求。Yi等[52]研究表明,羟乙基纤维素(HEC)有助于减少针孔,避免晶体的异常生长(如图7所示),HEC促进针孔周围铜(220)晶面生长,维持致密(111)晶面的稳定生长,并促进晶粒的微晶化,提高镀层的力学性能。但是纤维素之类的添加剂电化学性较差,长时间电解处理,镀液容易出现浑浊、絮状物、沉淀,影响铜箔表面处理效果。
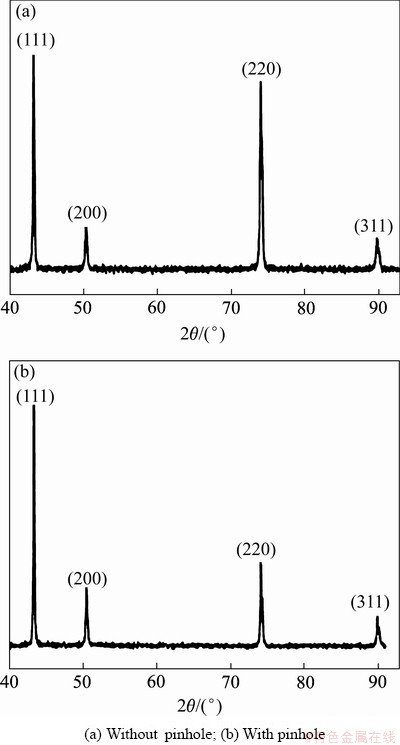
图7 铜箔表面的XRD谱[52]
Fig. 7 XRD patterns of copper foil surface[52]
蔡加勒等[53]和Healy等[54]][认为聚乙二醇作为一种非离子型表面活性剂,可显著降低电解液的表面张力,增加电解液的分散能力,而且聚乙二醇分子中疏水烷基链可吸附在电极表面,占据电极表面的活性位点,增大阴极极化,降低铜的沉积速率,但单独加入聚乙二醇时,抑制效果不明显;当Cl-与聚乙二醇组合加入时,由于 PEG-Cl--Cu+钝化膜的形成,铜沉积速率的降低将更明显,聚乙二醇在酸性镀铜溶液中的作用机理如图8所示[33]。
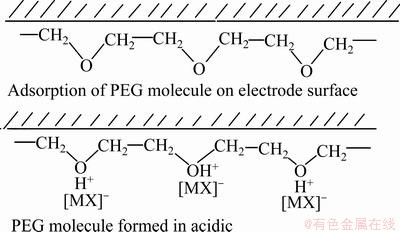
图8 聚乙二醇在酸性溶液中的镀铜机理[33]
Fig. 8 Copper plating mechanism of polyethylene glycol in acidic solution[33]
明胶[55-56]是由多种氨基酸脱水缩合形成的高分子化合物,是一种典型的表面活性剂。在电场的作用下,明胶胶粒会移向阴极,吸附在阴极表面并与Cu2+结合形成络合物,增大阴极极化,有利于新晶核的形成,阻碍Cu2+的沉积。Chang等[57]利用纯钛旋转圆盘电极,对比研究了酸性电解液中明胶含量对铜箔形貌、晶粒大小、择优取向等影响,铜箔光面的背散射衍射分析(EBSD)图像表明,在电沉积初期,明胶在平行于表面的侧向上抑制了铜晶粒的生长,有细化晶粒改善铜箔机械性能的作用,如图9所示[57]。任忠文[58]研究发现,明胶含量小于20 mg/L时,随着明胶含量的增加阴极过电位和溶液的分散能力随之增加,这是因为明胶是一种增极化剂,在电极表面可形成一层吸附膜,Cu2+必须穿过这层吸附膜,才能在电极上得电子还原;当明胶含量大于30 mg/L时,铜箔抗拉强度明显降低,这是由于高浓度的明胶粒子发生团聚掺杂在铜沉积过程中。
2.4 无机添加剂
2.4.1 氯离子
Cl-是影响铜电沉积的重要添加剂[59],它对铜沉积的影响机理如下[60]:1) 氯离子通过扩散作用吸附在阴极的活性位点上,与铜离子沉积的活性位点竞争,从而增加铜沉积的过电位,达到抑制铜离子放电的作用;2) 铜的电沉积分两步进行,首先Cu2+被还原为Cu+,然后在阴极Cu+继续被还原为Cu,Cu+易与Cl-形成不溶性的沉淀,覆盖在阴极表面阻止铜的进一步沉积;3) 由于Cl-的“桥连”作用,可以与一些添加剂或Cu2+形成络合物,增大阴极极化,抑制铜的沉积,达到细化晶粒的目的。
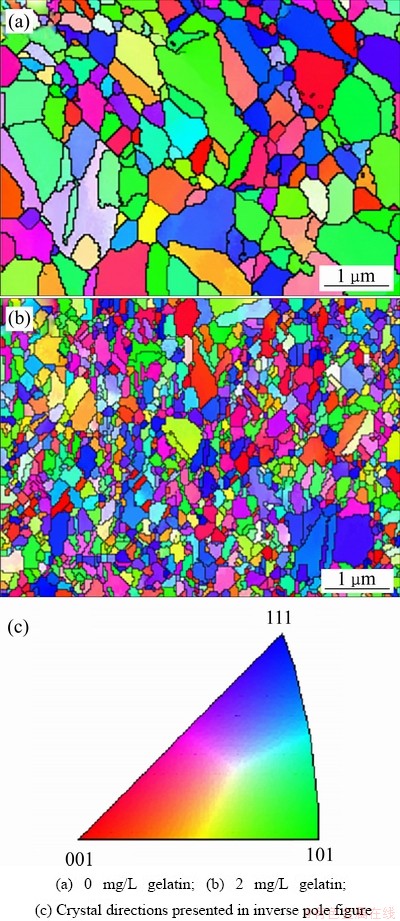
图9 铜箔电沉积的晶粒变化图[57]
Fig. 9 EBSD grain maps for copper foils electro- deposited[57]
有研究表明[61-67]:当PEG与Cl-组合使用时,由于PEG-Cl--Cu+钝化膜的形成使镀铜沉积速率降低两个数量级,当SPS-PEG-Cl-共同添加时,SPS通过吸附取代PEG形成的钝化膜,与PEG处于竞争关系,使铜成核速率和核数密度大大增加,其作用机理如图10所示[61]。
Gabrielli等[68]在含氯化物的硫酸铜溶液中通过电化学阻抗法提出了一种机制,该机制表明铜沉积过程中质量传输受限,主要是由于在电极上形成了不溶性的CuCl膜层,阻碍了电荷的传输速率。Shao等[69]研究发现,低浓度的氯离子增大溶液的电导率,可以起到去极化的作用,而高浓度的氯离子容易形成络合物,起到极化的作用,这主要是由于不同浓度的氯离子对双电层结构的影响不同。
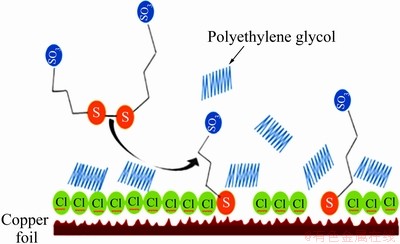
图10 SPS-PEG-Cl-体系的作用机理示意图[61]
Fig. 10 Schematic diagram of action mechanism of SPS-PEG-Cl- system[61]
2.4.2 稀土添加剂
稀土元素具有未充满的4f电子和较强的金属性[70],因此有效地利用稀土元素的这些特性,可以改善镀层的性能,而且稀土元素的标准电极电位较负,沉积铜箔中一般不含稀土,仅作用沉积过程,改善铜箔组织结构性能。
Maharana等[71]采用直流和脉冲两种方式,在酸性硫酸铜溶液中制备了铜基电沉积Cu-Y2O3复合镀层。为了明确纯铜与Cu-Y2O3复合镀层抗氧化性能的差异,实验分别在540 ℃和675 ℃条件下对纯铜及复合涂层进行20 h氧化,结果表明:复合涂层的抗氧化性能优于纯铜的,且540 ℃条件下10 kHz频率沉积的复合镀层质量增加变化最小,复合涂层较好的氧化响应可能是由于其致密的组织结构,如图11所示[71]。
杨胜奇等[72-73]研究发现,添加稀土CA的酸铜镀液可以提高产品的抗变色及抗腐蚀能力,降低镀铜层的孔隙,使镀层表面光亮。进一步经赫尔槽电镀表明,加稀土的镀液可以延长样品表面氧化变色的时间,在含非染料型光亮剂的电解液中加入稀土可以提高低电流区的走位能力,在含染料型光亮剂的电解液中加入稀土可以扩大光亮范围,研究发现含有稀土的光亮酸性镀铜液的使用期限延长,大大降低了应用成本。孔繁清等[74]在镀铜体系中加入稀土发光材料和分散剂(稀土氧化物或易共沉积的微粒)便可在紫外灯下观察到红光,表明稀土元素的加入可以促进共沉积,进而得到复合镀层。何田等[75] 研究了添加不同含量稀土RE对电解铜箔的组织及其性能的影响,结果表明稀土RE的加入可以细化晶粒,使晶粒更加均匀致密,同时,还能改善铜箔的力学性能;当加入3×10-5 RE时,力学性能和晶粒细化效果最优。
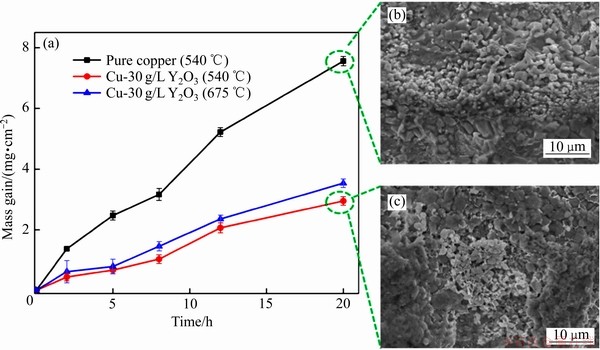
图11 纯铜及Cu-Y2O3 复合镀层质量变化和表面形貌[71]
Fig. 11 Mass change(a) and surface morphologies((b), (c)) of pure copper and Cu-Y2O3 composite coating[71]
2.4.3 其他无机盐
张世超等[76]研究了钨酸钠及硫酸钛作用铜箔表面晶粒形貌的影响,研究发现硫酸钛与镀层的形状及形核速度有关,硫酸钛浓度越高,形核速度越快,晶粒就越密集,但含量过高会形成大量的多级枝晶,容易造成线路板蚀刻不完全,使线路板出现残铜,过低则使粗化晶粒过少,降低铜箔抗剥离强度,而钨酸钠只与镀层晶粒的大小有关,随着钨酸钠含量的增加,晶粒逐渐变小,通过该方法得到的粗化铜箔表面粗糙度Ra最大可以达到0.18 μm,比未粗化试样提高200%。杨祥魁等[77]通过在粗化过程中加入磷钼酸钠,使其与铜离子络合形成杂多酸盐,改变铜离子的电化学析出电位,形成的铜钼合金在“山峰”沉积受到了阻化作用后向“山谷”移动,使粗化沉积的铜瘤状颗粒在峰顶和峰谷均匀分布,这种均匀瘤状结构的70 μm的HTE铜箔,在Tg175的板材上将抗剥离强度从普通铜箔的11.47 N/cm左右提高到15.98 N/cm以上,传统工艺处理铜箔与该工艺处理铜箔表面形貌如图12所示。
黄永发等[78]采用钨酸钠和硫酸亚锡等新型环保复合添加剂,开发出一种无砷粗化工艺,通过在镀液中加入锡粒可有效防止添加剂Sn2+的氧化变质,达到与含砷粗化等同的效果。冯绍彬等[79]等在粗化工艺中,采用钼酸铵作为无机添加剂,研究了粗化前后铜箔各性能参数的变化。SEM及各项性能参数结果显示,粗化后铜结晶呈蘑菇状生长,且成核数增加,同时粗化后铜箔真实表面积近似未处理铜箔的2倍,抗拉强度可由未处理时的0.62 MPa提高到1.41 MPa。周启伦等[80]利用硫酸亚钛、硫酸钛和钼酸盐作为粗化添加剂,发现硫酸亚钛可增强电解液的深镀能力,硫酸钛可控制晶核的形成速度,游离的钼酸根可以控制晶粒的生长速度,但钼酸根的浓度需要控制在合适的范围内,若浓度过低容易出现晶粒脱落的现象。18 μm铜箔经该工艺处理后,表面粗糙度值依然能保持Rz≤3 μm,并且抗剥离强度能保证9.8 N/cm以上,满足超低轮廓铜箔的要求。
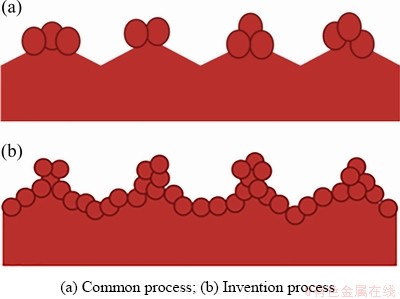
图12 此发明与传统工艺处理铜箔表面对比图
Fig. 12 Comparison of traditional copper foil surface treatment
3 结论与展望
电解铜箔表面处理技术是提升铜箔表面组织性能的有效方法,通过表面处理形成一层特殊的功能层,能够进一步提升铜箔的抗剥离强度、抗高温性、抗腐蚀性以及信号传输等性能。本文简要总结了当前电解铜箔表面技术的研究进展,分析了各处理工艺中添加剂的成分组合与影响机理。以此为基础,结合电子产业未来的发展需求,认为电解铜箔表面处理技术将重点聚焦以下几个方面。
1) 开发高效率、低能耗和短流程的生产工艺:针对现有电解铜箔表面处理工艺流程长成本高,按照产品性能的要求开发便捷高效的生产工艺,在能够降低生产成本的同时提高电解铜箔的性能。
2) 开发超薄载体铜箔表面处理技术:目前,国内研究较成熟的是18 μm和35 μm电解铜箔表面处理工艺,但是该工艺不适合应用于多层化线路板的内层线路精细化设计,因此,超薄载体铜箔的表面处理工艺将是未来的研究方向。
3) 开发具有纳米多孔道、高抗剥离强度和高抗氧化性的极薄锂电铜箔表面处理技术:以减小锂离子电池铜箔体积占比,提升动力续航能力。
4) 探索解决合金化工艺带来的电损耗和发热问题的方案:目前,电解铜箔表面处理技术中的合金化过程主要探索合金层对铜箔耐热性的影响,而针对高合金化工艺影响电解铜箔导电性的因素和由此带来的电损耗和发热问题研究甚少。
REFERENCES
[1] 田民波. 印制电路板技术的最新发展动向[J]. 印制电路信息, 2015(10): 10-15.
TIAN Min-bo. Recent development trend of PCB technologies[J]. Printed Circult Information, 2015(10): 10-15.
[2] 徐树民, 胡旭日, 王维河, 等. 电解铜箔的灰色表面处理工艺: 中国, 1962944A[P]. 2009-12-09.
XU Shu-min, HU Xu-ri, WANG Wei-he, et al. The gray surface treatment technology of electrolytic copper foil: China, CN1962944A[P]. 2009-12-09.
[3] 金荣涛. 电解铜箔产业发展与分析[J]. 印制电路信息, 2004(12): 17-20.
JIN Rong-tao. Development and analyse of electrodeposited copper foil industry[J]. Printed Circult Information, 2004(12): 17-20.
[4] MATSUDA M, SAKAI H, TOMONAGA S, et al. Electrodeposited copper foil, its manufacturing method, surface-treated electrodeposited copper foil using the electrodeposited copper foil, and copper- clad laminate and printed wiring board using the surface-treated electrodeposited copper foil: US, 8722199B2[P]. 2014-05-13.
[5] UNO T, OKUNO Y, TSURUTA T, et al. Surface-treated copper foil, and copper-clad laminate and printed wiring board using same: US, 20200029444A1[P]. 2020-01-23.
[6] BARD A J, FAULKNER L R. 电化学方法-原理和应 用[M]. 2版. 邵元华, 朱果逸, 董献堆, 等, 译. 北京: 化学工业出版社, 2005: 38-40.
BARD A J, FAULKNER L R. Electrochemical methods fundamentals and applications[M]. 2nd ed. SHAO Yuan-hua, ZHU Guo-yi, DONG Xian-dui, et al, transl. Beijing: Chemical Industry Press, 2005: 38-40.
[7] 金荣涛. 电解铜箔生产[M]. 长沙: 中南大学出版社, 2010: 158-160.
JIN Rong-tao. Production of electrolytic copper foil[M]. Changsha: Central South University Press, 2010: 158-160.
[8] YAMAMOTO T, KATAOKA T, HIRASAWA Y, et al. Surface treated copper foil, electrodeposited copper foil with carrier, manufacture method for the electrodeposited copper foil with carrier, and copper clad laminate: US, 20030148136A1[P]. 2003-08-07.
[9] 简志超, 彭永忠. 用于PCB基板的高耐热性电解铜箔的表面处理[J].有色金属工程, 2015, 5(2): 20-22.
JIAN Zhi-chao, PENG Yong-zhong. Surface treatment of high heat resistance electrolytic copper foil used for PCB[J]. Nonferrous Metals Engineering, 2015, 5(2): 20-22.
[10] 张 东, 石 晨, 张晓鹤, 等. 电解铜箔表面低粗化处理方法: 中国, 101067212A[P]. 2007-11-07.
ZHANG Dong, SHI Chen, ZHANG Xiao-he, et al. Electrolytic copper foil surface low coarsening treatment method: China, CN101067212A[P]. 2007-11-07.
[11] 胡旭日, 王海振, 徐好强, 等. 无添加剂体系中电解铜箔的多步粗化[J]. 电镀与涂饰, 2015, 34(1): 20-24.
HU Xu-ri, WANG Hai-zhen, XU Hao-qiang, et al. Multistep roughening of electrolytic copper foil in additive-free bath[J]. Electroplating & Finishing, 2015, 34(1): 20-24.
[12] 付 强, 付 毅, 张宏洋. 一种电解铜箔表面的微细粗化处理工艺: 中国, CN110205656A[P]. 2019-09-06.
FU Qiang, FU Yi, ZHANG Hong-yang. The invention relates to a micro-coarsening process for the surface of electrolytic copper foil: China, CN110205656A[P]. 2019-09-06.
[13] 何成群, 赵原森, 柴 云, 等. 一种电解铜箔生产中的表面处理工艺: 中国, CN103088379A[P]. 2013-05-08.
HE Cheng-qun, ZHAO Yuan-sen, CHAI Yun, et al. The invention relates to a surface treatment process in the production of electrolytic copper foil: China, CN103088379A[P]. 2013-05-08.
[14] IZΜMI A, KAKARA T, OTSUKI M W, et al. In situ residual stress analysis in a phenolic resin and copper composite material during curing[J]. Polymer, 2019, 182: 121857.
[15] 郑衍年. 电解铜箔表面处理工艺与结晶形态[J]. 印制电路信息, 2004(10): 14-16.
ZHENG Yan-nian. Copper foil surface treatment and crystalline forms[J]. Printed Circult Information, 2004(10): 14-16.
[16] 李应恩, 樊斌峰, 王建智, 等. 一种提高电解铜箔高温抗剥离性能面处理工艺: 中国, CN105018978A[P]. 2015-11-04.
LI Ying-en, FAN Bin-feng, WANG Jian-zhi, et al. The invention relates to a surface treatment process for improving the stripping resistance of electrolytic copper foil at high temperature: China, CN105018978[P]. 2015-11-04.
[17] 杨培霞, 安茂忠, 胡旭日, 等. 印制板用电解铜箔后处理工艺的研究[J]. 电镀与涂饰, 2005, 24(8): 42-45.
YANG Pei-xia, AN Mao-zhong, HU Xu-ri, et al. A post-treatment technics on electrolytic copper foil used for printed board[J]. Electroplating & Finishing, 2005, 24(8): 42-45.
[18] 谭育慧, 王 艳, 章 朦, 等. 电解铜箔表面电沉积Zn-Ni-P-La合金工艺[J]. 应用化学, 2015, 32(4): 458-463.
TAN Yu-hui, WANG Yan, ZHANG Meng, et al. Electrodeposited Zn-Ni-P-La alloys coating for electrolytic copper foil[J]. Chinese Journal of Applied Chemistry, 2015, 32(4): 458-463.
[19] ALBALAT R, GóMEZ E, MüLLER C, et al. Electrodeposition of zinc-nickel alloy coatings: influence of a phenolic derivative[J]. Journal of Applied Electrochemistry, 1990, 20(4): 635-639.
[20] ABEDINI B, PARVINI A N, YAZDANI S, et al. Electrodeposition and corrosion behavior of Zn-Ni-Mn alloy coatings deposited from alkaline solution[J]. Transactions of Nonferrous Metals Society of China, 2020, 30(2): 548-558.
[21] 李应恩, 樊斌锋, 王建智, 等. 锂电池用铜箔无铬防氧化技术的研究[J]. 中外企业家, 2016(18): 131-132.
LI Ying-en, FAN Bin-feng, WANG Jian-zhi, et al. Research on chromium-free oxidation prevention technology of copper foil for lithium batteries[J]. Chinese and Foreign Entrepreneurs, 2016(18): 131-132.
[22] 林家宝. 一种环保型电解铜箔无铬钝化处理液和处理方法: 中国, CN107151809A[P]. 2017-09-12.
LIN Jia-bao. The invention relates to an environmentally friendly electrolytic copper foil chromium-free passivation solution and a treatment method: China, CN107151809A[P]. 2017-09-12.
[23] 杨少坤. 新型环保铜箔表面钝化处理工艺研究[J]. 地球, 2013(3): 189-190.
YANG Shao-kun. Study on passivation treatment technology of new environmentally-friendly copper foil surface[J].The Earth, 2013(3): 189-190.
[24] 张金涛, 胡吉明, 张鉴清, 等. 金属涂装预处理新技术与涂层性能研究方法进展[J]. 表面技术, 2005, 34(1): 1-4.
ZHANG Jin-tao, HU Ji-ming, ZHANG Jian-qing, et al. Progress in novel surface pretreatment and studying method of origanic coated metals[J]. Surface technology, 2005, 34(1): 1-4.
[25] BORGES J N, BELMONTE T, GUILLOT J, et al. Functionalization of copper surfaces by plasma treatments to improve adhesion of epoxy resins[J]. Plasma Processes and Polymers, 2009, 6(S1): s490-s495.
[26] PALANIVEL V, ZHU D, VAN OOIJ W J. Nanoparticle- filled silane films as chromate replacements for aluminum alloys[J]. Progress in Organic Coatings, 2003, 47(3/4): 384-392.
[27] TSUCHIDA K, KΜMAGAI M, AKASE F. Surface treatment for copper foil: US, 6835241B2[P]. 2004-12-28.
[28] VAN OOIJ W J, ZHU D Q, PRASAD G, et al. Silane based chromate replacements for corrosion control, paint adhesion, and rubber bonding[J]. Surface Engineering, 2000, 16(5): 386-396.
[29] 胡旭日, 王维河, 王海振, 等. 一种电解铜箔表面处理剂的制备方法: 中国, CN104099061A[P]. 2014-10-15.
HU Xu-ri, WANG Wei-he, WANG Hai-zhen, et al. Preparation method of electrolytic copper foil surface treatment agent: China, CN104099061A[P]. 2014-10-15.
[30] LI Y S, TRAN T, XU Y, et al. Spectroscopic studies of trimetoxypropylsilane and bis(trimethoxysilyl)ethane sol-gel coatings on aluminum and copper[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2006, 65(3/4): 779-786.
[31] Tani M, Sasaki S, Uenishi K. Adhesion improvement on smooth Cu wiring surfaces of printed circuit boards[J]. Transactions of The Japan Institute of Electronics Packaging, 2011, 4(1): 24-30.
[32] BALAJI J, ROH S H, EDISON T N J I, et al. Sol-gel based hybrid silane coatings for enhanced corrosion protection of copper in aqueous sodium chloride[J]. Journal of Molecular Liquids, 2020, 302: 112551.
[33] 张立茗, 方景礼, 袁国伟, 等. 实用电镀添加剂[M]. 北京: 化学工业出版社, 2007.
ZHANG Li-ming, FANG Jing-li, YUAN Guo-wei, et al. Practical plating additive[M]. Beijing: Chemical Industry Press, 2007.
[34] XIONG S, LIANG D, BA Z, et al. Adsorption behavior of thiadiazole derivatives as anticorrosion additives on copper oxide surface: Computational and experimental studies[J]. Applied Surface Science, 2019, 492: 399-406.
[35] 郑雅杰, 李春华, 邹伟红. 三乙醇胺和EDTA·2Na盐双络合体系快速化学镀铜工艺研究[J]. 材料导报, 2006, 20(10): 159-162.
ZHENG Ya-jie, LI Chun-hua, ZOU Wei-hong. Study on electroless copper plating technology of high plating rate in triethanolamine and EDTA·2Na dual-chelating-agent system[J]. Materials Reports, 2006, 20(10): 159-162.
[36] LIN Y M, YEN S C. Effects of additives and chelating agents on electroless copper plating[J]. Applied Surface Science, 2001, 178(1/4): 116-126.
[37] LEE C Y, LIN P C, YANG C H, et al. Significantly improving the etching characteristics of electroplated Cu films through microstructure modification[J]. Surface and Coatings Technology, 2020, 386: 125471.
[38] 肖 宁, 邓志江, 滕艳娜, 等. 整平剂对酸性电镀硬铜的影响[J]. 电镀与涂饰, 2015, 34(19): 1082-1087.
XIAO Ning, DENG Zhi-jiang, TENG Yan-na, et al. Effects of different leveling agents on hard copper electroplating in acidic sulfate electrolyte[J]. Electroplating & Finishing, 2015, 34(19): 1082-1087.
[39] LIN C C, YEN C H, LIN S C, et al. Interactive effects of additives and electrolyte flow rate on the microstructure of electrodeposited copper foils[J]. Journal of The Electrochemical Society, 2017, 164(13): D810-D817.
[40] DAHMS W, JONAT M, SENGE G, et al. Water bath and method for electrolytic deposition of copper coatings: US, 6425996[P]. 2002-07-30.
[41] Kμmagai M, Hanafusa M. Copper electrolytic solution containing amine compound having specific skeleton and organosulfur compound as additives, and electrolytic copper foil produced using the same: US, 7005055B2[P]. 2006-02-28.
[42] FABIAN C P, RIDD M J, SHEEHAN M E. Assessment of activated polyacrylamide and guar as organic additives in copper electrodeposition[J]. Hydrometallurgy , 2007, 86(1/2): 44-55.
[43] 丁辛城, 彭代明, 陈梓侠, 等. 新型整平剂TS-L对铜电沉积的影响[J]. 电镀与涂饰, 2016, 35(11): 556-559.
DING Xin-cheng, PENG Dai-ming, CHEN Zi-xia, et al. Effect of novel leveling agent TS-L on electrodeposition of copper[J]. Electroplating & Finishing, 2016, 35(11): 556-559.
[44] VARVARA S, MURESAN L, NICOARA A, et al. Kinetic and morphological investigation of copper electrodeposition from sulfate electrolytes in the presence of an additive based on ethoxyacetic alcohol and triethyl-benzyl-ammonium chloride[J]. Materials Chemistry and Physics, 2001, 72(3): 332-336.
[45] VARVARA S, MURESAN L, POPESCU I C, et al. Comparative study of copper electrodeposition from sulphate acidic electrolytes in the presence of IT-85 and of its components[J]. Journal of Applied Electrochemistry, 2005, 35(1): 69-76.
[46] VARVARA S, MURESAN L, POPESCU I C, et al. Copper electrodeposition from sulfate electrolytes in the presence of hydroxyethylated 2-butyne-1,4-diol[J]. Hydrometallurgy, 2004, 75(1/4): 147-156.
[47] CHEN T C, TSAI Y L, HSU C F, et al. Effects of brighteners in a copper plating bath on throwing power and thermal reliability of plated through holes[J]. Electrochimica Acta, 2016, 212: 572-582.
[48] LEE H, TSAI S T, WU P H, et al. Influence of additives on electroplated copper films and their solder joints[J]. Materials Characterization, 2019, 147: 57-63.
[49] 徐文柱. 表面活性剂在电镀中的应用[J]. 过滤与分离, 2014, 24(4): 40-46.
XU Wen-zhu. The application of surfactants in electroplating[J]. Journal of Filtration & Separation, 2014, 24(4): 40-46.
[50] 郭国才. 表面活性剂在电镀中的应用[J]. 电镀与环保, 2006, 26(3): 15-16.
GUO Guo-cai. Application of surfactant in electroplating[J]. Electroplating & Pollution Control, 2006, 26(3): 15-16.
[51] XUE J Q, WU Q, WANG Z Q, et al. Function of additives in electrolytic preparation of copper powder[J]. Hydro- metallurgy, 2006, 82(3/4): 154-156.
[52] YI G, CAI F, PENG W, et al. Experimental an analysis of pinholes on electrolytic copper foil and their prevention[J]. Engineering Failure Analysis, 2012, 23: 76-81.
[53] 蔡加勒, 张爱强, 周绍民. 聚乙二醇聚合度对其吸附和阻化Cu2+放电的影响[J]. 高等学校化学学报, 1988, 9(1): 57-61.
CAI Jia-le, ZHANG Ai-qiang, ZHOU Shao-min. Effects of polymerization degree of polyglycols on their adsorption and inhibition of Cu2+ discharge[J]. Chemical Journal of Chinese Universities, 1988, 9(1): 57-61.
[54] HEALY J P, PLETCHER D, GOODENOUGH M. The chemistry of the additives in an acid copper electroplating bath[J]. Journal of Electroanalytical Chemistry, 1992, 338(1/2): 179-187.
[55] ZHANG D, YANG H. Gelatin-stabilized copper nanoparticles: Synthesis, morphology, and their surface- enhanced Raman scattering properties[J]. Physica B: Condensed Matter, 2013, 415: 44-48.
[56] 张文海, 陈俊民. 明胶在冶金中的应用[J]. 明胶科学与技术, 1983(1): 1-10.
ZHANG Wen-hai, CHEN Jun-min. Application of gelatin in metallurgy[J]. The Science and Technology of Gelatin, 1983(1): 1-10.
[57] CHANG T, JIN Y, WEN L, et al. Synergistic effects of gelatin and convection on copper foil electrodeposition[J]. Electrochimica Acta, 2016, 211: 245-254.
[58] 任忠文. 对电解铜箔生产明胶加入方法的讨论[J]. 印制电路信息, 2002(10): 24-25.
REN Zhong-wen. The method of adding gelatin from electrolytic copper foil was discussed[J]. Printed Circult Information, 2002(10): 24-25.
[59] KONDO K, MURAKAMI H. Crystal growth of electrolytic Cu foil[J]. Journal of the Electrochemical Society, 2004, 151(7): C514-C518.
[60] KOH L T, YOU G Z, LI C Y, FOO P D. Investigation of the effects of byproduct components in Cu plating for advanced interconnect metallization[J]. Microelectronics Journal, 2002, 33(3): 229-234.
[61] YOON Y, KIM H, KIM T Y, et al. Selective determination of PEG-PPG concentration in Cu plating bath with cyclic voltammetry stripping using iodide ion[J]. Electrochimica Acta, 2020, 339: 135916.
[62] SHEN H, KIM H C, SUNG M, et al. Thermodynamic aspects of bis(3-sulfopropyl) disulfide and 3-mercapto-1- propanesulfonic acid in Cu electrodeposition[J]. Journal of Electroanalytical Chemistry, 2018, 816: 132-137.
[63] LEE A, KIM M J, CHOS S, et al. High strength Cu foil without self-annealing prepared by 2M5S-PEG-SPS[J]. Korean Journal of Chemical Engineering, 2019, 36(6): 981-987.
[64] ZHENG M, WILLEY M, WEST A C. Electrochemical nucleation of copper on ruthenium[J]. Electrochemical and Solid-State Letters, 2005, 8(10): C151-C154.
[65] MOFFAT T P, WHEELER D, JOSELL D. Electrodeposition of copper in the SPS-PEG-Cl additive system[J]. Journal of The Electrochemical Society, 2004, 151(4): C262-C271.
[66] 辜 敏, 李 强, 鲜晓红, 等. PEG-Cl-添加剂存在下的铜电结晶过程研究[J]. 化学学报, 2007, 65(10): 881-886.
GU Min, LI Qiang, XIAN Xiao-hong, et al. Electrocrystallization of copper in the presence of PEG-Cl- additive[J]. Acta Chimica Sinica, 2007, 65(10): 881-886.
[67] HUANG L, YANG F Z, XU S K, et al. Electrochemical nucleation and growth of copper on HOPG in presence of PEG and chloride ions as additives[J]. Transactions of the IMF, 2006, 84(1): 47-51.
[68] GABRIELLI C, MOCOTEGUY P, PERROT H, et al. Mechanism of copper deposition in a sulphate bath containing chlorides[J]. Journal of Electroanalytical Chemistry, 2004, 572 (2): 367-375.
[69] SHAO W B, PATTANAIK G, ZANGARI G. Influence of chloride anions on the mechanism of copper electrodeposition from acidic sulfate electrolytes[J]. Journal of the Electrochemical Society, 2007, 154(4): D201-D207.
[70] LIU G, HUANG Z, WANG L, et al. Effects of Ce4+ on the structure and corrosion resistance of electroless deposited Ni-Cu-P coating[J]. Surface and Coatings Technology, 2013, 222: 25-30.
[71] MAHARANA H S, BASU A. Surface-mechanical and oxidation behavior of electro-co-deposited Cu-Y2O3 composite coating[J]. Surface and Coatings Technology, 2016, 304: 348-358.
[72] 杨胜奇. 稀土在金属表面处理工艺中的应用技术(3)—— 稀土对提高酸性亮铜镀层低区走位和抗变色能力的影响[J]. 材料保护, 2008, 41(5): 81-83.
YANG Sheng-qi. Application of rare earth in surface treatment of metal(3)—Effects of rare earth on improving the low zone orientation and resistance to discoloration of acid brigh copper coating[J]. Materials Protection, 2008, 41(5): 81-83.
[73] 杨胜奇, 张弘伟, 汪建忠. 稀土添加剂在光亮酸性镀铜中的应用[J].材料保护, 2003, 36(4): 67-67.
YANG Sheng-qi, ZHANG Hong-wei, WANG Jian-zhong. Application of rare earth additives in bright acid copper plating[J]. Materials Protection, 2003, 36(4): 67-67.
[74] 孔繁清, 闫慧忠, 赵增祺, 等. 稀土发光材料在化学复合镀中应用的研究[J]. 稀土, 2002, 23(4): 43-45.
KONG Fan-qing, YAN Hui-zhong, ZHAO Zeng-qi, et al. Composite electroless plating of the copper and rare earth luminescent material[J]. Chinese Rare Earths, 2002, 23(4): 43-45.
[75] 何 田, 易光斌, 蔡芬敏, 等. 添加RE对电解铜箔组织与性能的影响[J]. 特种铸造及有色合金, 2010, 30(7): 658-660.
HE Tian, YI Guang-bin, CAI Fen-min, et al. Effect of additive RE on microstructure and properties of electrolytic copper foil[J]. Special-cast and Non-ferrous Alloys, 2010, 30(7): 658-660.
[76] 张世超, 石伟玉, 白致铭. 铜箔表面粗化工艺的研究[J]. 电镀与精饰, 2005, 27(5): 1-3.
ZHANG Shi-chao, SHI Wei-yu, BAI Zhi-ming. Study on copper foil surface roughening technology[J]. Electroplating & Finishing, 2005, 27(5): 1-3.
[77] 杨祥魁, 徐树民, 王维河, 等. 一种电解铜箔表面的微细粗化处理工艺: 中国, CN106011965A[P]. 2016-10-12.
YANG Xiang-kui, XU Shu-min, WANG Wei-he, et al. A invention relates to a fine and coarsening process for the surface of electrolytic copper foil: China, CN106011965A[P]. 2016-10-12.
[78] 黄永发, 王 平, 唐云志, 等. 一种新型电解铜箔无砷粗化工艺研究[J]. 有色金属科学与工程, 2012, 3(2): 1-4.
HUANG Yong-fa, WANG Ping, TANG Yun-zhi, et al. A research on the arsenic-free coarsening technology of copper foil[J]. Nonferrous Metals Science and Engineering, 2012, 3(2): 1-4.
[79] 冯绍彬, 李振兴, 胡芳红, 等. 铜箔表面电镀铜粗化工艺[J].材料保护, 2010, 43(7): 24-26.
FENG Shao-bin, LI Zhen-xing, HU Fang-hong, et al. Coarsening process of copper plating on copper foil surface[J]. Materials Protection, 2010, 43(7): 24-26.
[80] 周启伦, 郑惠军, 黄国平, 等. 一种电解铜箔用添加剂及甚低轮廓电解铜箔表面处理工艺: 中国, CN102560584A[P]. 2012-07-11.
ZHOU Qi-lun, ZHENG Hui-jun, HUANG Guo-ping, et al. A invention relates to an additive for electrolytic copper foil and a surface treatment process for very low profile electrolytic copper foil: China, CN102560584A[P]. 2012-07-11.
Research progress of electrolytic copper foil surface treatment technology and additives
SHI Hui-juan1, LU Bing-hu2, FAN Xiao-wei1, LI Da-shuang2, ZHENG Xiao-wei2, LIU Yao1, TAN Yu-hui1, TANG Yun-zhi1
(1.Faculty of Materials Metallurgy and Chemistry, Jiangxi University of Science and Technology, Ganzhou 341000, China;
2.Anhui Tongguan Copper Foil Co., Ltd., Chizhou 247100, China)
Abstract: With the development of high-end industries, such as 5G communications and new-energy vehicles, the higher requirements for the capabilities of electrolytic copper foil products were put forward. Using an extremely important technology, the electrolyticcopper foil surface treatment is regarded as a primary way for solving copper foil green production problem and obtaining high performance coating copper foil. Here, the present domestic and foreign research status of copper foil surface treatment including coarsing process, curing process, alloying process, passivation process, silanization process, etc, were documented and summarized. Especially, the composition of the electrolyte in each process and the influencing factors of electrodeposition were presented and analyzed, respectively. Moreover, we reviewed the research status and classification of additives in coarsening progress, and emphasized the mechanism of each additives including grain refiner, leveling agent, brightener, surfactant and inorganic additive, as well as the effects of additives on morphology and properties of copper foil. Finally, the future development direction of copper foil in China was pointed out. We believe that this review will provide a reference for our country independent development of high-performance copper foil production technology.
Key words: electrolytic copper foil; surface treatment; additive; function mechanism; structure; performance
Foundation item: Projects(21671086, No. 21761013) supported by the National Natural Science Foundation of China; Project(Ganshikefa〔2019〕60) supported by Ganzhou Key Research and Development Program, China
Received date: 2020-06-30; Accepted date: 2021-03-16
Corresponding author: TANG Yun-zhi; Tel: +86-15879731367; Email: 9120060053@jxust.edu.con
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(21671086,21761013);赣州市重点研发计划资助项目(赣市科发〔2019〕60号)
收稿日期:2020-06-30;修订日期:2021-03-16
通信作者:唐云志,教授,博士;电话:15879731367;E-mail:9120060053@jxust.edu.cn
摘 要:随着5G通讯、新能源汽车等高端产业的发展,对电解铜箔产品性能提出更高的要求。表面处理技术是铜箔生产中极为重要的一项工艺技术,是解决铜箔绿色环保生产和获得高性能电解铜箔的主要途径。本文从国内外铜箔研究现状出发,归纳了包含粗化、固化、合金化、钝化、硅烷化等工艺流程的表面处理技术,并对每道工序中电解液的成分以及电沉积的影响因素进行分类总结,综述了粗化工序中添加剂的分类及研究现状与技术进展,重点阐述了包括晶粒细化剂、整平剂、光亮剂、表面活性剂与无机盐等各添加剂的作用机理及其对铜箔组织形貌和性能变化的影响规律,展望了我国铜箔的发展方向,为我国自主开发高性能铜箔生产技术提供参考。
[1] 田民波. 印制电路板技术的最新发展动向[J]. 印制电路信息, 2015(10): 10-15.
[2] 徐树民, 胡旭日, 王维河, 等. 电解铜箔的灰色表面处理工艺: 中国, 1962944A[P]. 2009-12-09.
[3] 金荣涛. 电解铜箔产业发展与分析[J]. 印制电路信息, 2004(12): 17-20.
[6] BARD A J, FAULKNER L R. 电化学方法-原理和应 用[M]. 2版. 邵元华, 朱果逸, 董献堆, 等, 译. 北京: 化学工业出版社, 2005: 38-40.
[7] 金荣涛. 电解铜箔生产[M]. 长沙: 中南大学出版社, 2010: 158-160.
[9] 简志超, 彭永忠. 用于PCB基板的高耐热性电解铜箔的表面处理[J].有色金属工程, 2015, 5(2): 20-22.
[10] 张 东, 石 晨, 张晓鹤, 等. 电解铜箔表面低粗化处理方法: 中国, 101067212A[P]. 2007-11-07.
[11] 胡旭日, 王海振, 徐好强, 等. 无添加剂体系中电解铜箔的多步粗化[J]. 电镀与涂饰, 2015, 34(1): 20-24.
[12] 付 强, 付 毅, 张宏洋. 一种电解铜箔表面的微细粗化处理工艺: 中国, CN110205656A[P]. 2019-09-06.
[13] 何成群, 赵原森, 柴 云, 等. 一种电解铜箔生产中的表面处理工艺: 中国, CN103088379A[P]. 2013-05-08.
[15] 郑衍年. 电解铜箔表面处理工艺与结晶形态[J]. 印制电路信息, 2004(10): 14-16.
[16] 李应恩, 樊斌峰, 王建智, 等. 一种提高电解铜箔高温抗剥离性能面处理工艺: 中国, CN105018978A[P]. 2015-11-04.
[17] 杨培霞, 安茂忠, 胡旭日, 等. 印制板用电解铜箔后处理工艺的研究[J]. 电镀与涂饰, 2005, 24(8): 42-45.
[18] 谭育慧, 王 艳, 章 朦, 等. 电解铜箔表面电沉积Zn-Ni-P-La合金工艺[J]. 应用化学, 2015, 32(4): 458-463.
[21] 李应恩, 樊斌锋, 王建智, 等. 锂电池用铜箔无铬防氧化技术的研究[J]. 中外企业家, 2016(18): 131-132.
[22] 林家宝. 一种环保型电解铜箔无铬钝化处理液和处理方法: 中国, CN107151809A[P]. 2017-09-12.
[23] 杨少坤. 新型环保铜箔表面钝化处理工艺研究[J]. 地球, 2013(3): 189-190.
[24] 张金涛, 胡吉明, 张鉴清, 等. 金属涂装预处理新技术与涂层性能研究方法进展[J]. 表面技术, 2005, 34(1): 1-4.
[29] 胡旭日, 王维河, 王海振, 等. 一种电解铜箔表面处理剂的制备方法: 中国, CN104099061A[P]. 2014-10-15.
[33] 张立茗, 方景礼, 袁国伟, 等. 实用电镀添加剂[M]. 北京: 化学工业出版社, 2007.
[35] 郑雅杰, 李春华, 邹伟红. 三乙醇胺和EDTA·2Na盐双络合体系快速化学镀铜工艺研究[J]. 材料导报, 2006, 20(10): 159-162.
[38] 肖 宁, 邓志江, 滕艳娜, 等. 整平剂对酸性电镀硬铜的影响[J]. 电镀与涂饰, 2015, 34(19): 1082-1087.
[43] 丁辛城, 彭代明, 陈梓侠, 等. 新型整平剂TS-L对铜电沉积的影响[J]. 电镀与涂饰, 2016, 35(11): 556-559.
[49] 徐文柱. 表面活性剂在电镀中的应用[J]. 过滤与分离, 2014, 24(4): 40-46.
[50] 郭国才. 表面活性剂在电镀中的应用[J]. 电镀与环保, 2006, 26(3): 15-16.
[53] 蔡加勒, 张爱强, 周绍民. 聚乙二醇聚合度对其吸附和阻化Cu2+放电的影响[J]. 高等学校化学学报, 1988, 9(1): 57-61.
[56] 张文海, 陈俊民. 明胶在冶金中的应用[J]. 明胶科学与技术, 1983(1): 1-10.
[58] 任忠文. 对电解铜箔生产明胶加入方法的讨论[J]. 印制电路信息, 2002(10): 24-25.
[66] 辜 敏, 李 强, 鲜晓红, 等. PEG-Cl-添加剂存在下的铜电结晶过程研究[J]. 化学学报, 2007, 65(10): 881-886.
[72] 杨胜奇. 稀土在金属表面处理工艺中的应用技术(3)—— 稀土对提高酸性亮铜镀层低区走位和抗变色能力的影响[J]. 材料保护, 2008, 41(5): 81-83.
[73] 杨胜奇, 张弘伟, 汪建忠. 稀土添加剂在光亮酸性镀铜中的应用[J].材料保护, 2003, 36(4): 67-67.
[74] 孔繁清, 闫慧忠, 赵增祺, 等. 稀土发光材料在化学复合镀中应用的研究[J]. 稀土, 2002, 23(4): 43-45.
[75] 何 田, 易光斌, 蔡芬敏, 等. 添加RE对电解铜箔组织与性能的影响[J]. 特种铸造及有色合金, 2010, 30(7): 658-660.
[76] 张世超, 石伟玉, 白致铭. 铜箔表面粗化工艺的研究[J]. 电镀与精饰, 2005, 27(5): 1-3.
[77] 杨祥魁, 徐树民, 王维河, 等. 一种电解铜箔表面的微细粗化处理工艺: 中国, CN106011965A[P]. 2016-10-12.
[78] 黄永发, 王 平, 唐云志, 等. 一种新型电解铜箔无砷粗化工艺研究[J]. 有色金属科学与工程, 2012, 3(2): 1-4.
[79] 冯绍彬, 李振兴, 胡芳红, 等. 铜箔表面电镀铜粗化工艺[J].材料保护, 2010, 43(7): 24-26.
[80] 周启伦, 郑惠军, 黄国平, 等. 一种电解铜箔用添加剂及甚低轮廓电解铜箔表面处理工艺: 中国, CN102560584A[P]. 2012-07-11.


